Clearly Transparent Nanopaper from Highly Concentrated Cellulose Nanofiber Dispersion Using Dilution and Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cellulose Pulp
2.2. Nanofibrillation
2.3. Conditioning of Cellulose Nanofiber Dispersion
2.4. Nanopaper
2.5. Characterization
2.6. Silver Nanowire and Transparent Conductive Lines
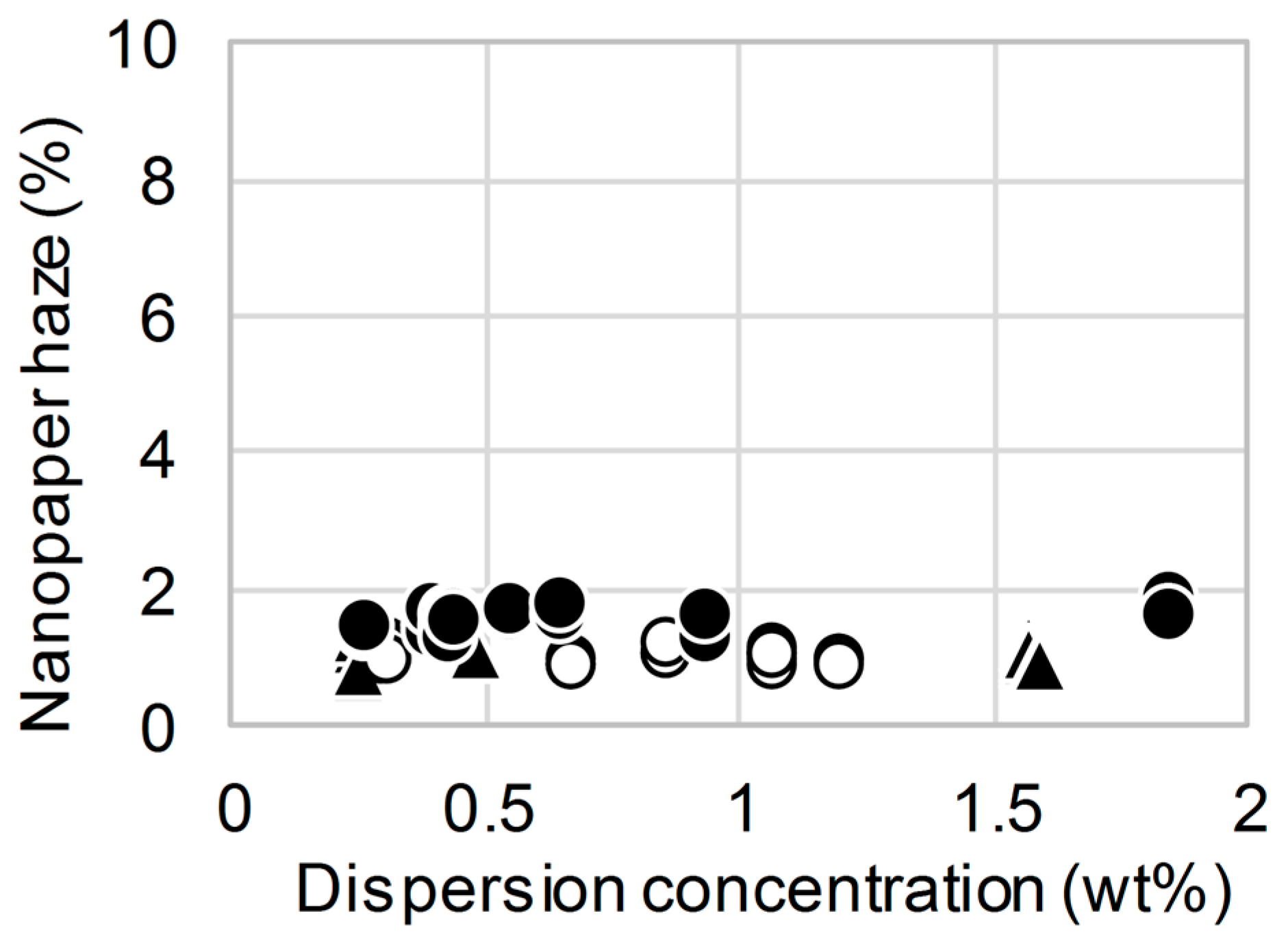
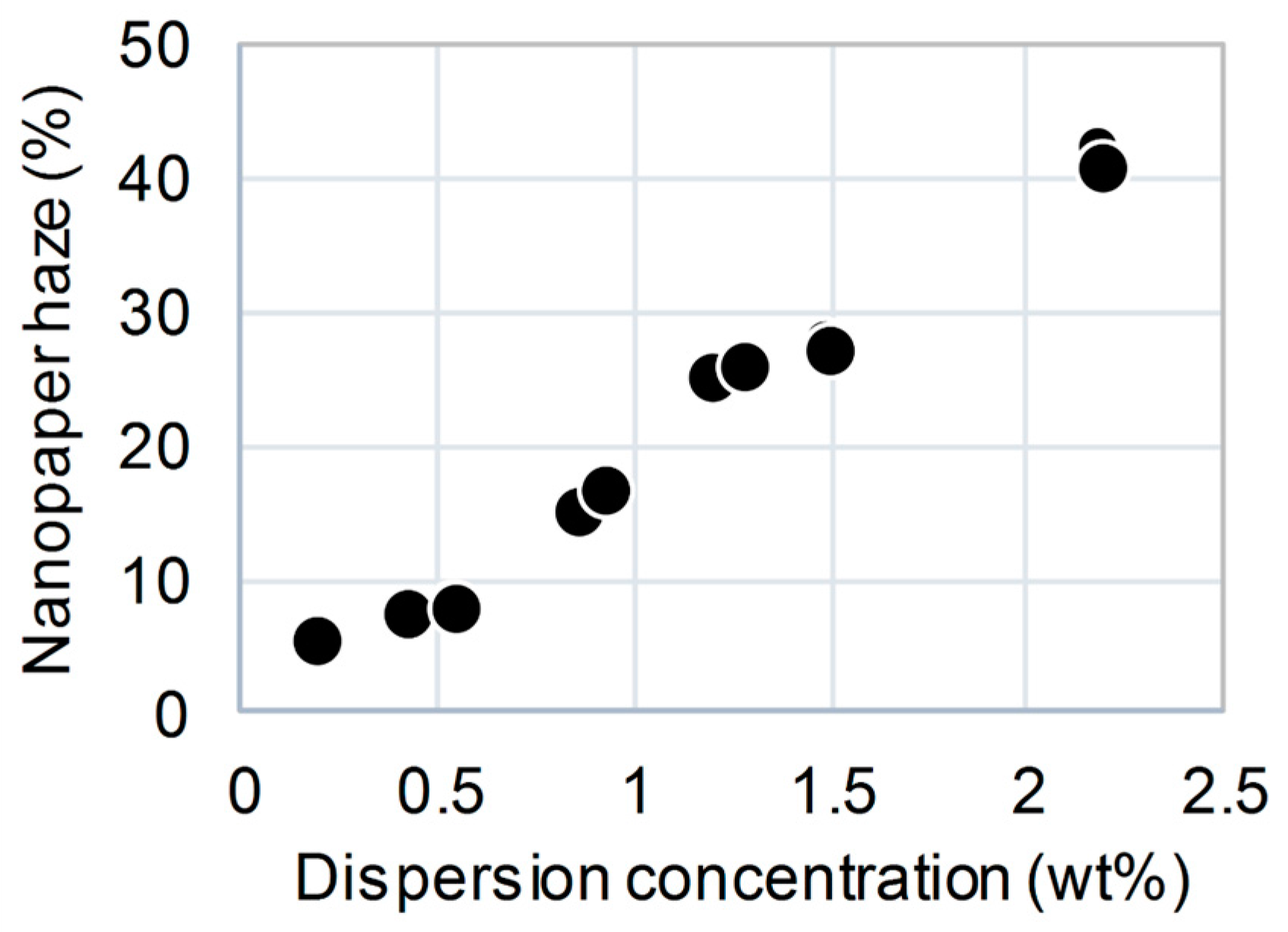
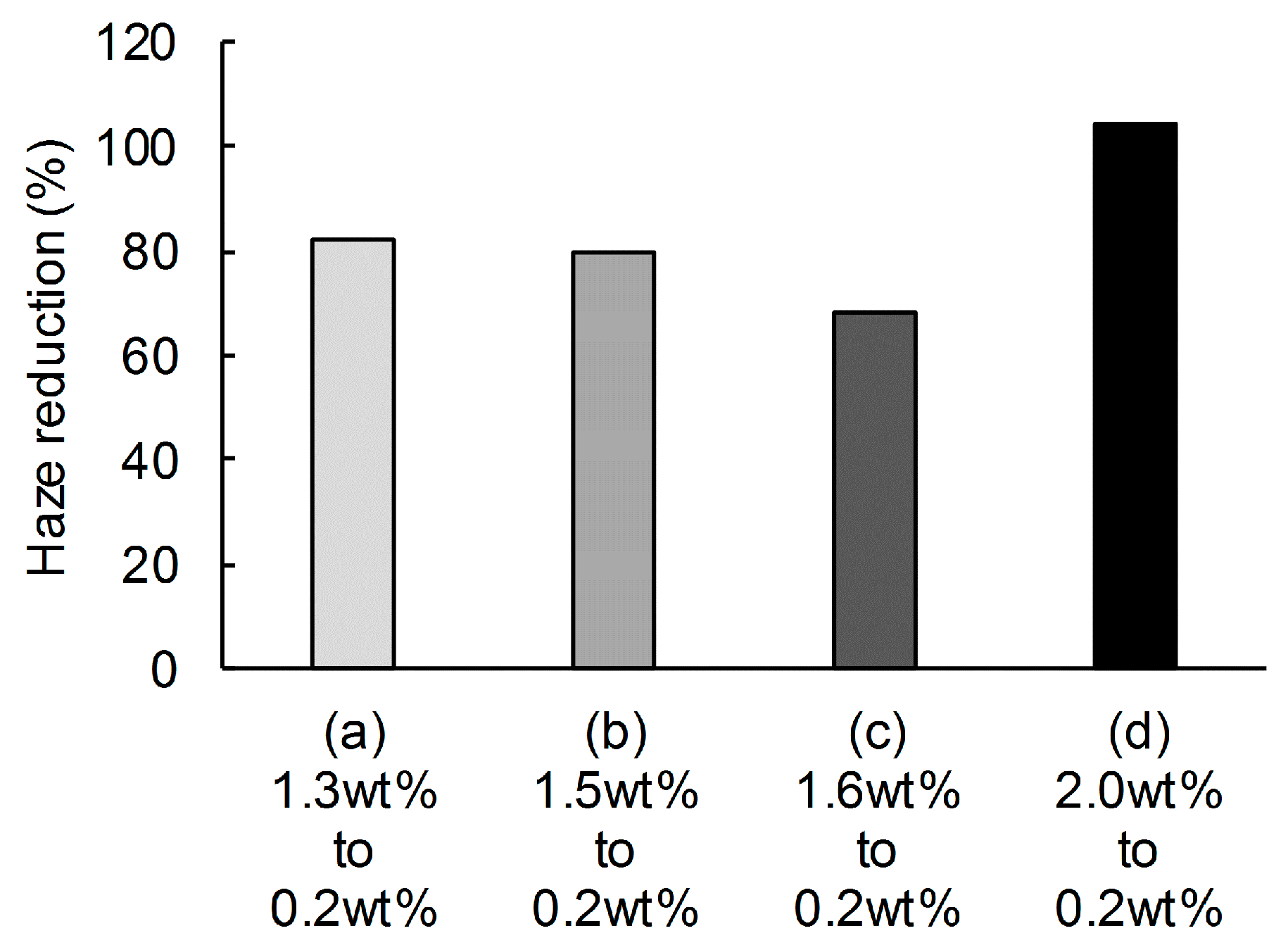
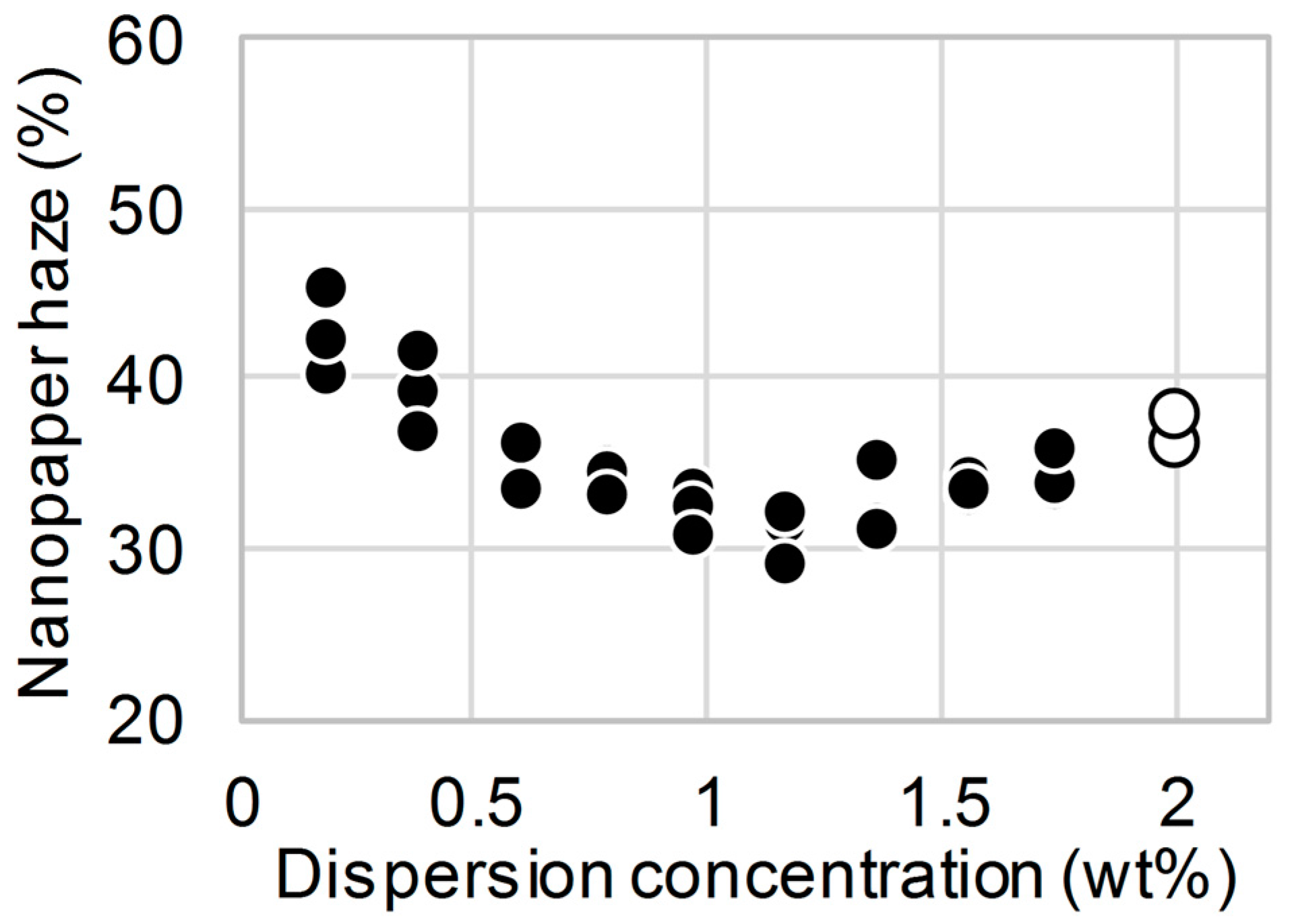
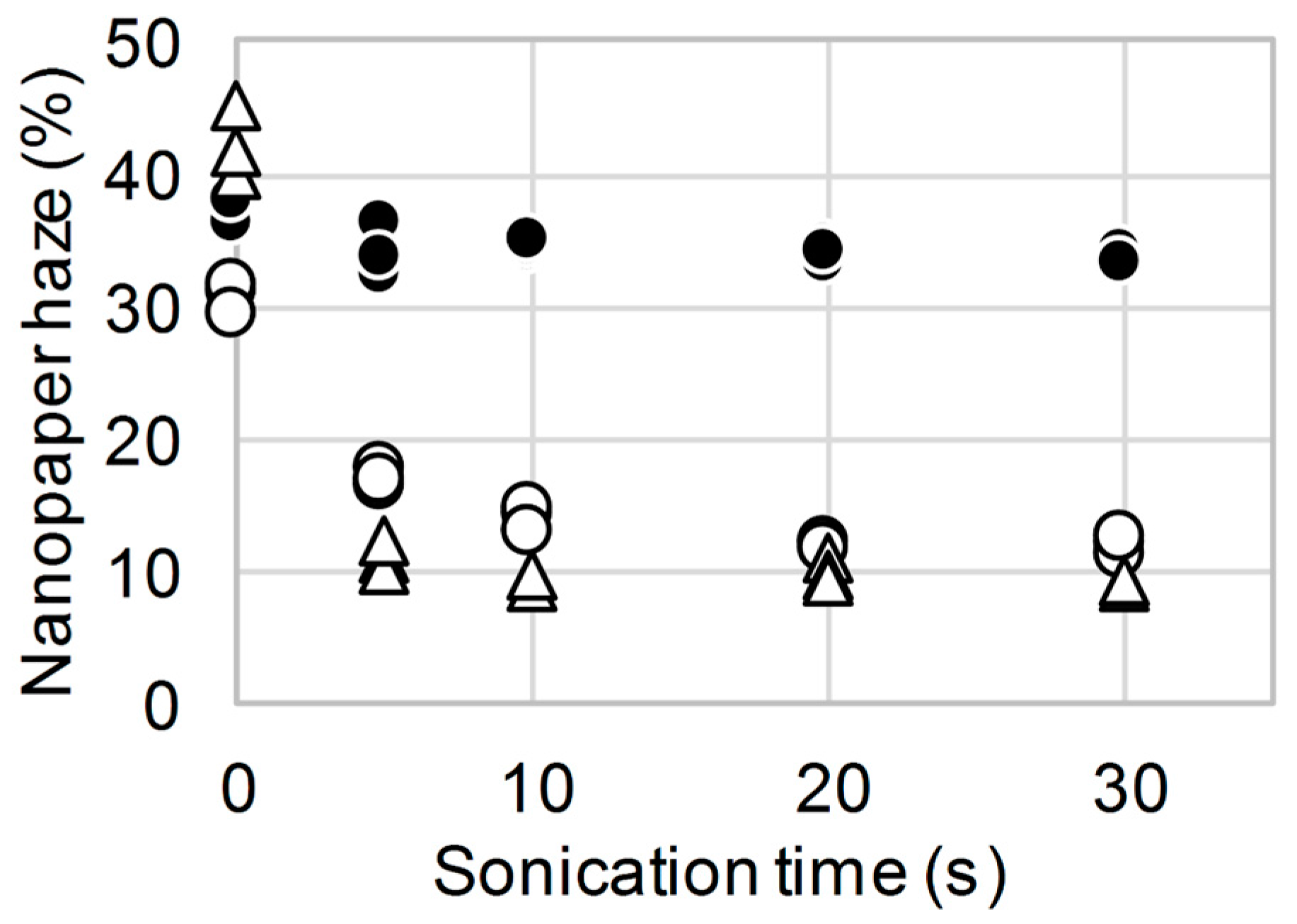
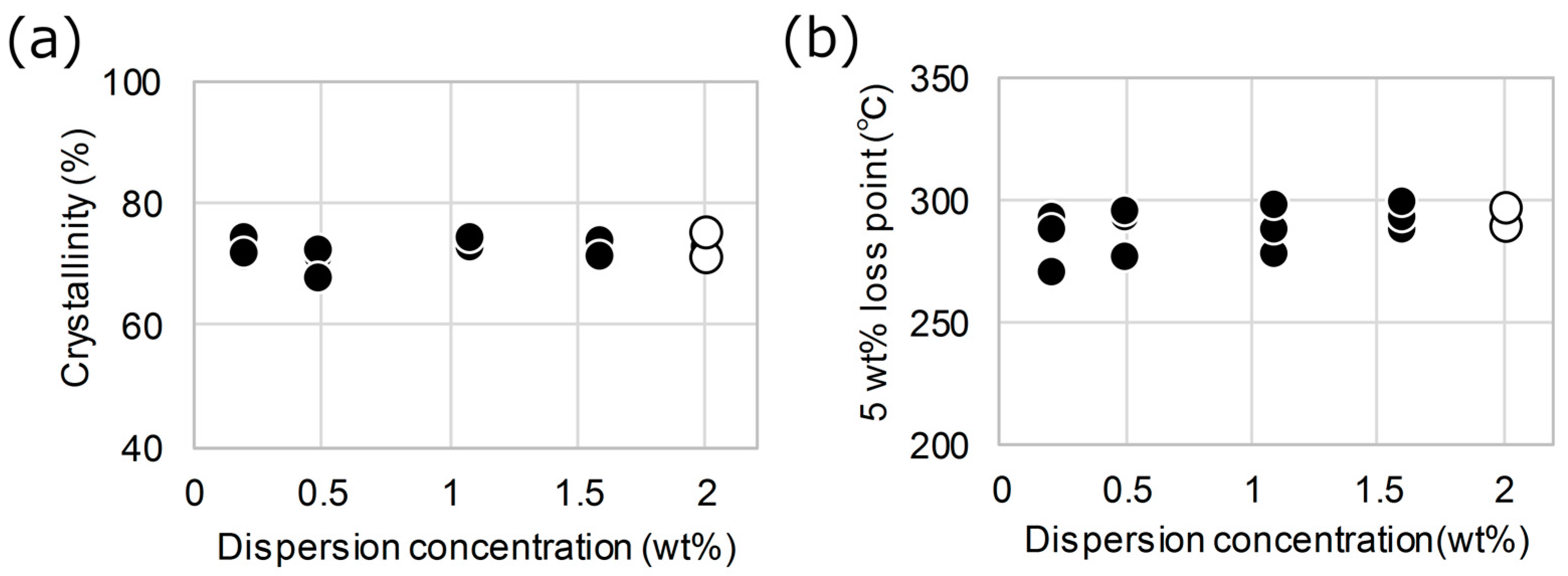
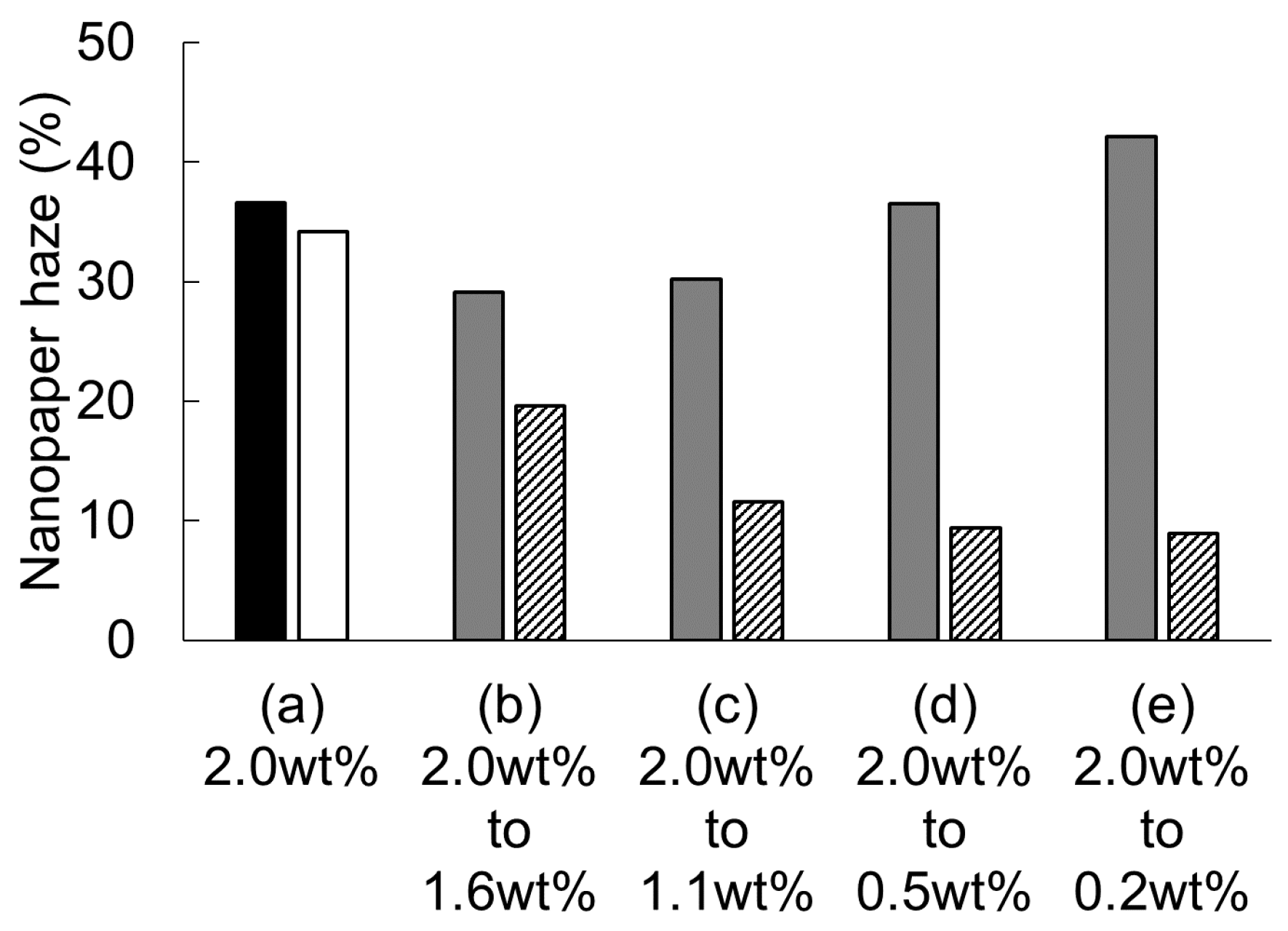
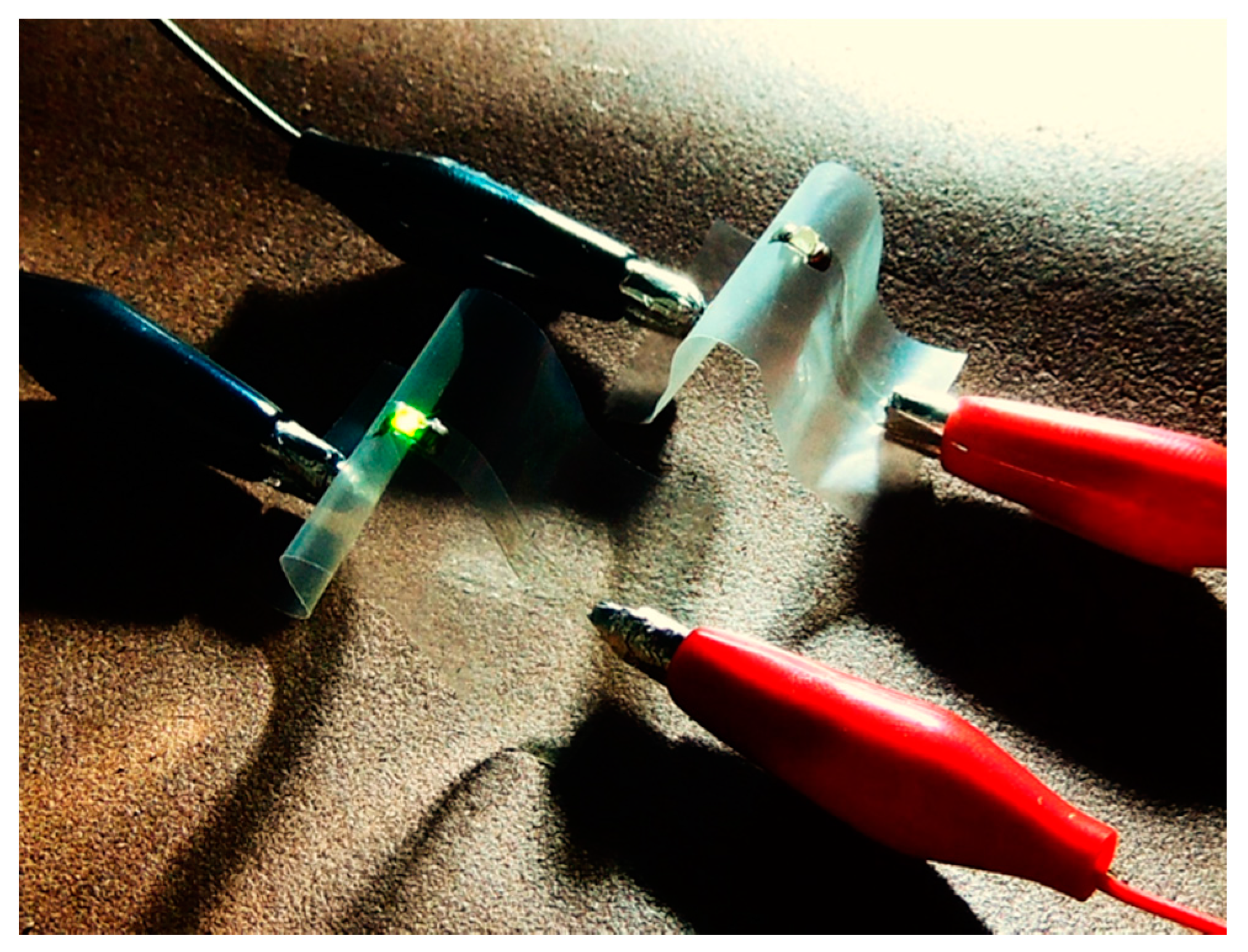
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically transparent nanofiber paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Nogi, M.; Kim, C.; Sugahara, T.; Inui, T.; Takahashi, T.; Suganuma, K. High thermal stability of optical transparency in cellulose nanofiber paper. Appl. Phys. Lett. 2013, 102, 181911. [Google Scholar] [CrossRef]
- Inui, T.; Koga, H.; Nogi, M.; Komoda, N.; Suganuma, K. A miniaturized flexible antenna printed on a high dielectric constant nanopaper composite. Adv. Mater. 2015, 27, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Celano, U.; Nagashima, K.; Koga, H.; Nogi, M.; Zhuge, F.; Meng, G.; He, Y.; De Boeck, J.; Jurczak, M.; Vandervorst, W.; et al. All-nanocellulose nonvolatile resistive memory. NPG Asia Mater. 2016, 8, e310. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Z.; Preston, C.; Lia, Y.; Hu, L. Transparent paper: Fabrications, properties, and device applications. Energy Environ. Sci. 2014, 7, 269–287. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Preston, C.; Hu, L. Development, application and commercialization of transparent paper. Transl. Mater. Res. 2014, 1, 015004. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent nanopaper-based flexible organic thin-film transistor array. Adv. Funct. Mater. 2014, 24, 1657–1663. [Google Scholar] [CrossRef]
- Koga, H.; Nogi, M.; Komoda, N.; Nge, T.T.; Sugahara, T.; Suganuma, K. Uniformly connected conductive networks on cellulose nanofiber paper for transparent paper electronics. NPG Asia Mater. 2014, 6, e93. [Google Scholar] [CrossRef]
- Nagashima, K.; Koga, H.; Celano, U.; Zhuge, F.; Kanai, M.; Rahong, S.; Meng, G.; He, Y.; De Boeck, J.; Jurczak, M.; et al. Cellulose nanofiber paper as an ultra flexible nonvolatile memory. Sci. Rep. 2014, 4, 5532. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Karakawa, M.; Komoda, N.; Yagyu, H.; Nge, T.T. Transparent conductive nanofiber paper for foldable solar cells. Sci. Rep. 2015, 5, 17254. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Chang, T.H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Yan, C.; Foo, C.Y.; Lee, P.S. Foldable electrochromics enabled by nanopaper transfer method. Adv. Funct. Mater. 2015, 25, 4203–4210. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Kim, C.; Nogi, M.; Suganuma, K. Electrically conductive lines on cellulose nanopaper for flexible electrical devices. Nanoscale 2013, 5, 9289–9295. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, F.; Denneulina, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G.; Tobjörk, D.; Österbacka, R. Inkjet-printed silver-nanoparticles on nano-engineered cellulose films for electrically conducting structures and organic transistors—Concept and challenges. J. Nanopart. Res. 2012, 14, 213. [Google Scholar] [CrossRef]
- Yagyu, H.; Saito, T.; Isogai, A.; Koga, H.; Nogi, M. Chemical Modification of Cellulose Nanofibers for the Production of Highly Thermal Resistant and Optically Transparent Nanopaper for Paper Devices. ACS Appl. Mater. Interfaces 2015, 7, 22012–22017. [Google Scholar] [CrossRef] [PubMed]
- Yagyu, H.; Ifuku, S.; Nogi, M. Acetylation of Optically Transparent Cellulose Nanopaper for High Thermal and Moisture Resistance in a Flexible Device Substrate. Flex. Print. Electron. 2017, 2, 14003. [Google Scholar] [CrossRef]
- Zhao, M.; Ansari, F.; Takeuchi, M.; Shimizu, M.; Saito, T.; Berglund, L.A.; Isogai, A. Nematic structuring of transparent and multifunctional nanocellulose papers. Nanoscale Horiz. 2018, 3, 28–34. [Google Scholar] [CrossRef]
- Isobe, N.; Kasuga, T.; Nogi, M. Clear transparent cellulose nanopaper prepared from concentrated dispersion by high-humidity drying. RSC Adv. 2018, 8, 1833–1837. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Tanaka, R.; Saito, T.; Hänninen, T.; Ono, Y.; Hakalahti, M.; Tammelin, T.; Isogai, A. Viscoelastic Properties of Core–Shell-Structured, Hemicellulose-Rich Nanofibrillated Cellulose in Dispersion and Wet-Film States. Biomacromolecules 2016, 17, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.P.; Feng, X.Q.; Gao, H. Ultrasonic technique for extracting nanofibers from nature materials. Appl. Phys. Lett. 2007, 90, 073112. [Google Scholar] [CrossRef]
- Nge, T.T.; Nogi, M.; Suganuma, K. Electrical functionality of inkjet-printed silver nanoparticle conductive tracks on nanostructured paper compared with those on plastic substrates. J. Mater. Chem. C. 2013, 1, 5235–5243. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasuga, T.; Isobe, N.; Yagyu, H.; Koga, H.; Nogi, M. Clearly Transparent Nanopaper from Highly Concentrated Cellulose Nanofiber Dispersion Using Dilution and Sonication. Nanomaterials 2018, 8, 104. https://doi.org/10.3390/nano8020104
Kasuga T, Isobe N, Yagyu H, Koga H, Nogi M. Clearly Transparent Nanopaper from Highly Concentrated Cellulose Nanofiber Dispersion Using Dilution and Sonication. Nanomaterials. 2018; 8(2):104. https://doi.org/10.3390/nano8020104
Chicago/Turabian StyleKasuga, Takaaki, Noriyuki Isobe, Hitomi Yagyu, Hirotaka Koga, and Masaya Nogi. 2018. "Clearly Transparent Nanopaper from Highly Concentrated Cellulose Nanofiber Dispersion Using Dilution and Sonication" Nanomaterials 8, no. 2: 104. https://doi.org/10.3390/nano8020104




