Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copper Nanoparticle Dissolution Experiments
2.3. Copper Nanoparticle and Cu(II) (aq) Adsorption Experiments
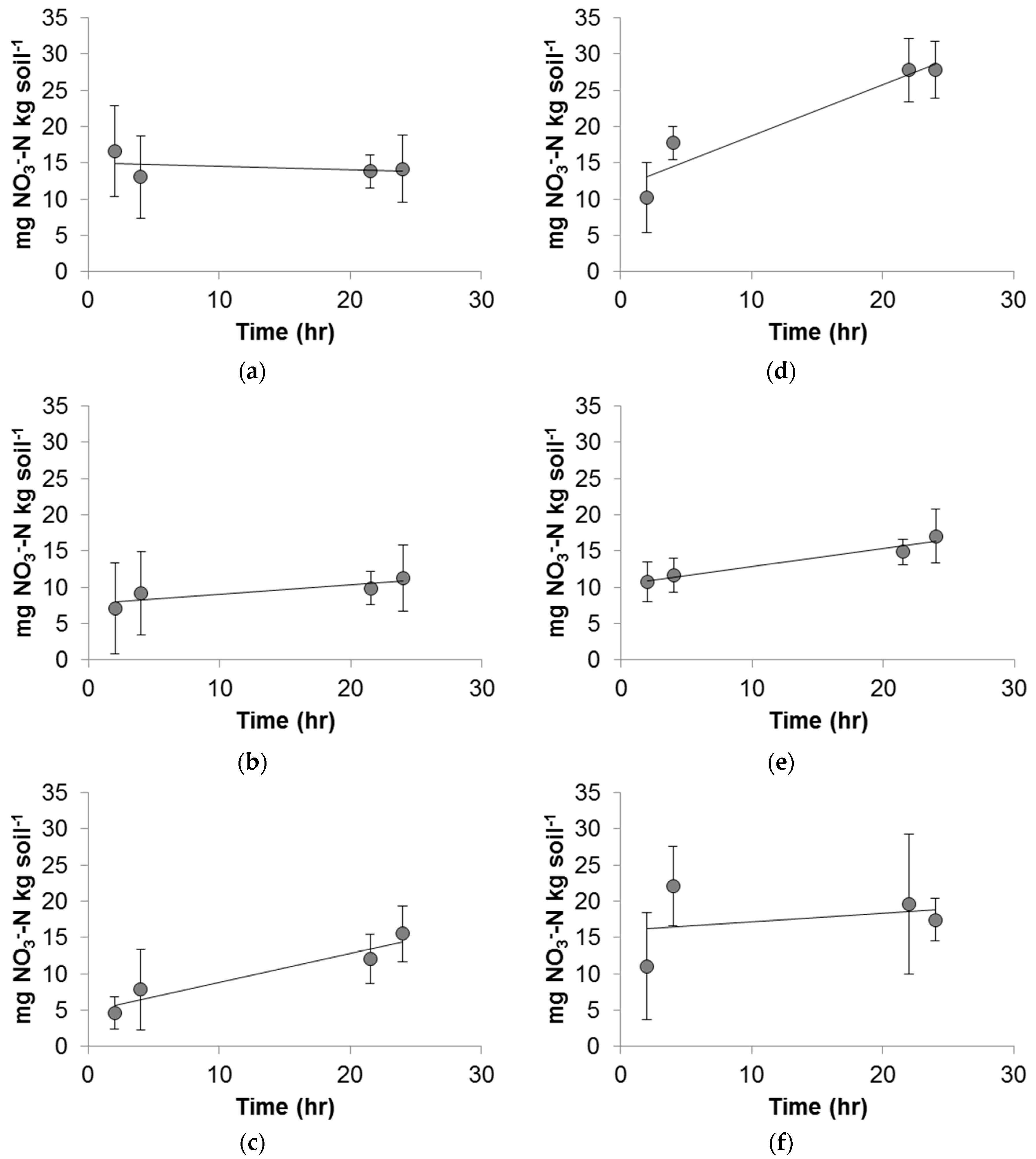
2.4. Batch Nitrification Kinetic Experiments
2.5. Statistical Analysis of Batch Nitrification Kinetic Experiments
2.6. X-Ray Absorption near Edge Structure Spectroscopy (XANES) Analysis
3. Results and Discussion
3.1. Copper Nanoparticle Dissolution Experiments
3.2. CuNP and Cu2+ (aq) Sorption Experiments
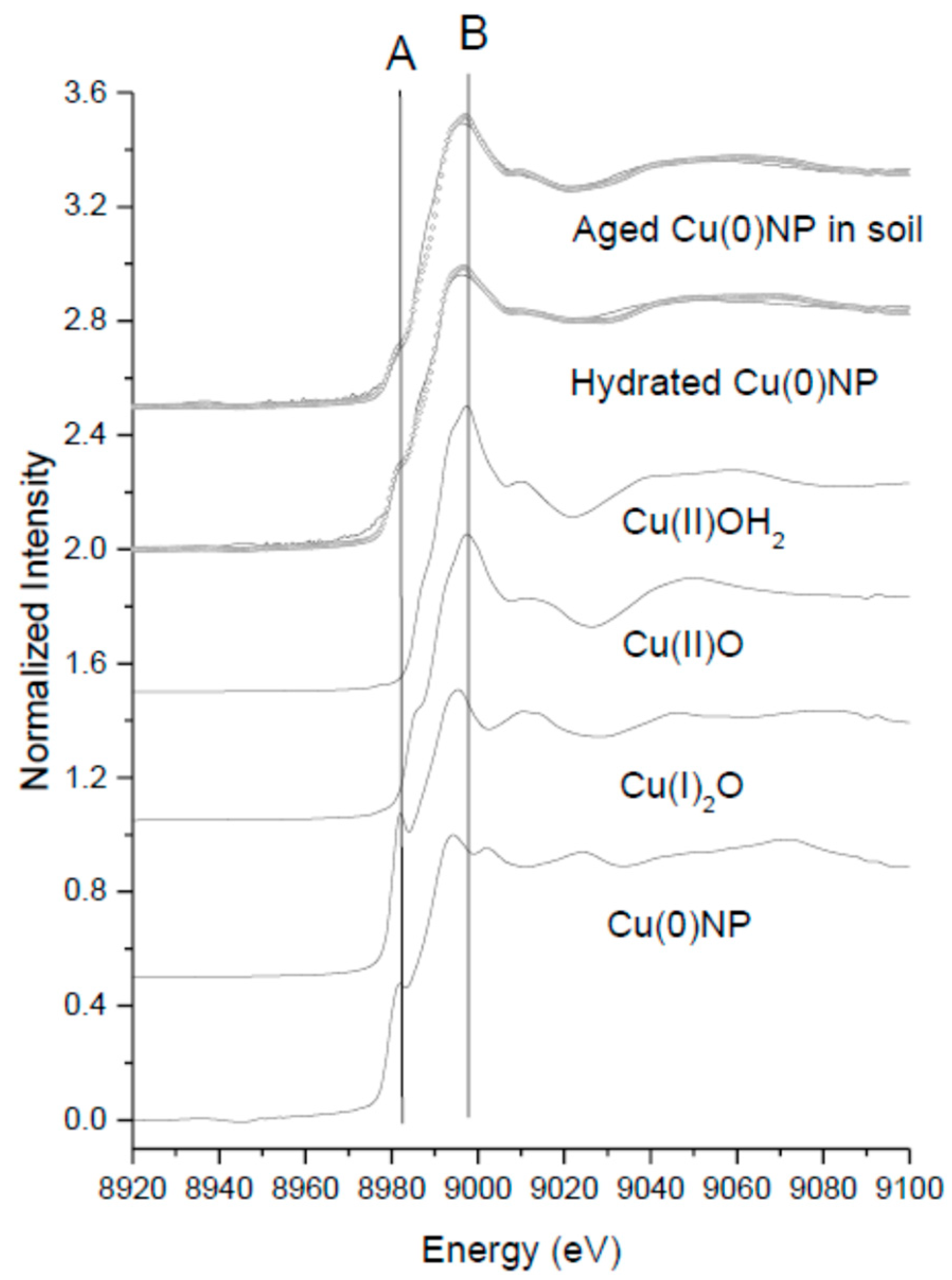
3.3. XANES Analysis
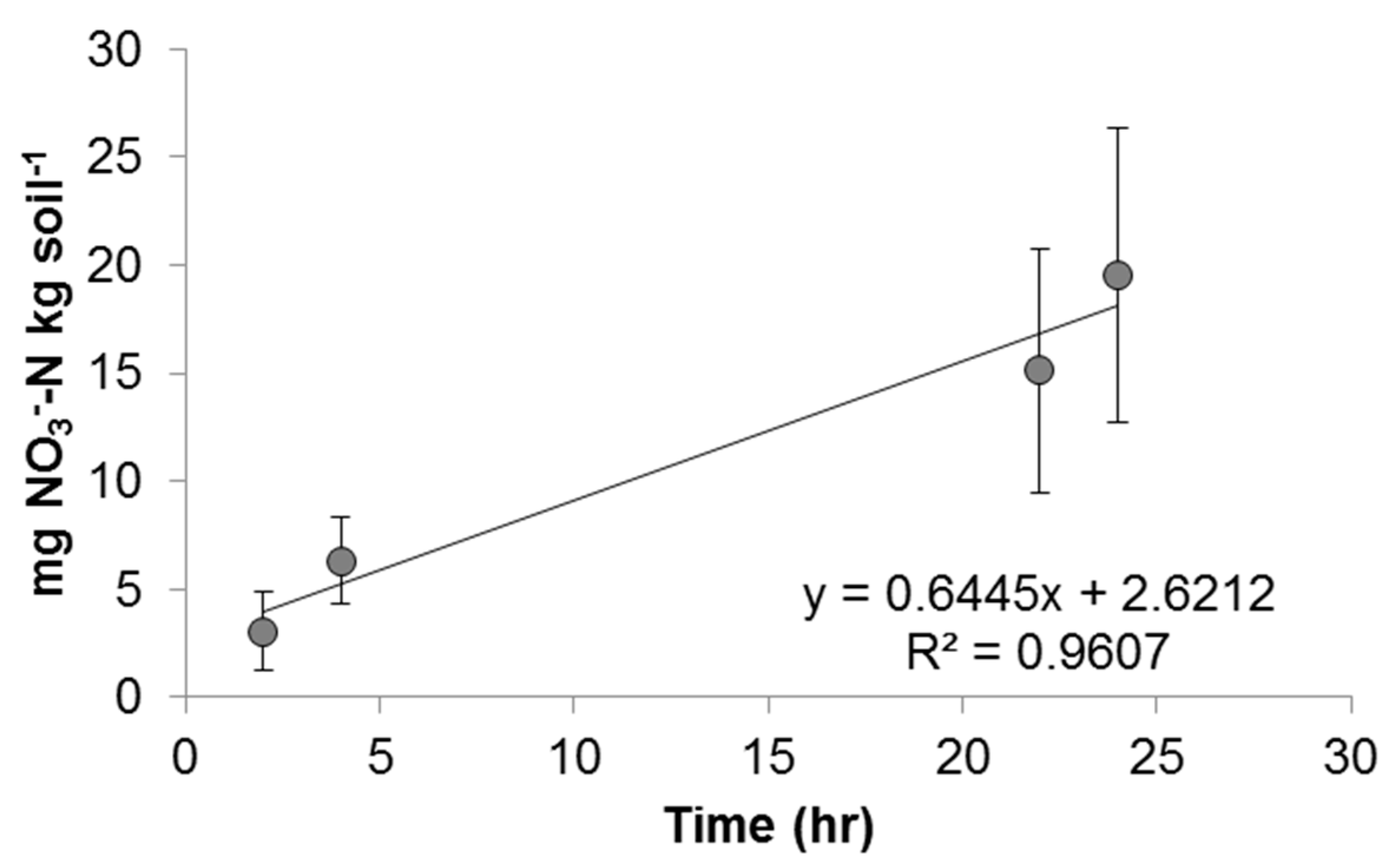
3.4. Batch Nitrification Kinetic Experiments
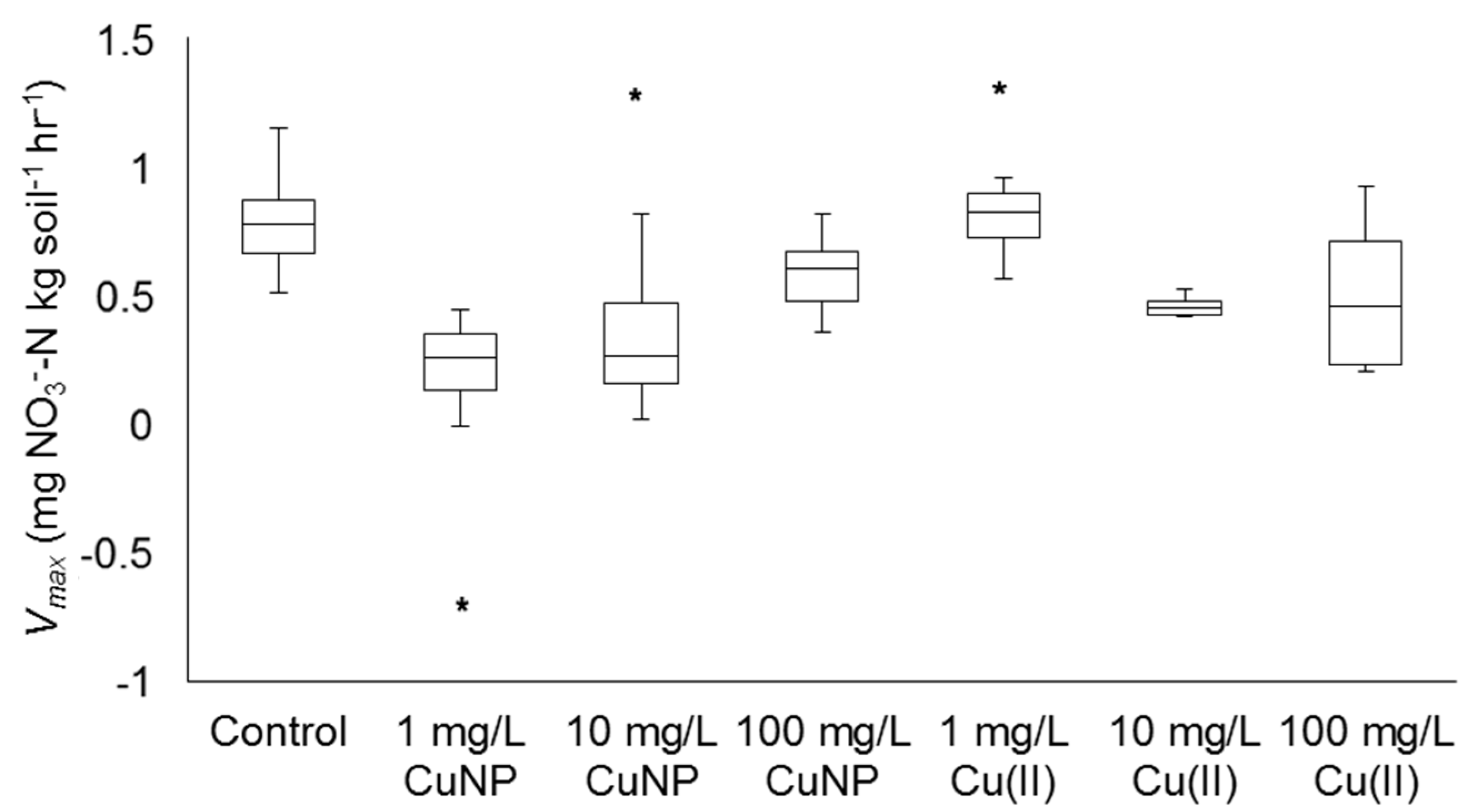
3.5. Impacts of Cu2+ and CuNPs on Soil Nitrification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CuNP | Copper nanoparticles |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| ISE | Ion selective electrode |
| MOPSO | 3-Morpholino-2-hydroxypropanesulfonic acid |
| NP | nanoparticles |
| XAS | X-ray absorption spectroscopy |
References
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Feng, Z.-B.; Zhang, F.-D.; Zhang, S.-Q.; He, X.-S. Preparation and Testing of Cementing and Coating Nano-Subnanocomposites of Slow/Controlled-Release Fertilizer. Agric. Sci. China 2006, 5, 700–706. [Google Scholar] [CrossRef]
- Ray, S.K.; Varadachari, C.; Ghosh, K. Novel Slow-Releasing Micronutrient Fertilizers. 2. Copper Compounds. J. Agric. Food Chem. 1997, 45, 1447–1453. [Google Scholar] [CrossRef]
- Barden, J.A.; Halfacre, R.G.; Parrish, D.J. Plant Science; McGraw-Hill: New York, NY, USA, 1987. [Google Scholar]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Lee, W.-M.; An, Y.-J.; Yoon, H.; Kweon, H.-S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Nagy, K.L.; Marcus, M.A.; Lanson, M.; Geoffroy, N.; Jacquet, T.; Kirpichtchikova, T. Formation of metallic copper nanoparticles at the soil–root interface. Environ. Sci. Technol. 2008, 42, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO Nanoparticle Dissolution and Toxicity to Wheat (Triticum aestivum) in Rhizosphere Soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Du, W.C.; Tan, W.J.; Yin, Y.; Ji, R.; Peralta-Videa, J.R.; Guo, H.Y.; Gardea-Torresdey, J.L. Differential effects of copper nanoparticles/microparticles in agronomic and physiological parameters of oregano (Origanum vulgare). Sci. Total Environ. 2018, 618, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- VandeVoort, A.R.; Arai, Y. Effect of silver nanoparticles on soil denitrification kinetics. Ind. Biotechnol. 2012, 8, 358–364. [Google Scholar] [CrossRef]
- VandeVoort, A.R.; Skipper, H.; Arai, Y. Macroscopic assessment of nanosilver toxicity to soil denitrification Kinetics. J. Environ. Qual. 2014, 43, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- VandeVoort, A.R.; Tappero, R.; Arai, Y. Residence time effects on phase transformation of nanosilver in reduced soils. Environ. Sci. Pollut. Res. 2014, 21, 7828–7837. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-Y.; Hoon Byeon, J.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shah, V.; Walker, V.K. Perturbation of an arctic soil microbial community by metal nanoparticles. J. Hazard. Mater. 2011, 190, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Braida, W.; Christodoulatos, C.; Wazne, M.; O’Connor, G. Nano-aluminum: Transport through sand columns and environmental effects on plants and soil communities. Environ. Res. 2008, 106, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Sikora, F.J.; Moore, K.P. Soil Test Methods from the Southeastern United States; Southern Cooperative Series Bulletin No. 419; Southern Extension and Research Activity Information Exchange Group 6 (SERA-IEG-6): Athens, GA, USA, 2014; ISBN 1-58161-419-5. [Google Scholar]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen Mineralization, Immobilization, and Nitrification. In Methods of Soil Analysis, Part 2. Microbiological and Bio-Chemical Properties; Weaver, R., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar]
- Masrahi, A.; VandeVoort, A.R.; Arai, Y. Effects of silver nanoparticle on soil-nitrification processes. Arch. Environ. Contam. Toxicol. 2014, 66, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Arai, Y. Aqueous interfacial chemistry of kaolinite for the removal of Cu(II) in the presence of birnessite: Kinetics and spectroscopic studies. Appl. Clay Sci. 2011, 53, 572–580. [Google Scholar] [CrossRef]
- Webb, S.M. SIXpack: A graphical user interface for XAS analysis using IFEFFIT. Phys. Scr. 2005, T115, 1011–1014. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivaska, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to Copper Nanoparticles Causes Gill Injury and Acute Lethality in Zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-W.; An, Y.-J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Guyen, O.T.N.; Oldenburg, S.J. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Chemistry of Soils, 2nd ed.; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Cabrero, A.; Fernandez, S.; Mirada, F.; Garcia, J. Effects of copper and zinc on the activated sludge bacteria growth kinetics. Water Res. 1998, 32, 1355–1362. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Lees, H. The effects of zinc and copper on soil nitrification. Biochem. J. 1948, 42, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Fait, G.; Broos, K.; Zrna, S.; Lombi, E.; Hamon, R. Tolerance of nitrifying bacteria to copper and nickel. Environ. Toxicol. Chem. 2006, 25, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 27, 2157–2164. [Google Scholar] [CrossRef] [PubMed]

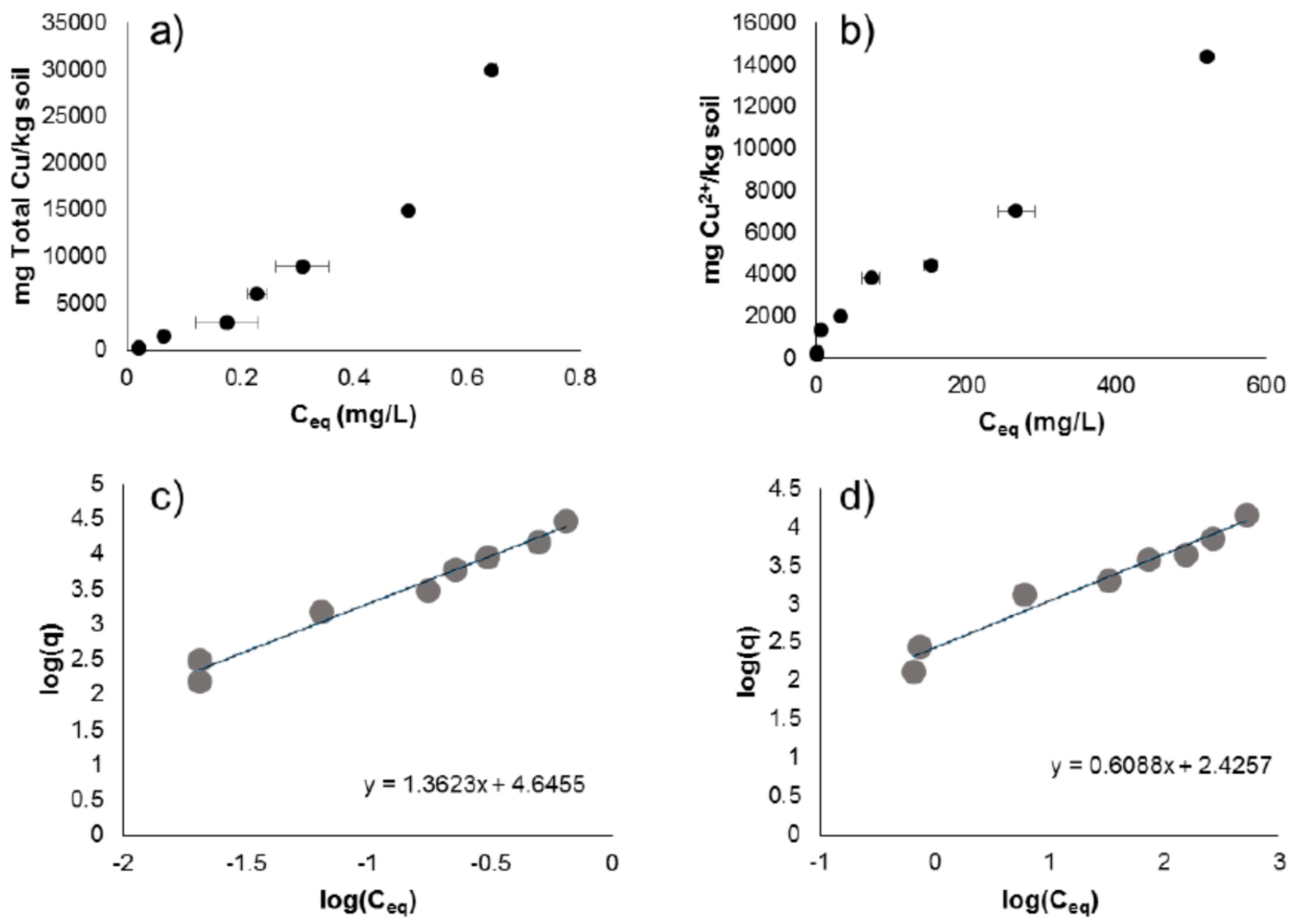
| Copper Species | Intercept | Kd | Slope | n | R2 | p |
|---|---|---|---|---|---|---|
| CuNP | 4.646 | 44,210 | 1.362 | 0.7341 | 0.9782 | <0.001 |
| Cu(II)SO4 | 2.426 | 266.5 | 0.6088 | 1.642 | 0.9644 | <0.001 |
| Sample | % Cu(0)NP | % Cu(II)O | % Cu(I)2O | % Cu(OH)2 | Reduced Chi Square |
|---|---|---|---|---|---|
| Bulk XAS Analysis | |||||
| Freshly hydrated Cu(0)NPs | 51.19 ± 0.5 | 20.98 ± 0.5 | 22.4 ± 0.5 | 6 ± 0.5 | 0.00101 |
| 4mo aged Cu(0)NPs in soil | 39.04 ± 0.7 | 11.03 ± 0.7 | - | 52.3 ± 0.7 | 0.00063 |
| [Cu]total and Cu Species | N | Average Vmax (mg NO3−-N kg Soil−1 h−1) Value | Average Intercept of Linear Regression | R2 of Linear Regression Line | p |
|---|---|---|---|---|---|
| Control | 17 | 0.645 (±0.190) | 2.62 | 0.961 | 0.020 |
| 1 mg/L Cu2+ | 9 | 0.703 (±0.236) | 11.7 | 0.908 | 0.045 |
| 10 mg/L Cu2+ | 8 | 0.247 (±0.0430) | 10.4 | 0.947 | 0.025 |
| 100 mg/L Cu2+ | 8 | 0.279 (±0.224) | 16.0 | 0.0837 | 0.683 |
| 1 mg/L CuNP | 12 | −0.0489 (±0.205) | 15.1 | 0.132 | 0.580 |
| 10 mg/L CuNP | 20 | 0.132 (±0.347) | 7.68 | 0.757 | 0.135 |
| 100 mg/L CuNP | 13 | 0.400 (±0.163) | 4.90 | 0.911 | 0.049 |
| [Cu]total and Cu Species | Control | 1 mg/L CuNP | 10 mg/L CuNP | 100 mg/L CuNP | 1 mg/L Cu2+ | 10 mg/L Cu2+ |
|---|---|---|---|---|---|---|
| 1 mg/L CuNP | 0.000285 | x | x | x | x | x |
| 10 mg/L CuNP | <0.0001 | 0.0637 | x | x | x | x |
| 100 mg/L CuNP | 0.00106 | 0.00255 | 0.00588 | x | x | x |
| 1 mg/L Cu2+ | 0.534 | <0.0001 | <0.0001 | 0.00506 | x | x |
| 10 mg/L Cu2+ | <0.0001 | <0.0001 | 0.565 | 0.00227 | 0.00035 | x |
| 100 mg/L Cu2+ | 0.0253 | 0.0424 | 0.382 | 0.382 | 0.0152 | 0.804 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VandeVoort, A.R.; Arai, Y. Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer. Nanomaterials 2018, 8, 927. https://doi.org/10.3390/nano8110927
VandeVoort AR, Arai Y. Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer. Nanomaterials. 2018; 8(11):927. https://doi.org/10.3390/nano8110927
Chicago/Turabian StyleVandeVoort, Allison Rick, and Yuji Arai. 2018. "Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer" Nanomaterials 8, no. 11: 927. https://doi.org/10.3390/nano8110927
APA StyleVandeVoort, A. R., & Arai, Y. (2018). Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer. Nanomaterials, 8(11), 927. https://doi.org/10.3390/nano8110927




