Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
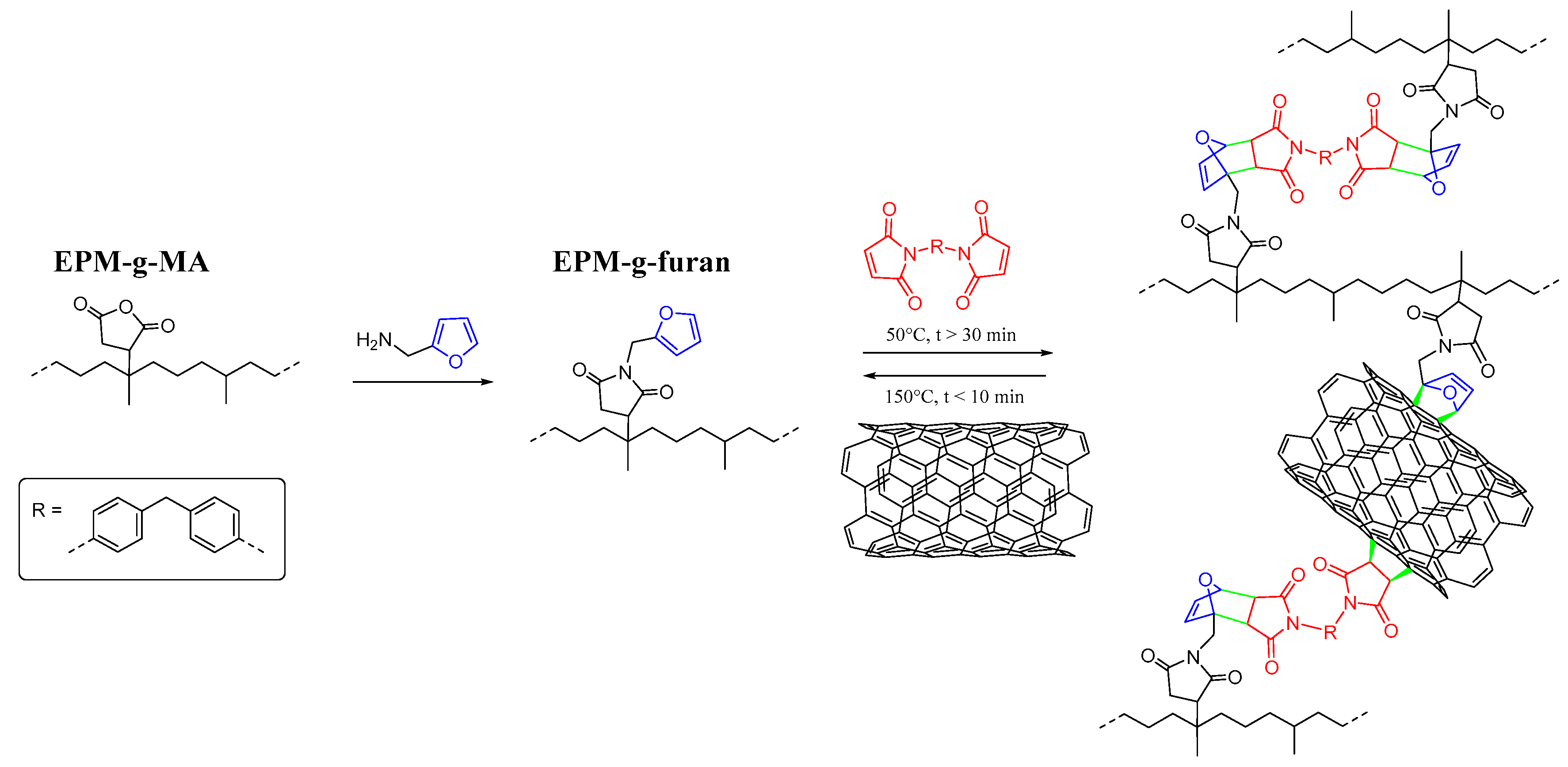
2.2.1. Furan-Functionalization of EPM-g-MA
2.2.2. Amine Modification of CNT
2.2.3. Solution Mixing and Cross-Linking of Nanocomposites
2.3. Characterization
- VR Volume fraction of rubber in swollen sample.
- VS Molar volume of solvent (decalin: 154 mL/mol at room temperature).
- ρ Density (0.860 g/mL for EPM-g-furan and 0.896 g/mL for decalin).
3. Results and Discussion
3.1. The Reinforcing Effect of CNT in Rubber Nanocomposites
3.1.1. Chemical Characterization
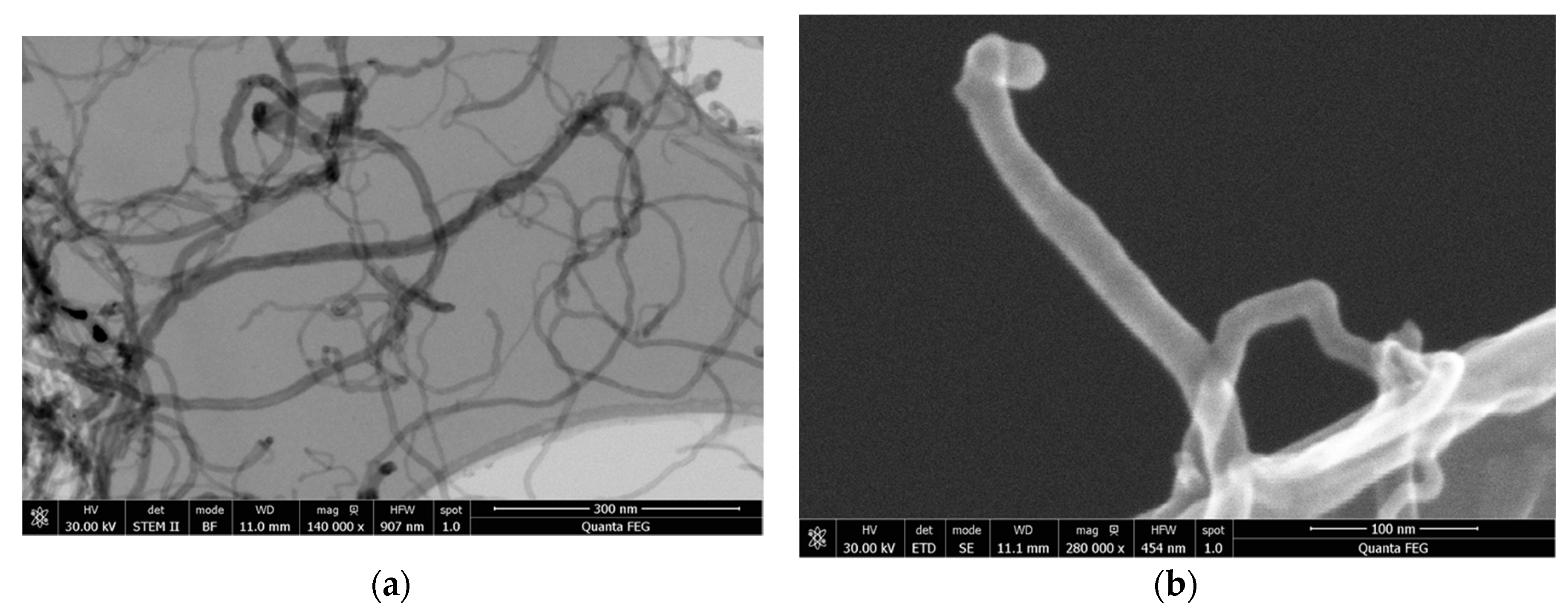
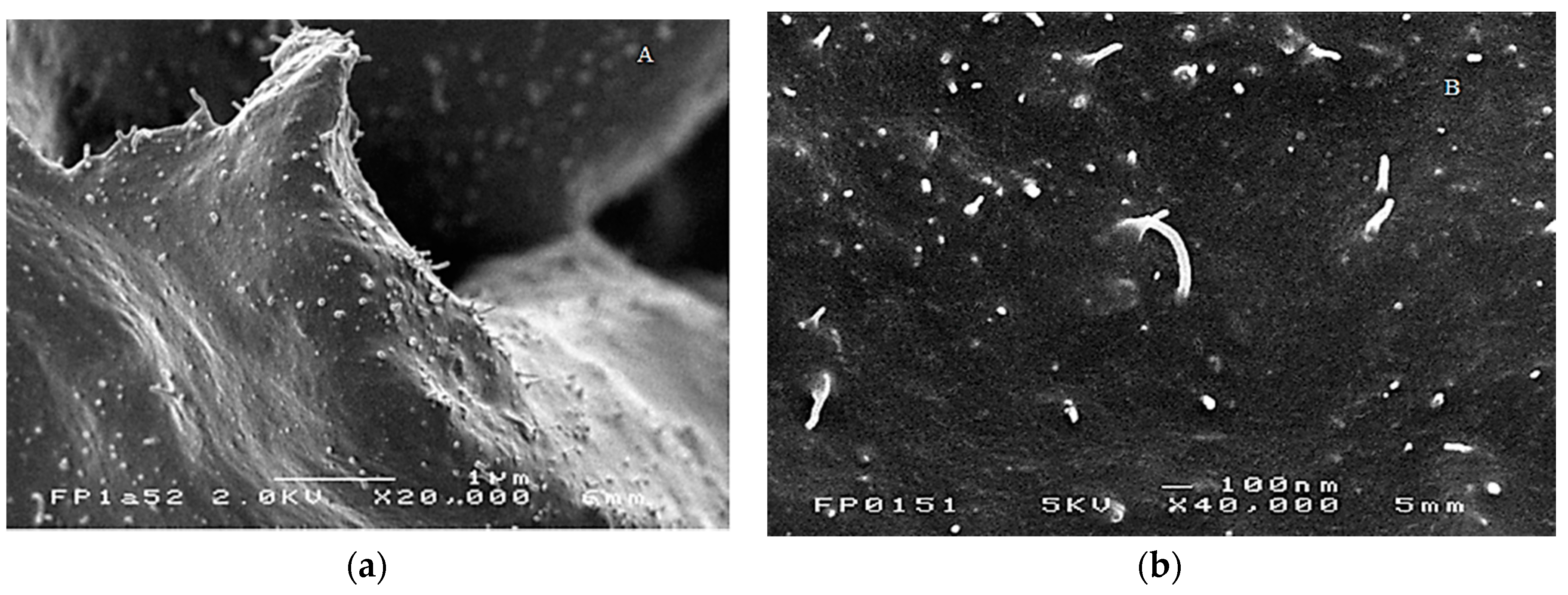
3.1.2. Morphological Characterization
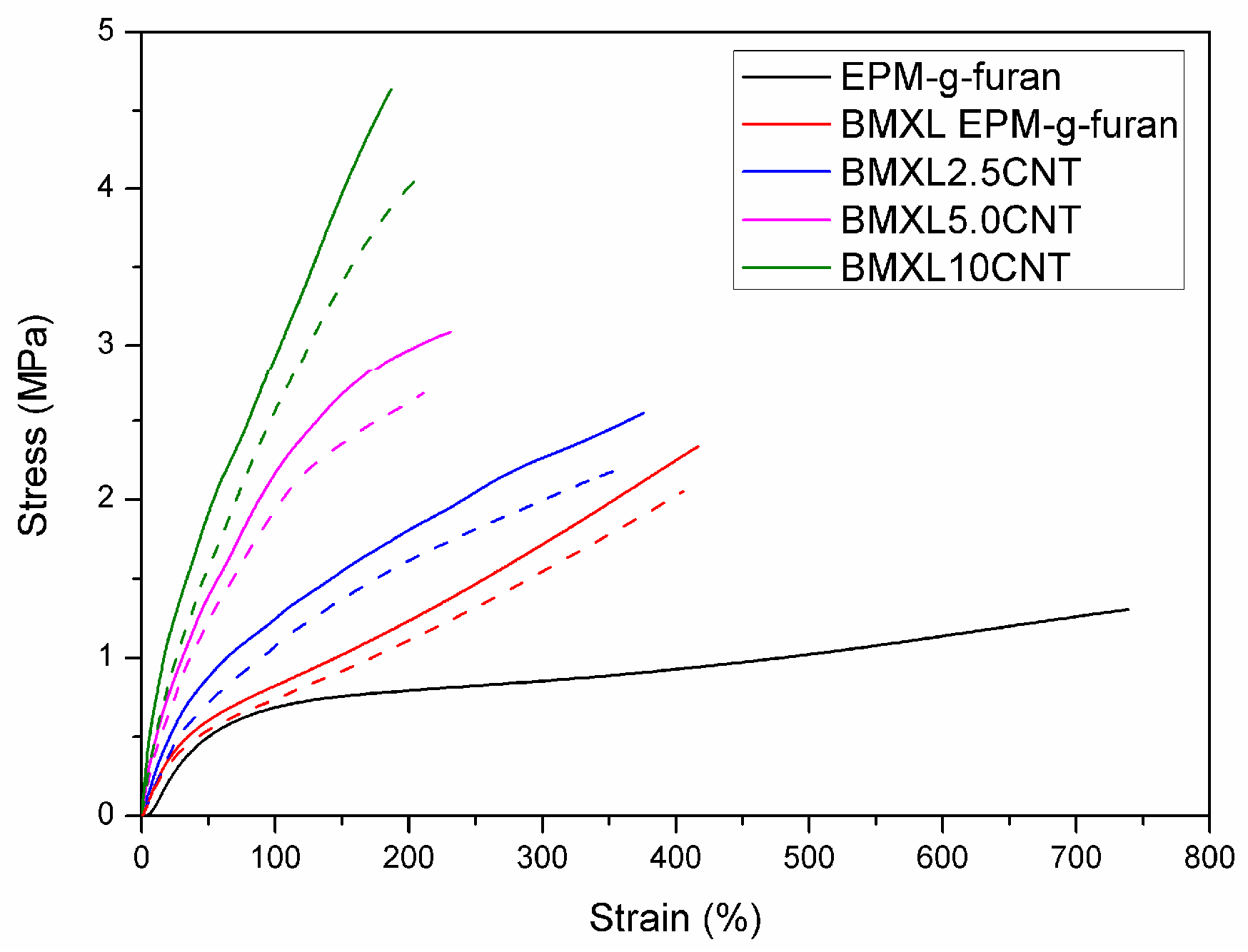
3.1.3. Tensile Properties of BM Cross-Linked EPM-g-Furan Nanocomposites with CNT
3.1.4. Tensile Properties of Rubber Nanocomposites with Various (Modified) CNT
3.2. Conductive Behaviour of BM Cross-Linked Rubber Nanocomposites
3.2.1. Percolation Threshold
3.2.2. Sensitivity Deformation Testing
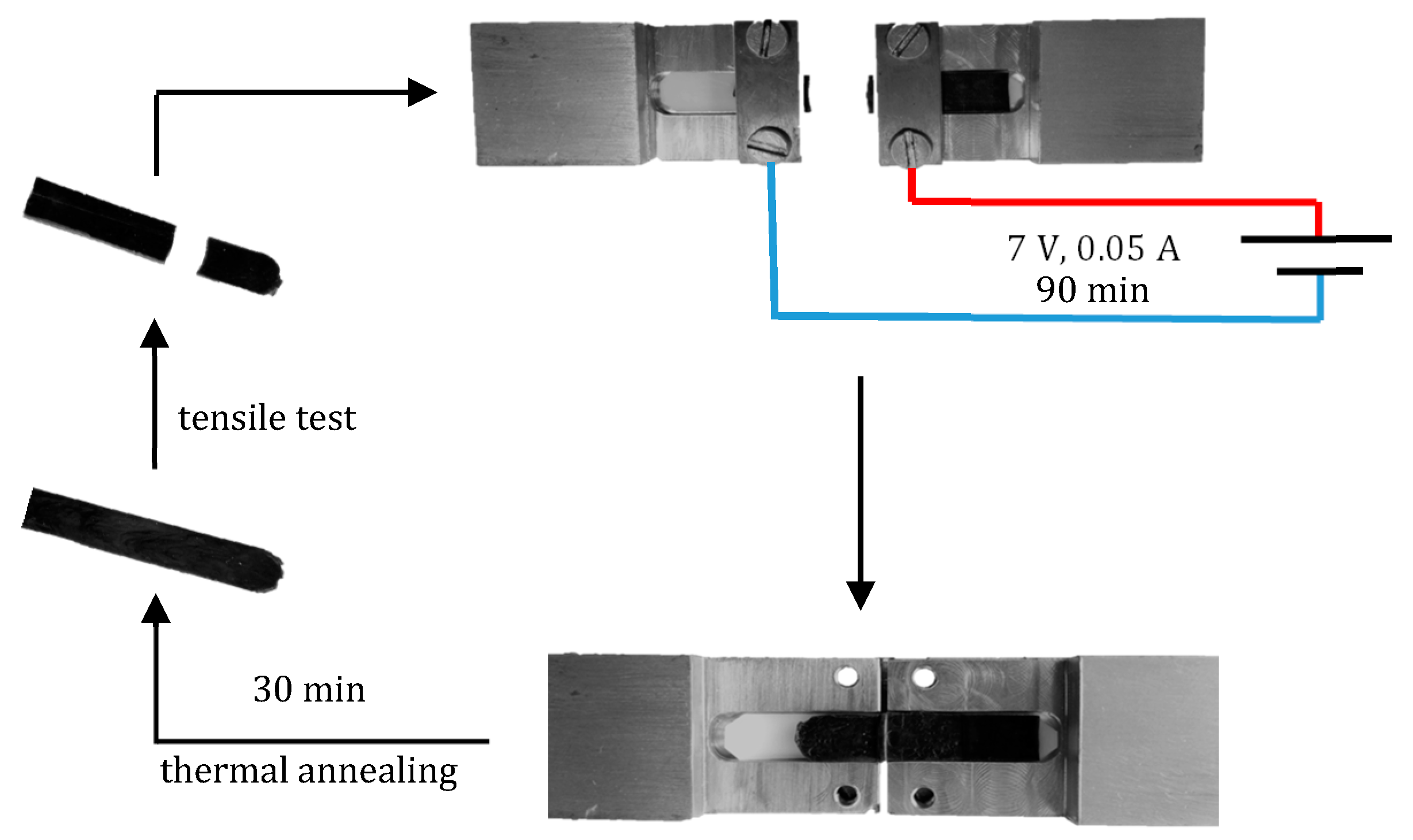
3.3. Crack-Healing by Welding
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
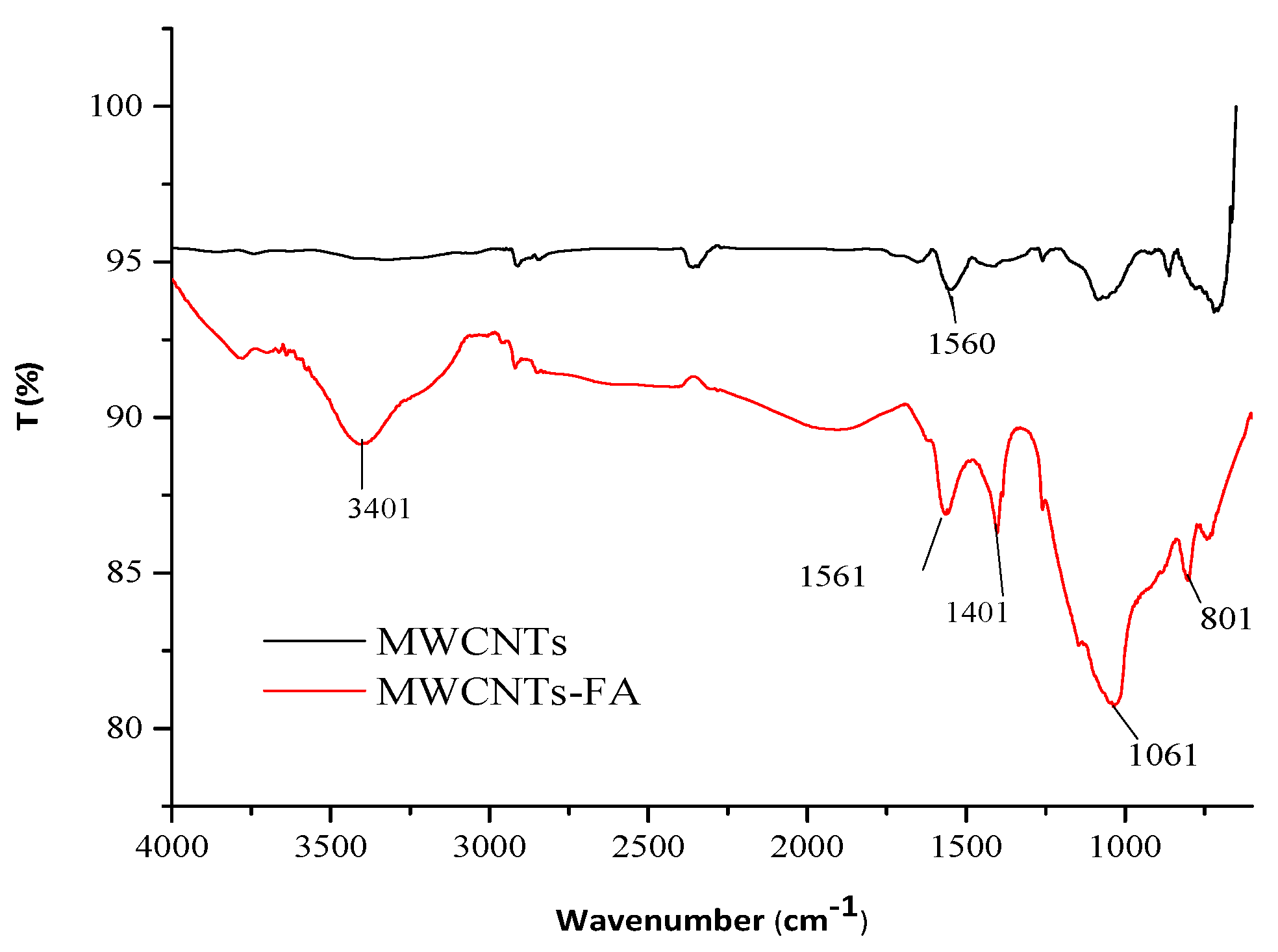
- 3400 cm−1, characteristic of the N-H bond stretching;
- 1060 cm−1, characteristic of a stretching of the C–O bond;
- 1404 cm−1 band relating to the bending of C–O bond;
- 1399 cm−1 relative to the stretching of the C–N bond;
- 801 cm−1 band relative to the bending of the N–H bond;
- 1561 and 1560 cm−1 can be observed the band of the C=C stretching of the graphitic structure.
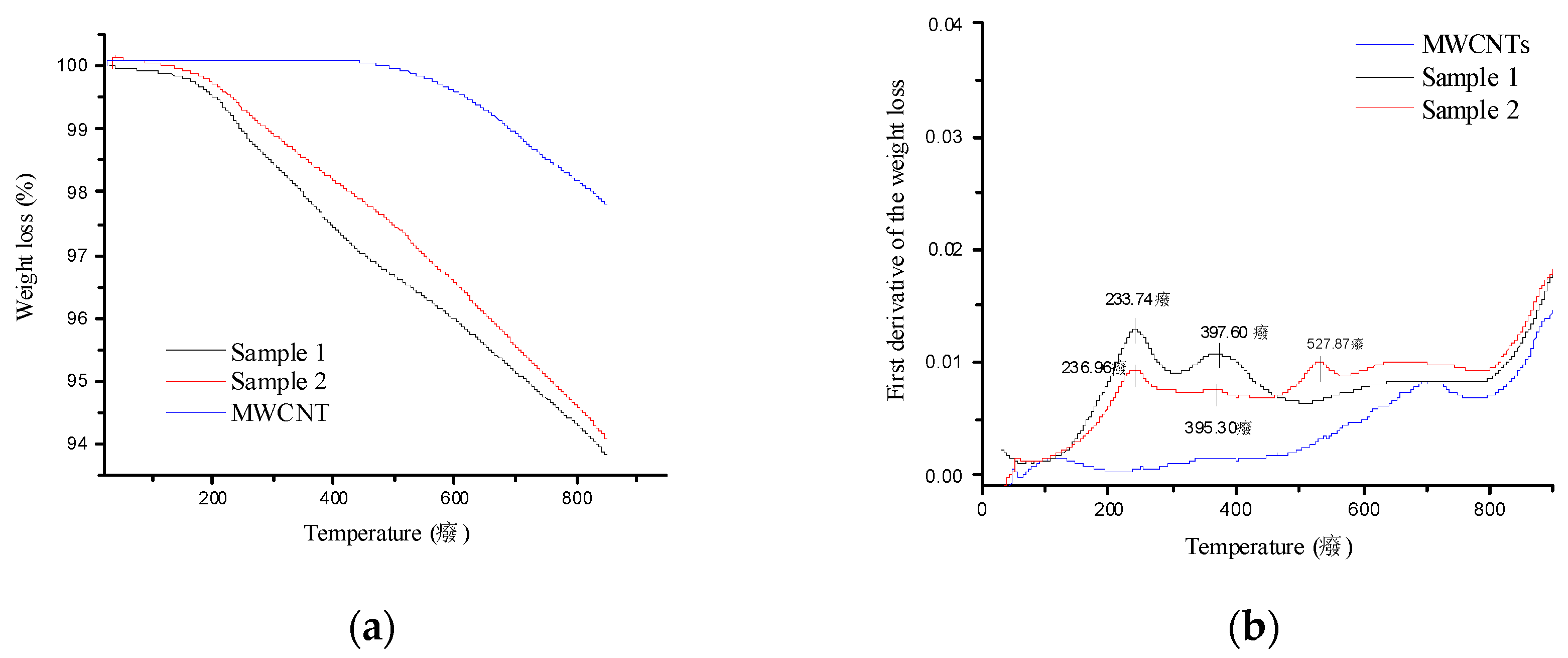
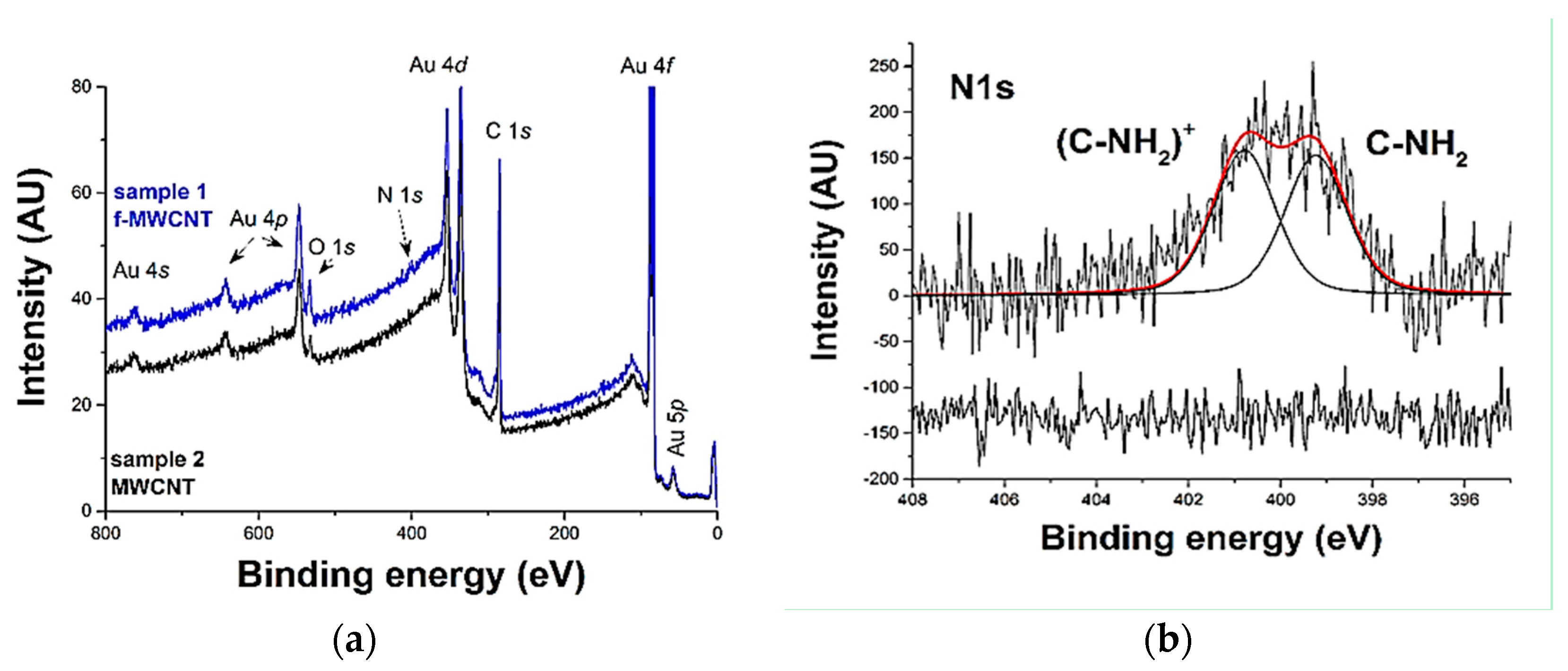
- Nitrogen of the amine with an energy of 401 eV;
- Nitrogen in the form of an oxidized species (or with a different chemical environment like an adsorbed amine) with energy equal to 399 eV.
| C | O | N | |
|---|---|---|---|
| non-functionalized CNT | 91 | 9 | 0 |
| 90 | 10 | 0 | |
| 89 | 11 | 0 | |
| FFA functionalized CNT | 93 | 4.9 | 2.0 |
| 93 | 5.1 | 1.6 | |
| 93 | 5.3 | 1.6 |
References
- Polgar, L.M.; van Essen, M.; Pucci, A.; Picchioni, F. Smart Rubbers: Synthesis and Applications; Smithers Rapra: Shawbury, UK, 2017; ISBN 978-1-91108-823-3. [Google Scholar]
- Ponnamma, D.; Sadasivuni, K.K.; Grohens, Y.; Guo, Q.; Thomas, S. Carbon nanotube based elastomer composites—An approach towards multifunctional materials. J. Mater Chem. C 2014, 2, 8446–8485. [Google Scholar] [CrossRef]
- Ajayan, P.; Stephan, O.; Colliex, C.; Trauth, D. Aligned Carbon Nanotube Arrays Formed by Butting A Polymer Resin-Nanotube Composite. Science 1994, 265, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- De Heer, W. Nanotubes and the Pursuit of Applications. MRS Bull. 2004, 29, 281–285. [Google Scholar] [CrossRef]
- Ciardelli, F.; Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites based on polyolefins and functional thermoplastic materials. Polym. Int. 2008, 57, 805–836. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Diez-Pascual, A.M.; Lazaro, E.; Vera, S.; Gomez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gun’ko, Y.K. Recent advances in research on carbon nanotube—Polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Grady, B.P. Recent developments concerning the dispersion of carbon nanotubes in polymers. Macromol. Rapid Commun. 2010, 31, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Toprakci, H.A.K.; Kalanadhabhatla, S.K.; Spontak, R.J.; Ghosh, T.K. Polymer Nanocomposites Containing Carbon Nanofibers as Soft Printable Sensors Exhibiting Strain-Reversible Piezoresistivity. Adv. Funct. Mater. 2013, 23, 5536–5542. [Google Scholar] [CrossRef]
- Lin, L.; Liu, S.; Zhang, Q.; Li, X.; Ji, M.; Deng, H.; Fu, Q. Towards Tunable Sensitivity of Electrical Property to Strain for Conductive Polymer Composites Based on Thermoplastic Elastomer. ACS Appl. Mater. Interfaces 2013, 5, 5815–5824. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ma, L.; Bao, R.; Qi, G.; Yang, W.; Xie, B.; Yang, M. A new approach to construct segregated structures in thermoplastic polyolefin elastomers towards improved conductive and mechanical properties. J. Mater. Chem. A 2015, 3, 5482–5490. [Google Scholar] [CrossRef]
- Bilotti, E.; Zhang, R.; Deng, H.; Baxendale, M.; Peijs, T. Fabrication and property prediction of conductive and strain sensing TPU/CNT nanocomposite fibres. J. Mater. Chem. 2010, 20, 9449–9455. [Google Scholar] [CrossRef]
- Costa, P.; Ferreira, A.; Sencadas, V.; Viana, J.C.; Lanceros-Mendez, S. Electro-Mechanical Properties of Triblock Copolymer Styrene-Butadiene-Styrene/Carbon Nanotube Composites for Large Deformation Sensor Applications. Sens. Actuators A Phys. 2013, 201, 458–467. [Google Scholar] [CrossRef]
- Pham, G.T.; Park, Y.; Liang, Z.; Zhang, C.; Wang, B. Processing and modeling of conductive thermoplastic/carbon nanotube films for strain sensing. Compos. Part B Eng. 2008, 39, 209–216. [Google Scholar] [CrossRef]
- Robert, C.; Feller, J.F.; Castro, M. Sensing skin for strain monitoring made of PC-CNT conductive polymer nanocomposite sprayed layer by layer. ACS Appl. Mater. Interfaces 2012, 4, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Bilotti, E.; Zhang, H.; Deng, H.; Zhang, R.; Fu, Q.; Peijs, T. Controlling the dynamic percolation of carbon nanotube based conductive polymer composites by addition of secondary nanofillers: The effect on electrical conductivity and tuneable sensing behaviour. Compos. Sci. Technol. 2013, 74, 85–90. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Carbon nanotube polymer coatings for textile yarns with good strain sensing capability. Sens. Actuators A Phys. 2012, 179, 83–91. [Google Scholar] [CrossRef]
- Bautista-Quijano, J.R.; Aviles, F.; Cauich-Rodriguez, J.V.; Schoenfelder, R.; Bachmatiuk, A.; Gemming, T.; Ruemmeli, M.H. Tensile piezoresistivity and disruption of percolation in singlewall and multiwall carbon nanotube/polyurethane composites. Synth. Met. 2013, 185, 96–102. [Google Scholar] [CrossRef]
- Deng, H.; Ji, M.; Yan, D.; Fu, S.; Duan, L.; Zhang, M.; Fu, Q. Towards tunable resistivity–strain behavior through construction of oriented and selectively distributed conductive networks in conductive polymer composites. J. Mater. Chem. A 2014, 2, 10048–10058. [Google Scholar] [CrossRef]
- Sperling, L.H.; Mishra, V. Study on superabsorbent of maleic anhydride/acrylamide semi-interpenetrated with poly(vinyl alcohol). Polym. Adv. Technol. 1996, 7, 197–208. [Google Scholar] [CrossRef]
- Polgar, L.M.; van Duin, M.; Broekhuis, A.A.; Picchioni, F. Use of Diels—Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Broekhuis, A.A.; Picchioni, F. Reversible polymer networks containing covalent and hydrogen bonding interactions. Eur. Polym. J. 2014, 50, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally Self-Healing Polymeric Materials: The Next Step to Recycling Thermoset Polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Araya-Hermosilla, R.; Pucci, A.; Araya-Hermosilla, E.; Pescarmona, P.; Raffa, P.; Polgar, L.; Moreno-Villoslada, I.; Flores, M.; Fortunato, G.; Broekhuis, A.; et al. An easy synthetic way to exfoliate and stabilize MWCNTs in a thermoplastic pyrrole-containing matrix assisted by hydrogen bonds. RSC Adv. 2016, 6, 85829–85837. [Google Scholar] [CrossRef]
- Posadas, P.; Fernandez-Torres, A.; Chamorro, C.; Mora-Barrantes, I.; Rodriguez, A.; Gonzalez, L.; Valentin, J.L. Study on peroxide vulcanization thermodynamics of ethylenevinyl acetate copolymer rubber using 2,2,6,6,-tetramethylpiperidinyloxyl nitroxide. Polym. Int. 2013, 62, 909–918. [Google Scholar] [CrossRef]
- Hirschl, C.; Biebl-Rydlo, M.; DeBiasio, M.; Muehleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater Sol. Cells 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. The entropy of the rotational conformations of (poly)isoprene molecules and its relationship to rubber elasticity and temperature increase for moderate tensile or compressive strains. J. Chem. Phys. 1943, 11. [Google Scholar] [CrossRef]
- Dikland, H.G.; Van Duin, M. Miscibility of EPM-EPDM Blends. Rubber Chem. Technol. 2003, 76, 495–506. [Google Scholar] [CrossRef]
- Giuliani, A.; Placidi, M.; Francesco, F.; Pucci, A. A new polystyrene-based ionomer/MWCNT nanocomposite for wearable skin temperature sensors. React. Funct. Polym. 2014, 76, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Zydziak, N.; Huebner, C.; Bruns, M.; Barner-Kowollik, C. One-Step Functionalization of Single-Walled Carbon Nanotubes (SWCNTs) with Cyclopentadienyl-Capped Macromolecules via Diels—Alder Chemistry. Macromolecules 2011, 44, 3374–3380. [Google Scholar] [CrossRef]
- Syrgiannis, Z.; Melchionna, M.; Prato, M. Covalent Carbon Nanotube Functionalization. In Encyclopedia of Polymeric Nanomaterials; Springer: Heidelberg, Germany, 2015; pp. 480–487. ISBN 978-3-642-29649-9. [Google Scholar]
- Xue, P.; Wang, J.; Bao, Y.; Li, Q.; Wu, C. Synergistic effect between carbon black nanoparticles and polyimide on refractive indices of polyimide/carbon black nanocomposites. New J. Chem. 2012, 36, 903–910. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Bokobza, L.; Belin, C. Effect of strain on the properties of a styrene—Butadiene rubber filled with multiwall carbon nanotubes. J. Appl. Polym. Sci. 2007, 105, 2054–2061. [Google Scholar] [CrossRef]
- Hirsch, A.; Backes, C. Carbon Nanotube Science. Synthesis, Properties and Applications. Von Peter J. F. Harris. Angew. Chem. 2009, 122, 1766–1767. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sinturel, C.; Bahloul, O.; Saboungi, M.; Thomas, S.; Salvetat, J. Improving reinforcement of natural rubber by networking of activated carbon nanotubes. Carbon 2008, 46, 1037–1045. [Google Scholar] [CrossRef]
- Thostenson, E.; Ren, Z.; Chou, T. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Polgar, L.M.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of cross-linking and de-cross-linking of EPM rubber with thermoreversible Diels-Alder chemistry. Eur. Polym. J. 2017, 90, 150–161. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-3-527-67020-8. [Google Scholar]

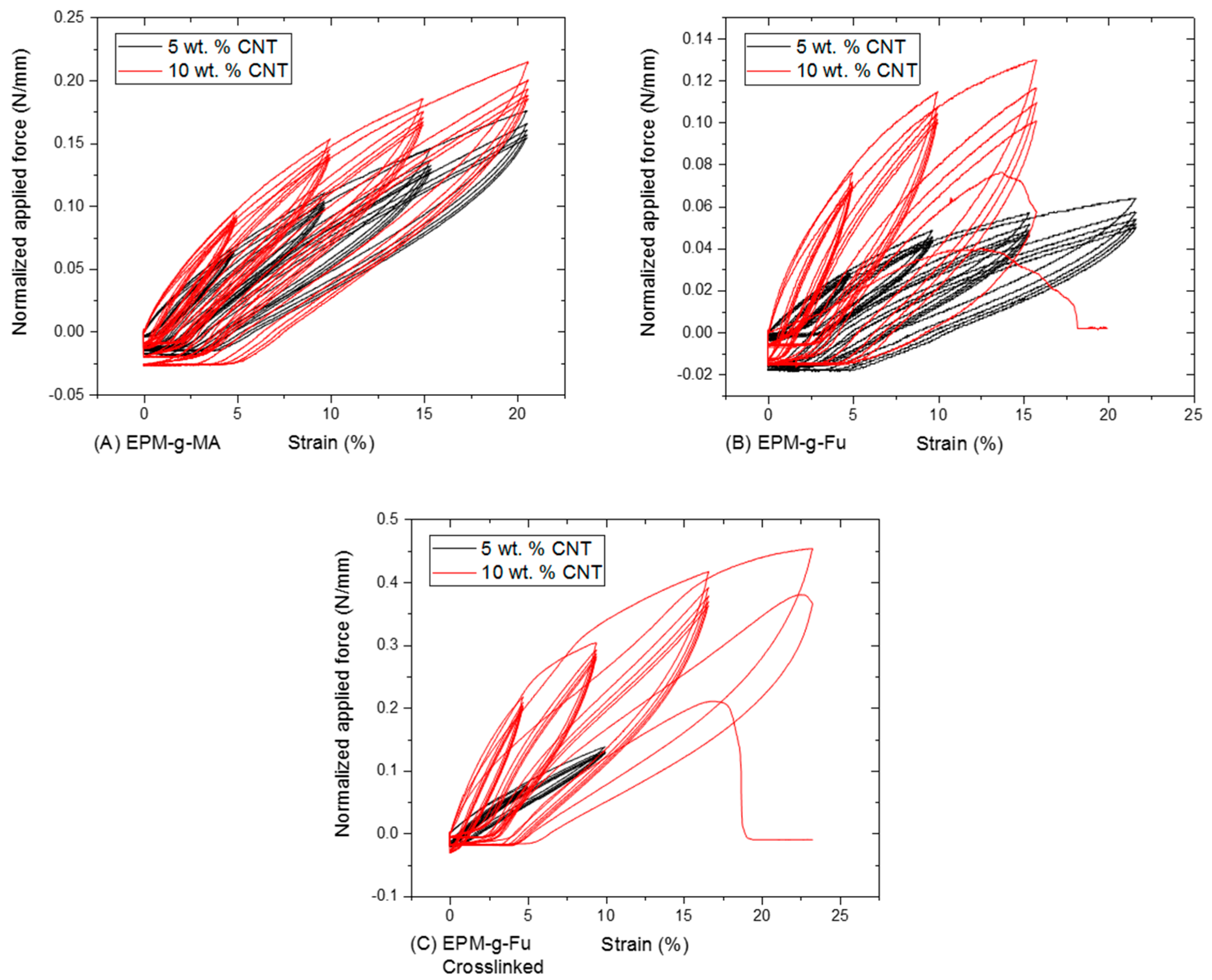

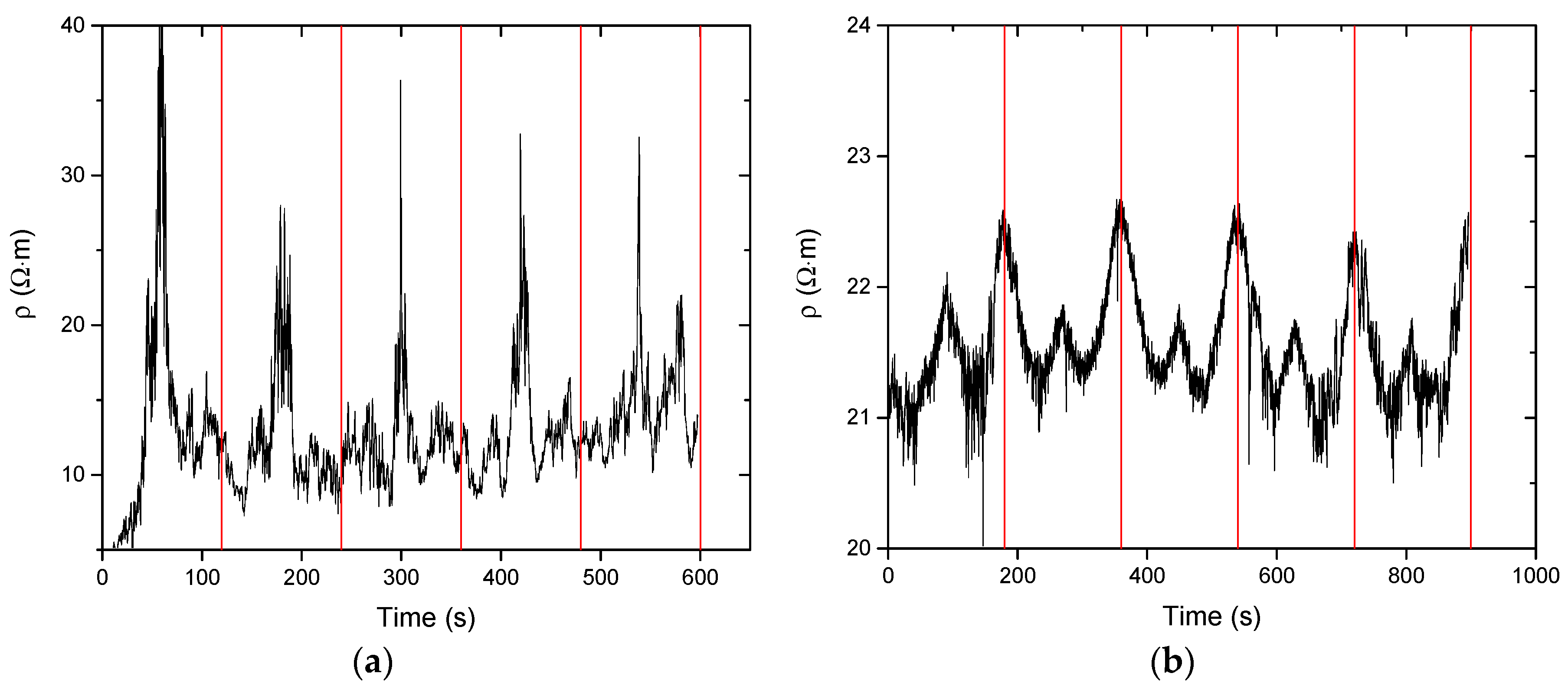
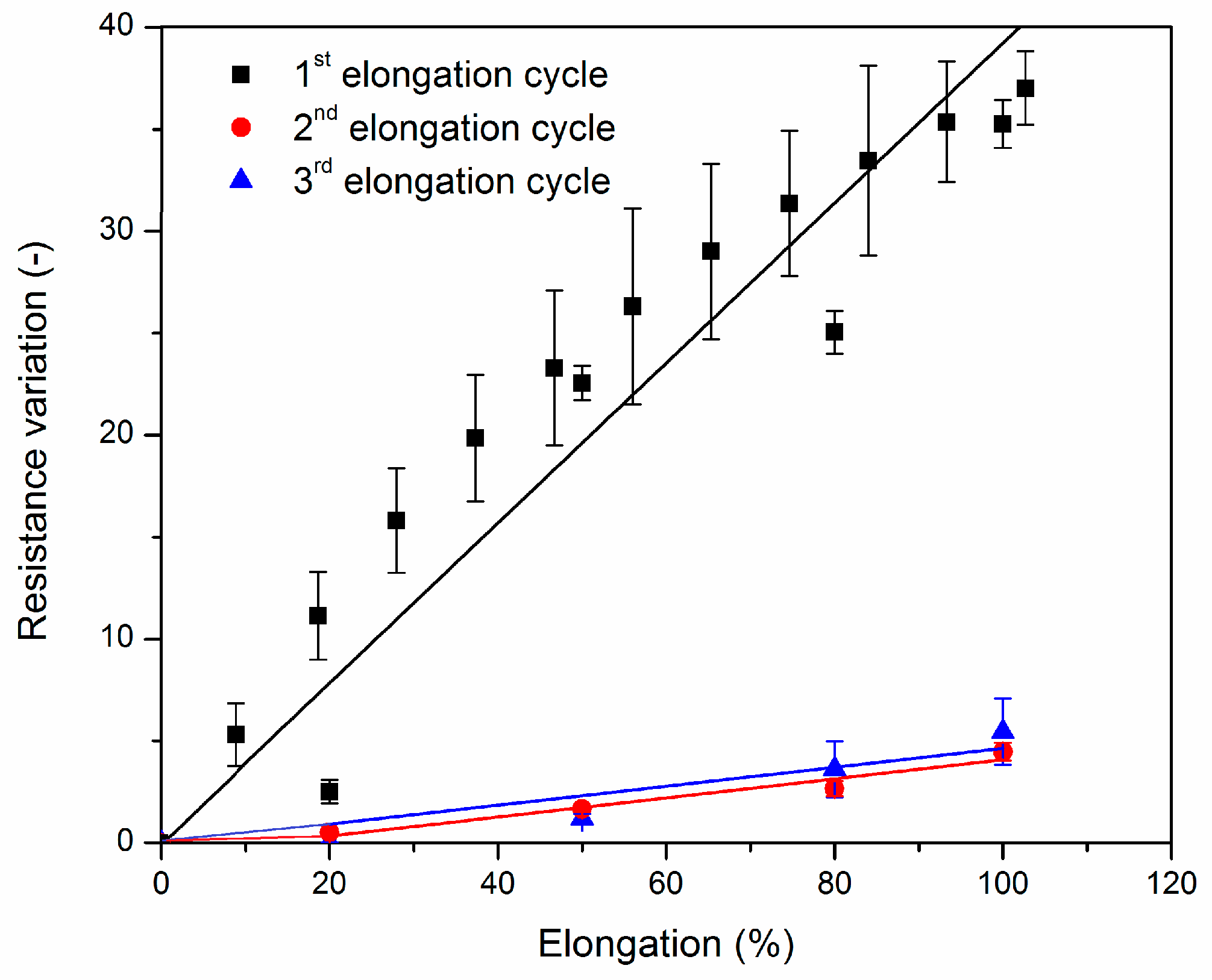
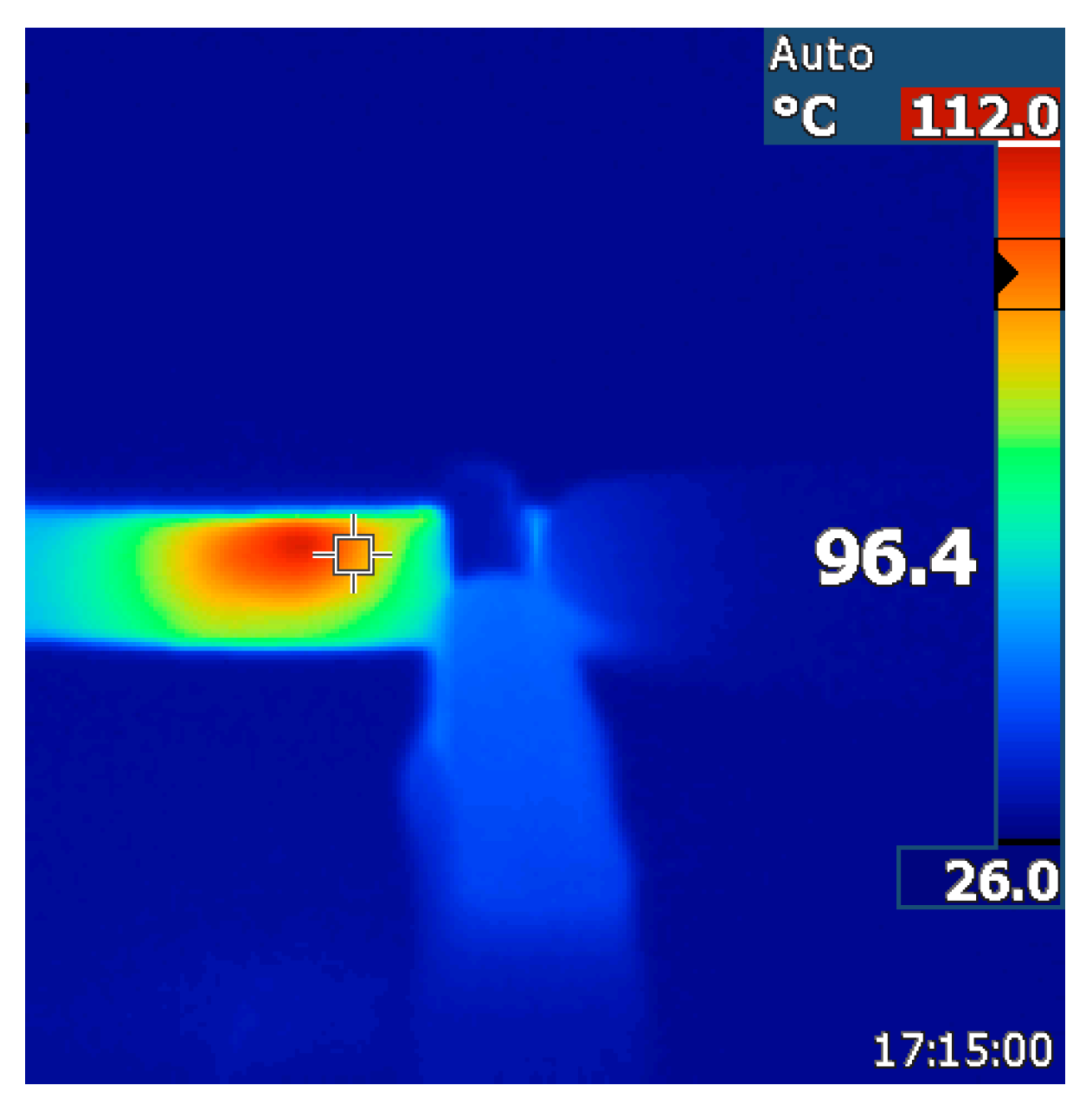
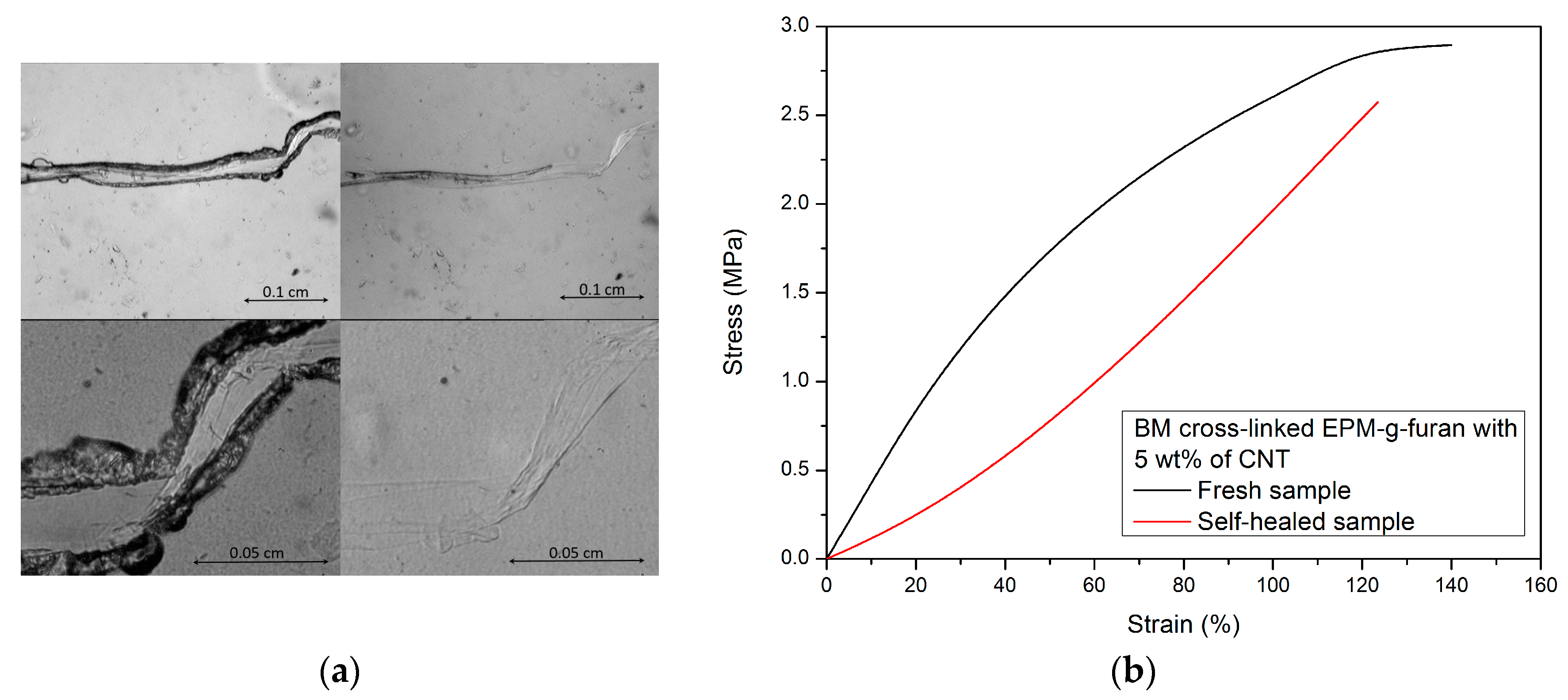
| EPM * Rubber | CNT (wt %) | BM † (g) | TGA ‡ Residue at 600 °C (%) | Gel Content (%) | Cross-Link Density (10−4 mol/mL) | CNT (wt %) |
|---|---|---|---|---|---|---|
| EPM-g-MA | - | - | 0.0 | 0 | - | - |
| 3.023 g EPM-g-MA | 2.4 | - | 1.7 | 25 | 0.23 | 2.4 |
| 3.047 g EPM-g-MA | 3.5 | - | 9.4 | 55 | 0.64 | 3.5 |
| EPM-g-furan | - | - | 0.0 | 0 | - | - |
| 3.019 g EPM-g-furan | 2.4 | - | 1.6 | 68 | 0.75 | 2.4 |
| 3.007 g EPM-g-furan | 3.4 | - | 9.5 | 82 | 0.95 | 3.4 |
| 3.010 g EPM-g-furan | - | - | 0.0 | 93 | 2.1 | - |
| 3.012 g EPM-g-furan | 2.4 | - | 1.5 | 95 | 5.2 | 2.4 |
| 3.036 g EPM-g-furan | 4.0 | 0.114 | 4.3 | 96 | 3.6 | 4.0 |
| 3.024 g EPM-g-furan | 4.8 | 0.114 | 4.1 | 98 | 4.0 | 4.8 |
| 3.146 g EPM-g-furan | 5.6 | 0.118 | 10.1 | 99 | 4.7 | 5.6 |
| 3.028 g EPM-g-furan | 6.5 | 0.113 | 7.7 | 99 | 6.3 | 6.5 |
| Filler Loading (wt %) | EPM-g-MA | EPM-g-Furan | BM Cross-Linked EPM-g-Furan | Reprocessed, Cross-Linked EPM-g-Furan | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tb (MPa) | Eb (%) | Tb (MPa) | Eb (%) | Tb (MPa) | Eb (%) | Tb (MPa) | Eb (%) | ||
| No CNT | 1.2 | 950 | 1.3 | 750 | 2.2 | 210 | 1.7 | 190 | |
| CNT | 2 | 2.5 | 250 | 2.5 | 250 | 3.1 | 200 | 2.9 | 190 |
| 5 | 3.7 | 200 | 3.8 | 180 | 5.2 | 140 | 5 | 130 | |
| 10 | 5 | 130 | 5.1 | 130 | 7 | 80 | 6.5 | 90 | |
| modified CNT | 2 | 2.5 | 240 | 2.6 | 220 | 3.0 | 210 | 2.9 | 200 |
| 5 | 5.3 | 180 | 5.7 | 150 | 6.3 | 120 | 6.2 | 110 | |
| 10 | 7.2 | 110 | 7.5 | 100 | 7.8 | 100 | 7.5 | 90 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polgar, L.M.; Criscitiello, F.; Van Essen, M.; Araya-Hermosilla, R.; Migliore, N.; Lenti, M.; Raffa, P.; Picchioni, F.; Pucci, A. Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes. Nanomaterials 2018, 8, 58. https://doi.org/10.3390/nano8020058
Polgar LM, Criscitiello F, Van Essen M, Araya-Hermosilla R, Migliore N, Lenti M, Raffa P, Picchioni F, Pucci A. Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes. Nanomaterials. 2018; 8(2):58. https://doi.org/10.3390/nano8020058
Chicago/Turabian StylePolgar, Lorenzo Massimo, Francesco Criscitiello, Machiel Van Essen, Rodrigo Araya-Hermosilla, Nicola Migliore, Mattia Lenti, Patrizio Raffa, Francesco Picchioni, and Andrea Pucci. 2018. "Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes" Nanomaterials 8, no. 2: 58. https://doi.org/10.3390/nano8020058






