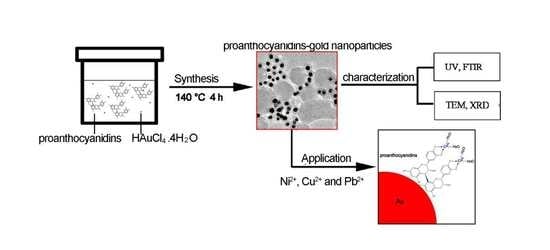
Green Synthesis, Characterization and Application of Proanthocyanidins-Functionalized Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pomegranate Peel Extract Protected Gold Nanoparticles
2.3. Synthesis of Proanthocyanidin-Functionalized Gold Nanoparticles
2.4. Removal of Heavy Metal Ions and Methylene Blue (MB)
2.5. Characterization of Gold Nanoparticles and Determination of the Concentration of Heavy Metal Ions
2.5.1. UV-Vis Absorption Spectra
2.5.2. Fourier Transform Infrared Spectroscopy
2.5.3. Transmission Electron Microscopy
2.5.4. X-ray Diffraction
2.5.5. Atomic Absorption Spectrometry
3. Results and Discussion
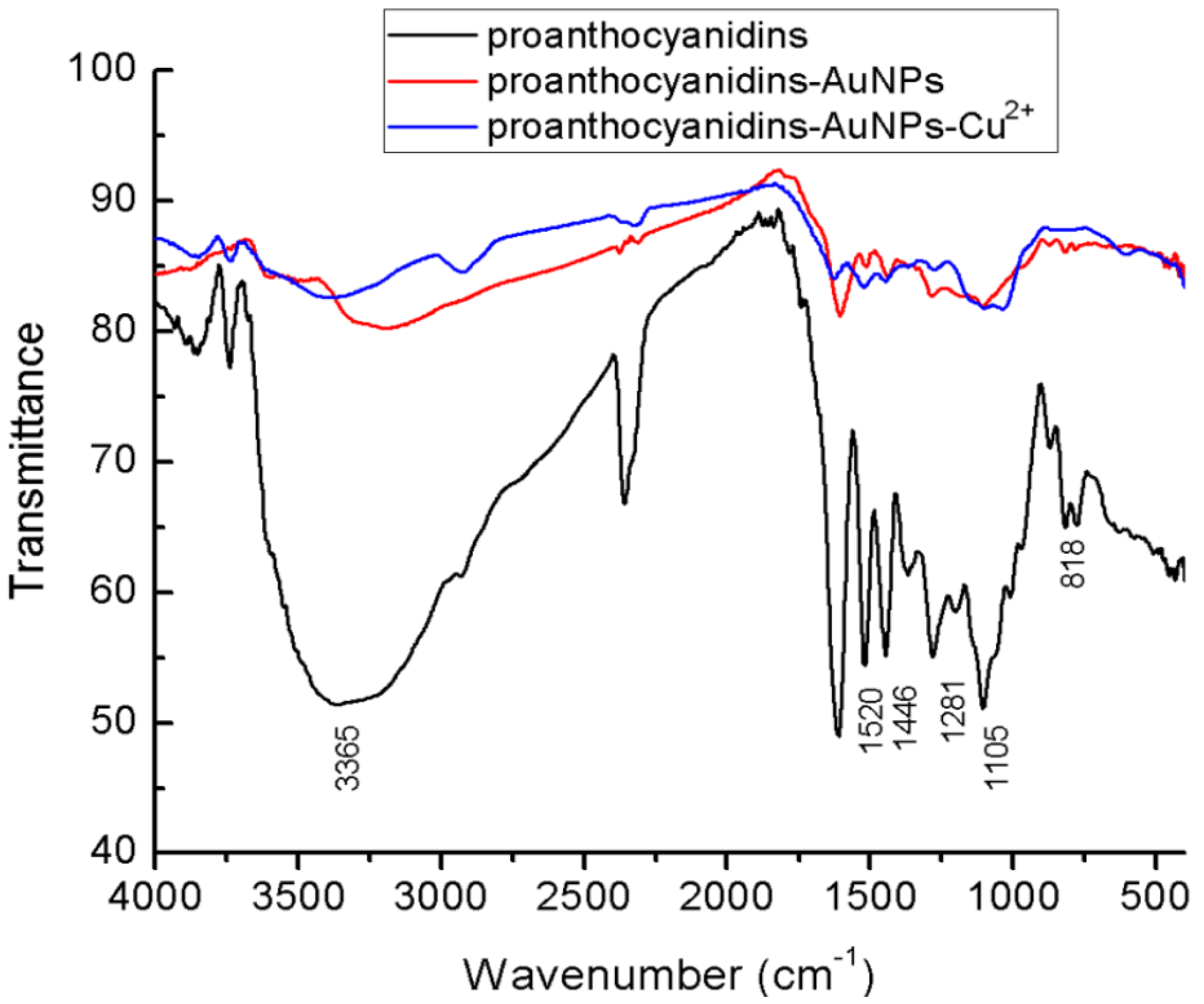
3.1. UV-Vis and FTIR Absorption Spectra
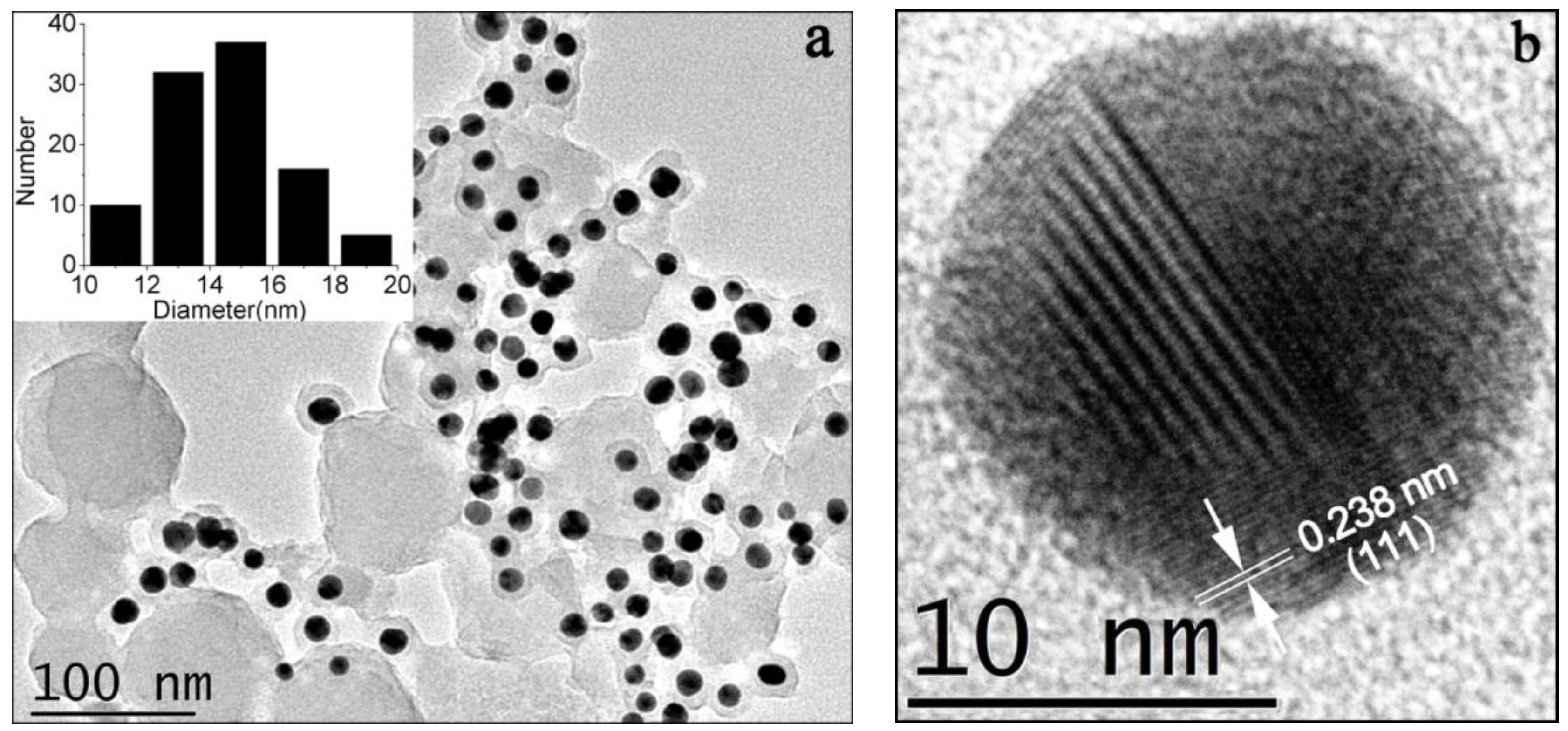
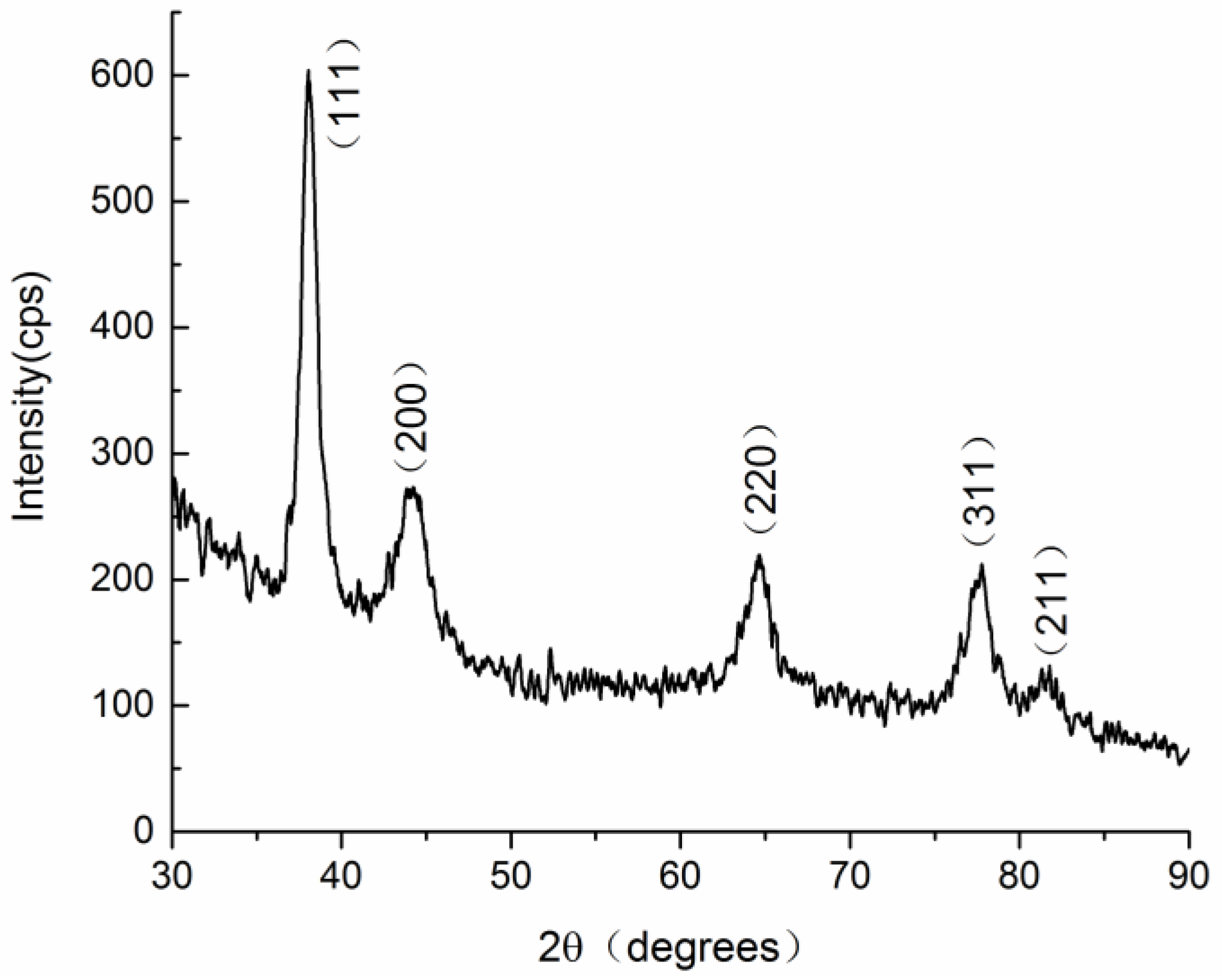
3.2. TEM and XRD Characterization
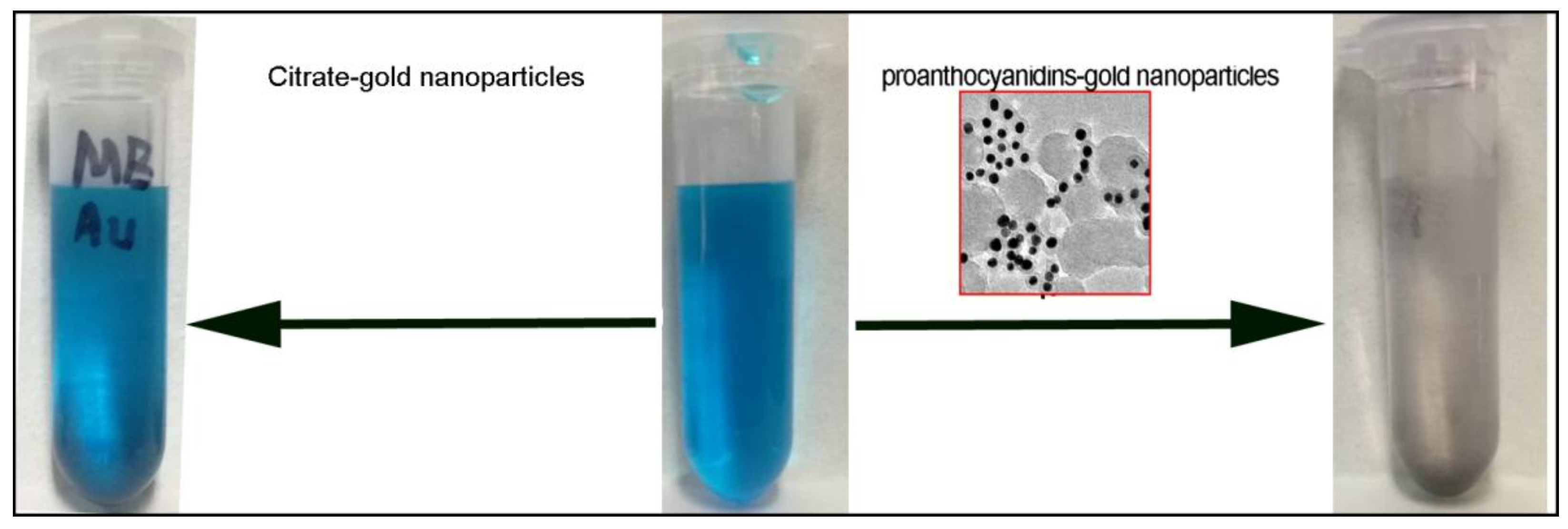
3.3. Formation Mechanism of Proanthocyanidins-Functionalized Gold Nanoparticles
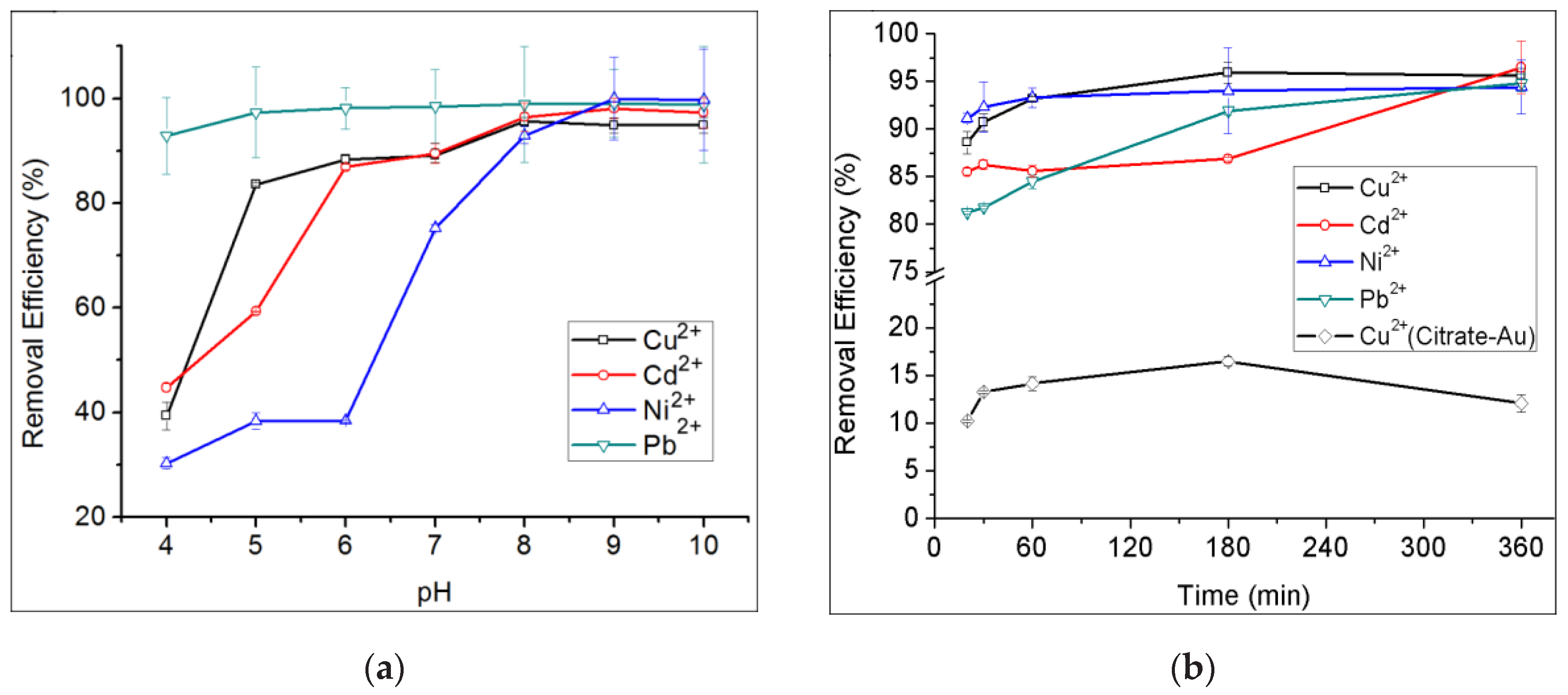
3.4. Removal of Dye and Heavy Metal Ions in Aqueous Solution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; EI-Sayed, I.H.; EI-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic Side Chains. Bioconj. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, Z.; Zu, Y.; Fu, Y.; Zhao, C.; Zhao, X.; Meng, R.; Tan, S. Synthesis of camptothecin-loaded gold nanomaterials. Appl. Surf. Sci. 2010, 256, 3917–3920. [Google Scholar] [CrossRef]
- Polavarapu, L.; Xu, Q.-H. A simple method for large scale synthesis of highly monodisperse gold nanoparticles at room temperature and their electron relaxation properties. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, S.; Klabunde, K.J.; Sorensen, C.M.; Dragieva, I. Gram-Scale synthesis of monodisperse gold colloids by the solvated metal atom dispersion method and digestive ripening and their organization into two- and three-dimensional structures. J. Am. Chem. Soc. 2002, 124, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant mediated green synthesis of CuO nanoparticles: Comparison of toxicity of engineered and plant mediated CuO nanoparticles towards Daphnia magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, R.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Botero, L.; Herrera, A.P.; Hinestroza, J.P. Oriented growth of -MnO2 nanorods using natural extracts from grape stems and apple peels. Nanomaterials 2017, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Foo, Y.Y.; Periasamy, V.; Kiew, L.V.; Kumar, G.G.; Malek, S.N.A. Curcuma mangga-mediated synthesis of gold nanoparticles: Characterization, stability, cytotoxicity, and blood compatibility. Nanomaterials 2017, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Camargo, A.C.D.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer Skin, mesocarp, divider Membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Shi, S.; Li, P.; Pan, Z.; Venkitasamy, C. Extraction kinetics and properties of proanthocyanidins from pomegranate Peel. Int. J. Food Eng. 2014, 10, 683–695. [Google Scholar] [CrossRef]
- Skerget, M.; Kotnik, P.; Hadolin, M.; Hras, A.R.; Simonic, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research. New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ferna´ndez, J.; Pérez-Juste, J.; Abajo, F.J.G.; Liz-Marzán, L.M. Seeded growth of submicron Au colloids with quadrupole plasmon resonance modes. Langmuir 2006, 22, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Hakanson, U.; Rogobete, L.; Sandoghdar, V. Enhancement of single-molecule fluorescence using a gold nanoparticle as an optical nanoantenna. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zu, Y.; Fu, Y.; Meng, R.; Guo, S.; Xing, Z.; Tan, S. Hydrothermal synthesis of histidine-functionalized single-crystalline gold nanoparticles and their pH-dependent UV absorption characteristic. Colloid Surf. B 2010, 76, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Foo, L.Y. Proanthocyanidins: Gross chemical structures by infrared spectra. Phytochemistry 1981, 20, 1397–1402. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Technol. 2007, 41, 235–247. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Determination of crystallite size in synthetic and natural hydroxyapatite: A comparison between XRD and TEM results. Adv. Mater. Res. 2013, 620, 28–34. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nulcleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Torreggiani, A.; Jurasekova, Z.; Sanchez-Cortes, S.; Tamba, M. Spectroscopic and pulse radiolysis studies of the antioxidant properties of (+)catechin: Metal chelation and oxidizing radical scavenging. J. Raman Spectrosc. 2008, 39, 265–275. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, G.; Zhang, Z.; Wang, A. A simple hydrothermal approach to modify palygorskite for high-efficient adsorption of methylene blue and Cu(II) ions. Chem. Eng. J. 2015, 265, 228–238. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Narband, N.; Uppal, M.; Dunnill, C.W.; Hyett, G.; Wilson, M.; Parkin, I.P. The interaction between gold nanoparticles and cationic and anionic dyes: Enhanced UV-visible absorption. Phys. Chem. Chem. Phys. 2009, 11, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Kitching, H.; Kenyon, A.J.; Parkin, I.P. The interaction of gold and silver nanoparticles with a range of anionic and cationic dyes. Phys. Chem. Chem. Phys. 2014, 16, 6050–6059. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Calderon, B.; Fullana, A. Heavy metal release due to aging effect during zero valent iron nanoparticles remediation. Water Res. 2015, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Weng, X.; Tong, Y.; Lin, W.; Chen, Z. Simultaneous removal of Pb (II) and Cd (II) from aqueous solution by green synthesized iron nanoparticles. Acta Sci. Circumst. 2015, 35, 3538–3544. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biao, L.; Tan, S.; Meng, Q.; Gao, J.; Zhang, X.; Liu, Z.; Fu, Y. Green Synthesis, Characterization and Application of Proanthocyanidins-Functionalized Gold Nanoparticles. Nanomaterials 2018, 8, 53. https://doi.org/10.3390/nano8010053
Biao L, Tan S, Meng Q, Gao J, Zhang X, Liu Z, Fu Y. Green Synthesis, Characterization and Application of Proanthocyanidins-Functionalized Gold Nanoparticles. Nanomaterials. 2018; 8(1):53. https://doi.org/10.3390/nano8010053
Chicago/Turabian StyleBiao, Linhai, Shengnan Tan, Qinghuan Meng, Jing Gao, Xuewei Zhang, Zhiguo Liu, and Yujie Fu. 2018. "Green Synthesis, Characterization and Application of Proanthocyanidins-Functionalized Gold Nanoparticles" Nanomaterials 8, no. 1: 53. https://doi.org/10.3390/nano8010053