Exploring Reaction Conditions to Improve the Magnetic Response of Cobalt-Doped Ferrite Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Structural and Chemical Characterization
2.2. Morphological Characterization
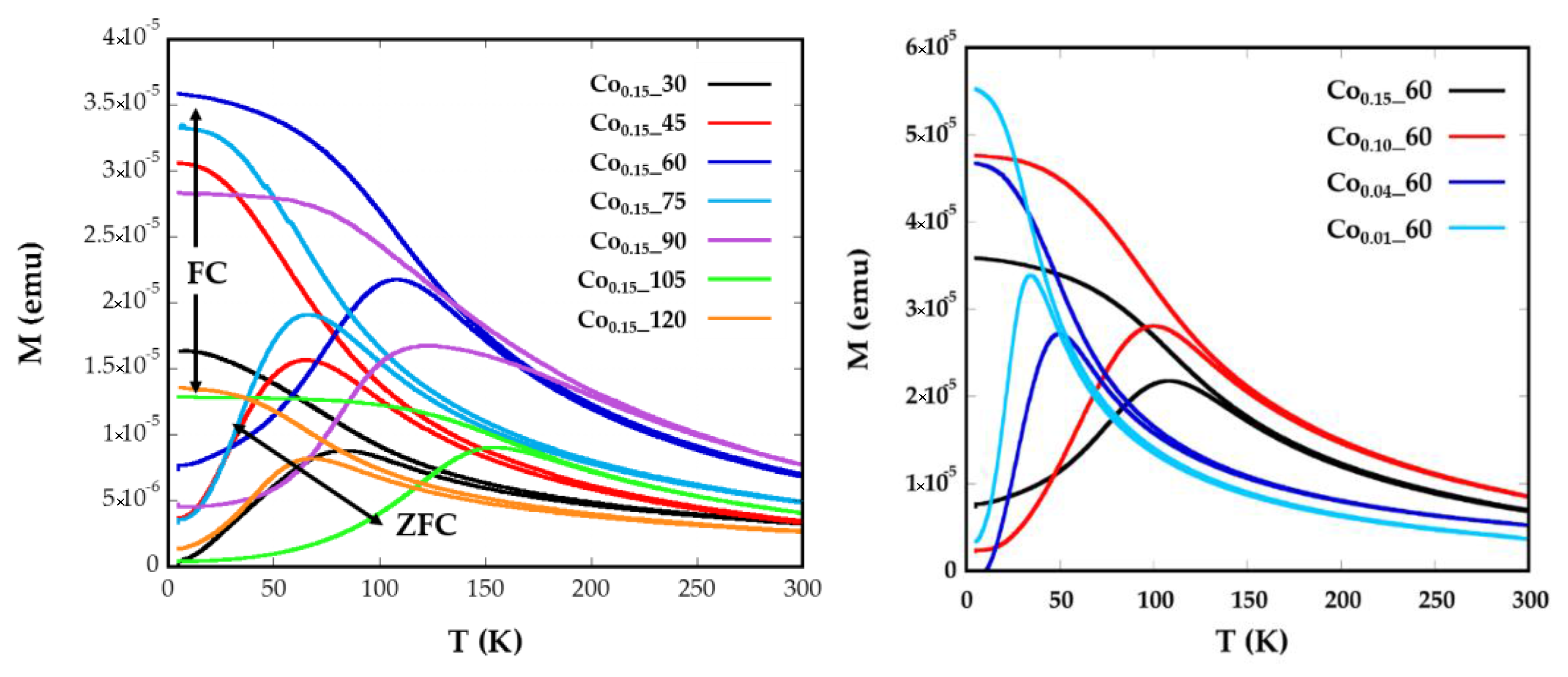
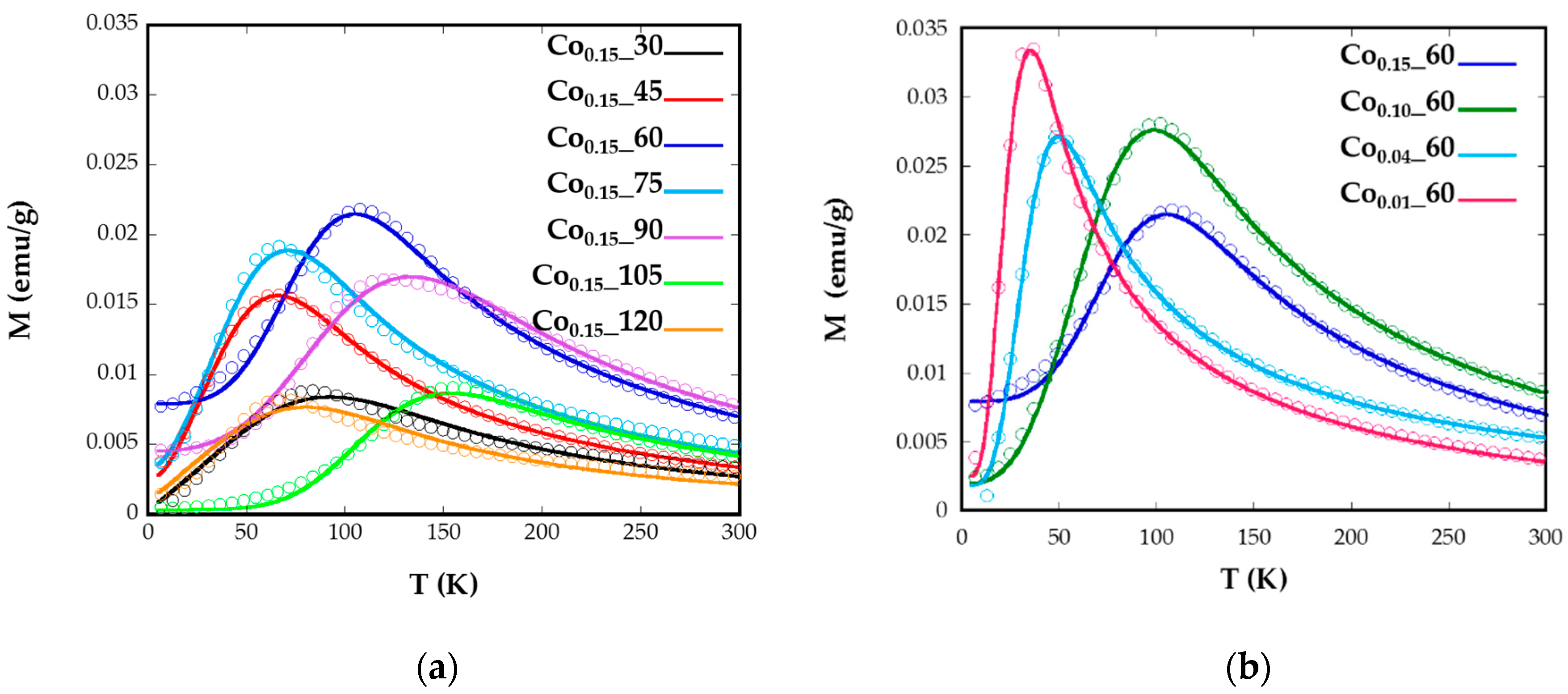
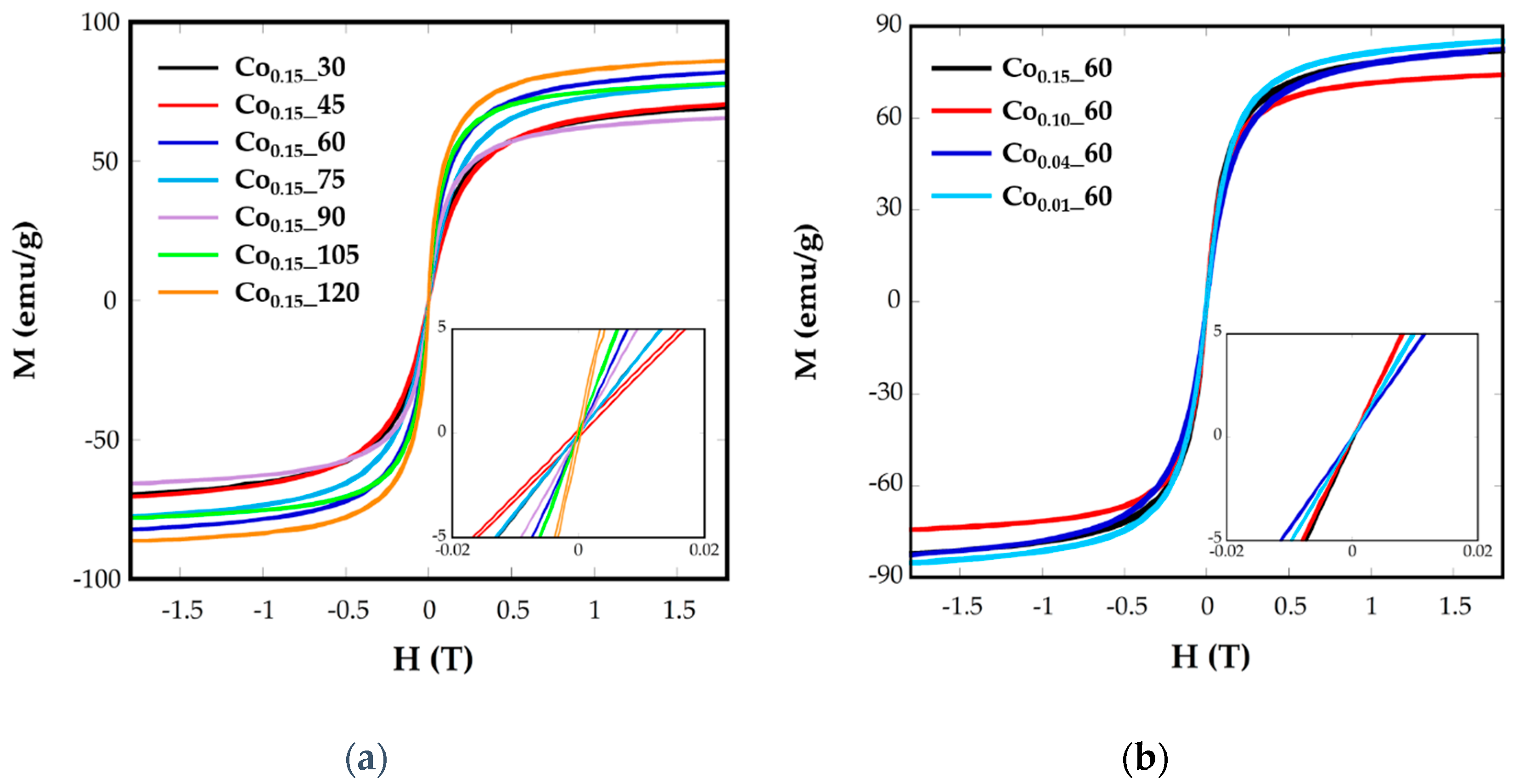
2.3. Magnetic Characterization
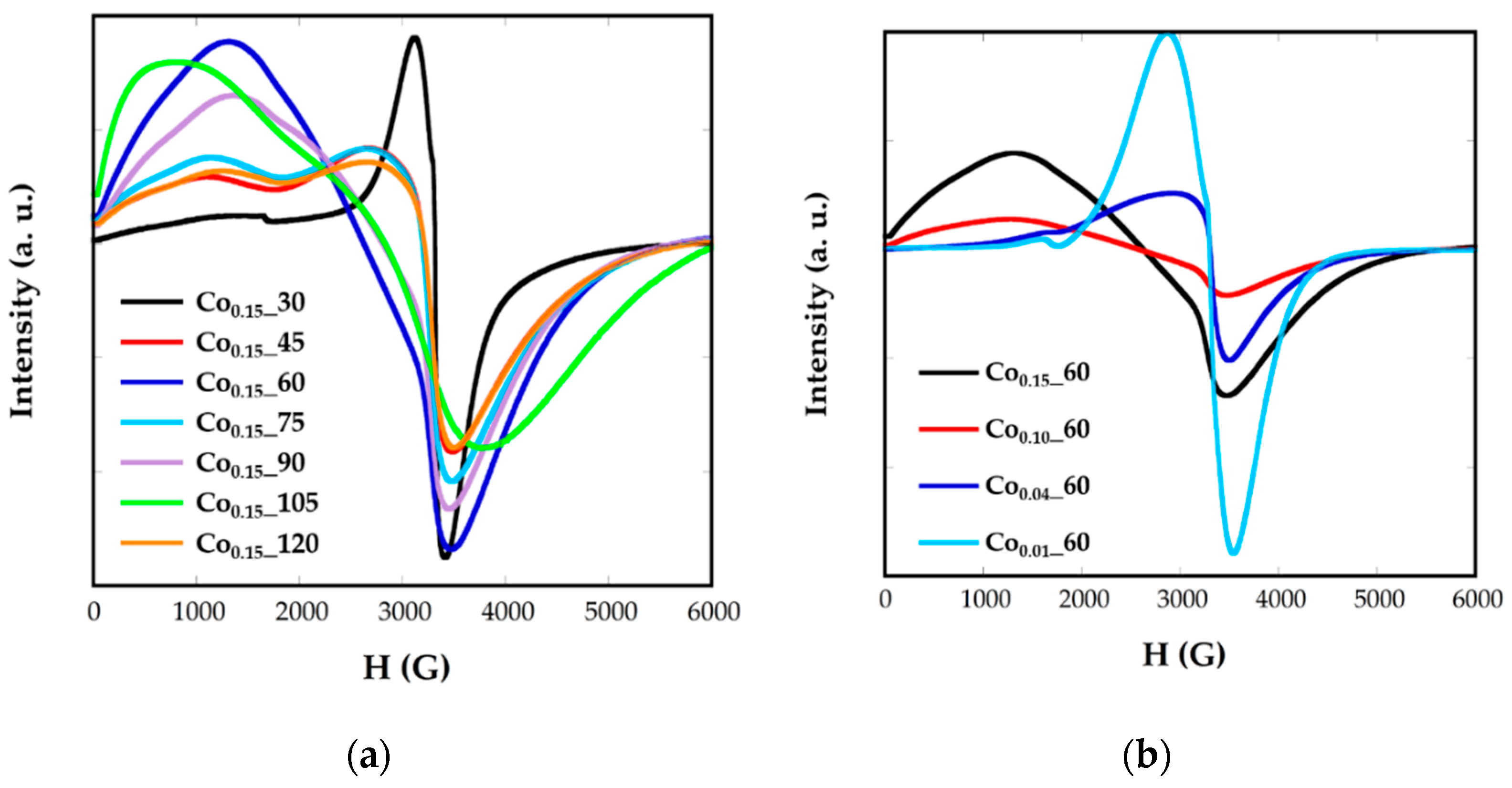
2.4. Electron Magnetic Resonance
3. Materials and Methods
3.1. Materials
3.2. Syntheiss of Cobalt-Doped Nanoparticles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nikitin, A.; Fedorova, M.; Naumenko, V.; Shchetinin, I.; Abakumov, M.; Erofeev, A.; Gorelkin, P.; Meshkov, G.; Beloglazkina, E.; Ivanenkov, Y.; et al. Synthesis, characterization and MRI application of magnetite water-soluble cubic nanoparticles. J. Magn. Magn. Mater. 2017, 441, 6–13. [Google Scholar] [CrossRef]
- Dilnawaz, D.; Singh, A.; Mohanty, C.; Sahoo, S.K. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706. [Google Scholar] [CrossRef] [PubMed]
- Agotegaray, M.A.; Campelo, A.E.; Zysler, R.D.; Gumilar, F.; Bras, C.; Gandini, A.; Minetti, A.; Massheimer, V.L.; Lassalle, V.L. Magnetic nanoparticles for drug targeting: From design to insights into systemic toxicity. Preclinical evaluation of hematological, vascular and neurobehavioral toxicology. Biomater. Sci. 2017, 5, 772–783. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, M.; Wang, D.; Zhang, Z.; Li, G. Magnetic separation techniques in sample preparation for biological analysis: A review. J. Pharm. Biomed. 2014, 101, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor magnetic hyperthermia induced by RGD-functionalized Fe3O4 nanoparticles, in an experimental model of colorectal liver metastases. J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rubio, I.; Insausti, M.; Garaio, E.; Gil de Muro, I.; Plazaola, F.; Rojo, T.; Lezama, L. Fe3O4 nanoparticles prepared by the seeded-growth route for hyperthermia: Electron magnetic resonance as a key tool to evaluate size distribution in magnetic nanoparticles. Nanoscale 2014, 6, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.; Jadhav, V.; Khot, V.; Zhu, X.; Xin, M.; Chen, H. Superparamagnetic MFe2O4 (M = Ni, Co, Zn, Mn) nanoparticles: Synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applications. J. Mater. Sci. Mater. Med. 2015, 26, 127. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gutierrez, V.; Virumbrales, M.; Saez-Puche, R.; Torralvo-Fernandez, M.J. Superparamagnetic behavior of MFe2O4 nanoparticles and MFe2O4/SiO2 composites (M: Co, Ni). J. Phys. Chem. C 2013, 117, 20927–20935. [Google Scholar] [CrossRef]
- Yafet, Y.; Kittle, C. Antiferromagnetic arrangements in ferrites. Phys. Rev. 1952, 87, 290–294. [Google Scholar] [CrossRef]
- Carta, D.; Casula, M.F.; Falqui, A.; Loche, D.; Mountjoy, G.; Sangregorio, C.; Corrias, A. A structural and magnetic investigation of the inversion degree in ferrite nanocrystals MFe2O4 (M = Mn, Co, Ni). J. Phys. Chem. C 2009, 113, 8606–8615. [Google Scholar] [CrossRef]
- Lu, L.T.; Dung, N.T.; Tung, L.D.; Thanh, C.T.; Quy, O.K.; Chuc, N.V.; Maenosonoe, S.; Thanh, N.T.K. Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: The influence of solvent, surfactant, reductant and synthetic conditions. Nanoscale 2015, 7, 19596–19610. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; de Montferrand, C.; Lalatonne, Y.; Motte, L.; Brioude, A. Effect of cobalt doping concentration on the crystalline structure and magnetic properties of monodisperse CoxFe3−xO4 nanoparticles within nonpolar and aqueous solvents. J. Phys. Chem. C 2012, 116, 4349–4355. [Google Scholar] [CrossRef]
- Fantechi, E.; Campo, G.; Carta, D.; Corrias, A.; de Julián Fernández, C.; Gatteschi, D.; Innocenti, C.; Pineider, F.; Rugi, F.; Sangregorio, C. Exploring the effect of co doping in fine maghemite nanoparticle. J. Phys. Chem. C 2012, 116, 8261–8270. [Google Scholar] [CrossRef]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3−xO4 Nanocubes for theranostic applications: Effect of cobalt content and particle size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Albino, M.; Lottini, E.; Sangregorio, C. Influence of cobalt dopping on the hyperthermin efficiency of magnetite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 365–371. [Google Scholar] [CrossRef]
- Crouse, C.A.; Barron, R.A. Reagent control over size, uniformity and composition of Co–Fe–O nanoparticles. J. Mater. Chem. 2008, 18, 4146–4153. [Google Scholar] [CrossRef]
- Shemer, G.; Tirosh, E.; Livneh, T.; Markovich, G. Tuning a coloidan synthesis to control Co2+ doping in ferrite nanocrystals. J. Phys. Chem. C 2007, 111, 14334–14338. [Google Scholar] [CrossRef]
- Calero-DdelC, V.L.; González, A.M.; Rinaldi, C. A statistical analysis to control the growth of cobalt ferrite nanoparticles synthesized by the thermodecomposition method. J. Manuf. Sci. Eng. 2010, 132. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Castellanos, M.; West, A.R. Deviation from Vegard’s law in oxide solid solutions. The systems Li2TiO3–MgO and Li2TiO3–Na2TiO3. J. Chem. Soc. Faraday 1980, 76, 2159–2169. [Google Scholar] [CrossRef]
- Ajroudi, L.; Mlikia, N.; Bessaisb, L.; Madigouc, V.; Villainc, S.; Lerouxc, C. Magnetic, electric and therma properties of cobalt ferrite nanoparticles. Mater. Res. Bull. 2014, 59, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Pattrick, R.A.D.; van der Laan, G.; Henderson, C.M.B.; Kuiper, P.; Dudzik, E.; Vaughan, D.J. Cation site occupancy in spinel ferrites studied by X-ray magnetic circular dichroism: Developing a method for mineralogist. Eur. J. Mineral. 2002, 14, 1095–1102. [Google Scholar] [CrossRef]
- Byrne, J.M.; Coker, V.S.; Moise, S.; Wincott, P.L.; Vaughan, D.J.; Tuna, F.; Arenholz, E.; van der Laan, G.; Pattrick, R.A.D.; Lloyd, J.R.; et al. Controlled cobalt doping in biogenic magnetite nanoparticles. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, S.; Panneerselvam, G.; Antony, M.P.; Philip, J. High temperature stability of surfactant capped CoFe2O4 nanoparticles. Mater. Chem. Phys. 2011, 130, 1300–1306. [Google Scholar] [CrossRef]
- Mameli, V.; Musinu, A.; Ardu, A.; Ennas, G.; Peddis, D.; Niznansky, D.; Sangregorio, C.; Innocenti, C.; Thanh, N.T.K.; Cannas, C. Studying the effect of Zn-substitution on the magnetic and hyperthermic properties of cobalt ferrite nanoparticles. Nanoscale 2016, 8, 10124–10137. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Falkner, J.C.; Yavuz, C.T.; Colvin, V.L. Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts. Chem. Commun. 2004, 20, 2306–2307. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.; Abdelraheem, W.H.; Han, C.; Nadagouda, M.N.; Sygellou, L.; Arfanis, M.K.; Falaras, P.; Sharma, V.K.; Dionysiou, D.D. Cobalt ferrite nanoparticles with controlled composition-peroxymonosulfate mediated degradation of 2-phenylbenzimidazole-5-sulfonic acid. Appl. Catal. B Environ. 2018, 221, 266–279. [Google Scholar] [CrossRef]
- Eom, Y.; Abbas, M.; Noh, H.Y.; Kim, C.G. Morphology-controlled synthesis of highly crystalline Fe3O4 and CoFe2O4 nanoparticles using a facile thermal decomposition method. RSC Adv. 2016, 6, 15861–15867. [Google Scholar] [CrossRef]
- Yang, H.; Ogawa, T.; Hasegawa, D.; Takahashi, M. Synthesis and magnetic properties of monodisperse magnetite nanocubes. Jpn. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Zeng, H.; Rice, P.M.; Wang, S.X.; Sun, S. Shape-Controlled Synthesis and Shape-Induced Texture of MnFe2O4 Nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459. [Google Scholar] [CrossRef] [PubMed]
- Stoner, E.C.; Wohlfarth, E.P.A. A Mechanism of magnetic hysteresis heterogeneous alloys. Philos. Trans. R. Soc. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Zhang, Q.; Castellanos-Rubio, I.; Munshi, R.; Orue, I.; Pelaz, B.; Gries, K.I.; Parak, W.J.; del Pino, P.; Pralle, A. Model driven optimization of magnetic anisotropy of exchange-coupled core-shell ferrite nanoparticles for maximal hysteretic loss. Chem. Mater. 2015, 27, 7380–7387. [Google Scholar] [CrossRef]
- Vargas, J.M.; Nunes, W.C.; Socolovsky, L.M.; Knobel, M.; Zanchet, D. Effect of dipolar interaction observed in iron-based nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 2–7. [Google Scholar] [CrossRef]
- Bruvera, I.J.; Mendoza, P.; Calatayud, M.P.; Goya, G.F.; Sanchez, F.H. Determination of the blocking temperature of magnetic nanoparticles: The good, the bad and the ugly. J. Appl. Phys. 2015, 118, 184304. [Google Scholar] [CrossRef]
- Usov, N.A. Numerical simulation of field-cooled and zero field-cooled processes for assembly of superparamagnetic nanoparticles with uniaxial anisotropy. J. Appl. Phys. 2011, 109, 023913. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J. Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Einsenstein, I.; Aharoni, A. Asymptotic superparamagnetic time constants for cubic anisotropy. II. Negative anisotropy constants. Phys. Rev. B 1977, 16, 1285–1290. [Google Scholar] [CrossRef]
- Slonczewski, J.C. Origin of Magnetic Anisotropy in Cobalt-Substituted Magnetite. Phys. Rev. 1958, 110, 1341–1348. [Google Scholar] [CrossRef]
- Shenker, H. Magnetic Anisotropy of Cobalt Ferrite and Nickel Cobalt Ferrite. Phys. Rev. 1957, 107, 1246–1249. [Google Scholar] [CrossRef]
- Nlebedim, I.C.; Snyder, J.E.; Moses, A.J.; Jiles, D.C. Anisotropy and Magnetostriction in Non-Stoichiometric Cobalt Ferrite. IEEE Trans. Magn. 2012, 48, 3084–3087. [Google Scholar] [CrossRef]
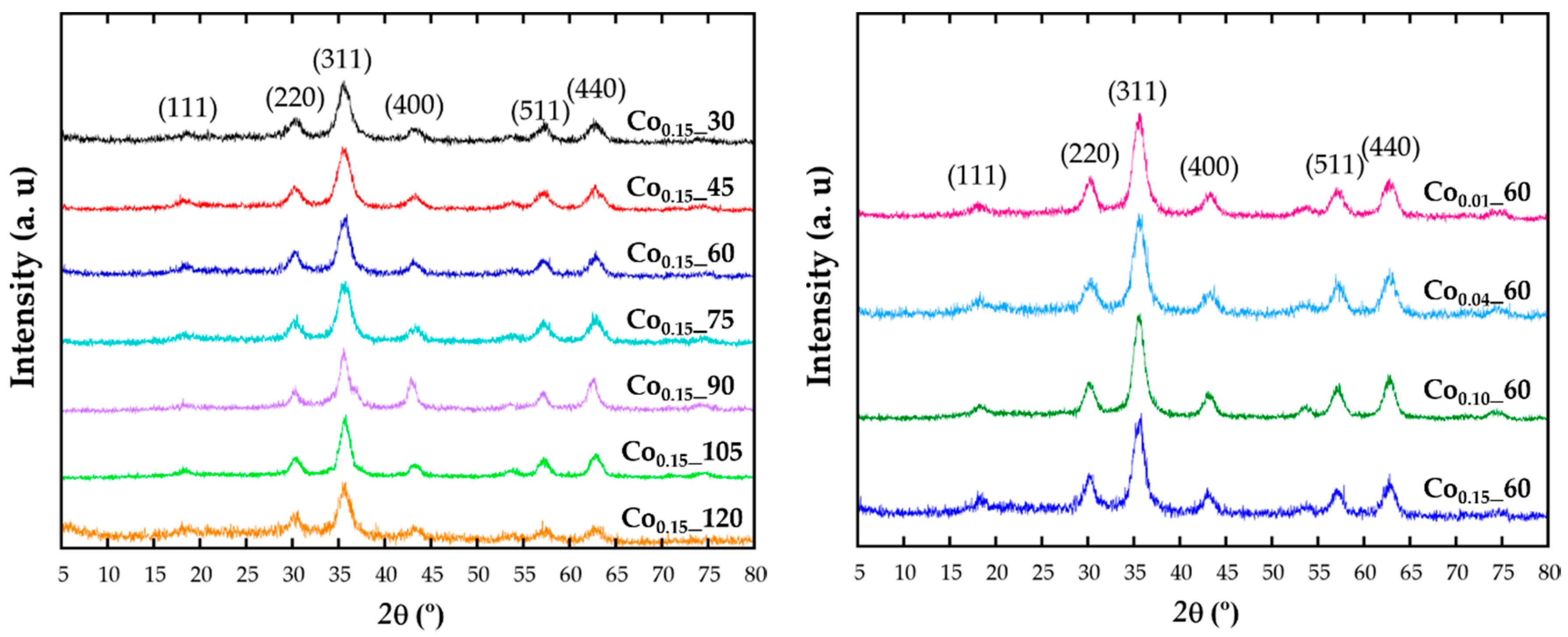

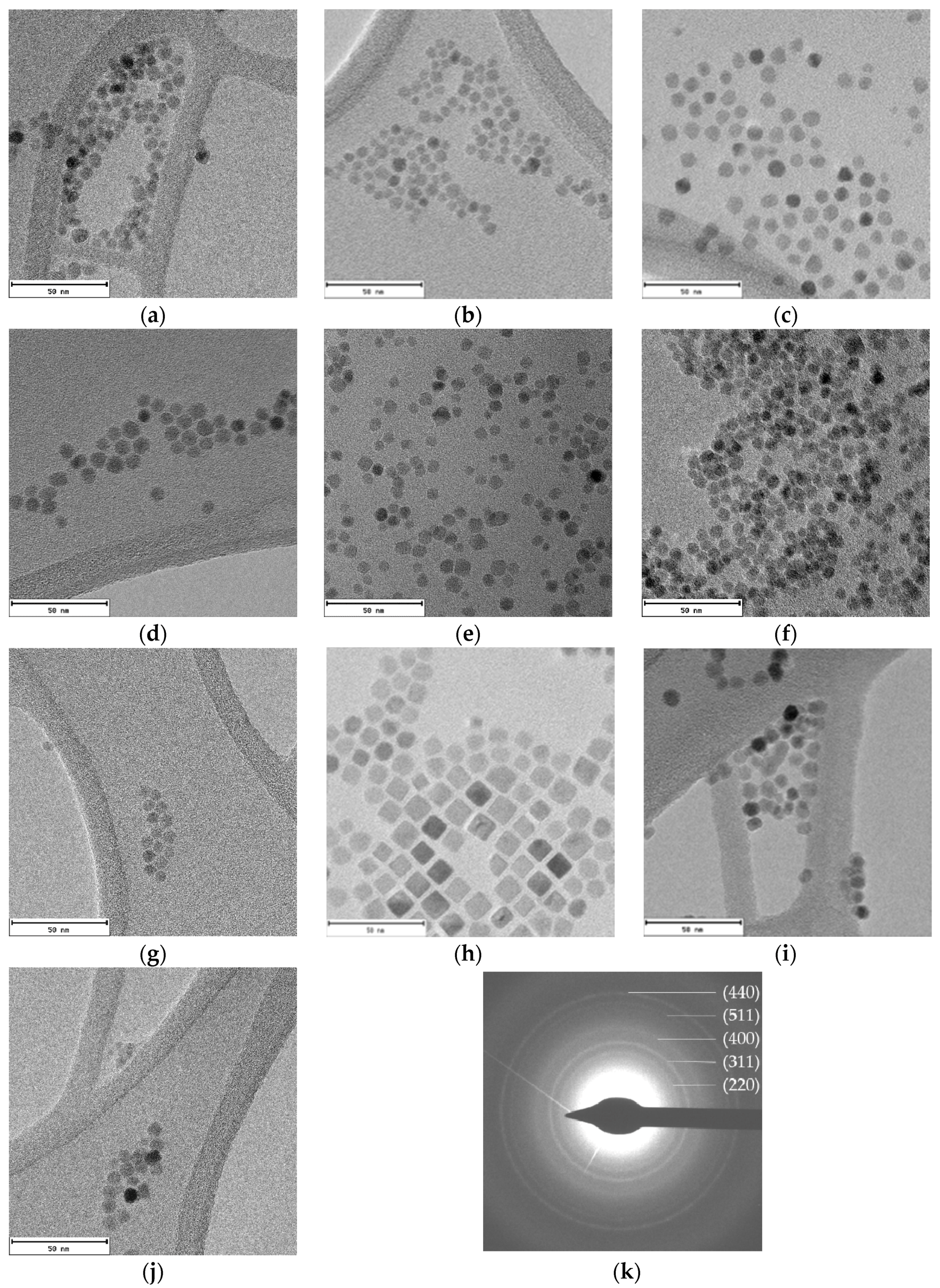
| Sample | a (Å) | ICP | TGA (%) | TEM (nm) |
|---|---|---|---|---|
| Co0.15_30 | 8.3757 (4) | Co0.08Fe2.92O4 | 28.08 | 6 (1) |
| Co0.15_45 | 8.3701 (3) | Co0.08Fe2.92O4 | 30.68 | 6 (1) |
| Co0.15_60 | 8.3780 (3) | Co0.14Fe2.86O4 | 43.82 | 8 (1) |
| Co0.10_60 | 8.3769 (2) | Co0.07Fe2.93O4 | 29.58 | 7 (1) |
| Co0.04_60 | 8.3700 (3) | Co0.03Fe2.97O4 | 23.31 | 7 (1) |
| Co0.01_60 | 8.3730 (2) | Co0.01Fe2.99O4 | 25.77 | 6 (1) |
| Co0.15_75 | 8.3671 (3) | Co0.1Fe2.9O4 | 40.85 | 7 (1) |
| Co0.15_90 | 8.4277(4) | Co0.09Fe2.11O4 | 31.33 | 11 (1) |
| Co0.15_105 | 8.3798 (2) | Co0.11Fe2.89O4 | 25.89 | 8 (1) |
| Co0.15_120 | 8.3671 (5) | Co0.16Fe2.84O4 | 36.981 | 6 (1) |
| Sample | ICP | <Tb> (K) | DZFC/FC (nm) | Keff (0 K) (KJ/m3) | Kc (KJ/m3) | Cox | Hc (5 K) (Oe) | Ms (emu/g) |
|---|---|---|---|---|---|---|---|---|
| Co0.15_60 | Co0.14Fe2.86O4 | 75.7 | 7.51 (1) | 138 | 553.2 | 0.05 | 5.300 | 82.15 |
| Co0.1_60 | Co0.07Fe2.93O4 | 66.7 | 7.68 (1) | 102 | 434 | 0.04 | 3650 | 74.25 |
| Co0.04_60 | Co0.03Fe2.97O4 | 34.8 | 7.39(1) | 54.9 | 219.6 | 0.02 | 999 | 82.59 |
| Co0.01_60 | Co0.01Fe2.99O4 | 24.6 | 6.86 (1) | 46.5 | 186.2 | 0.02 | 610 | 85.25 |
| Co0.15_30 | Co0.08Fe2.92O4 | 51.8 | 6.37 (2) | 115.6 | 462.4 | 0.05 | 3.300 | 69.66 |
| Co0.15_45 | Co0.08Fe2.92O4 | 47.8 | 6.14 (1) | 107 | 428 | 0.04 | 3.600 | 70.58 |
| Co0.15_75 | Co0.1Fe2.9O4 | 46.1 | 6.44 (1) | 102.4 | 409.6 | 0.04 | 3.310 | 77.48 |
| Co0.15_90 | Co0.09Fe2.11O4 | 96.5 | 10.7 (2) | 55.1 | 220.6 | 0.02 | 2.262 | 65.63 |
| Co0.15_105 | Co0.11Fe2.89O4 | 110.2 | 7.96 (1) | 208.8 | 835.2 | 0.08 | 6.360 | 77.93 |
| Co0.15_120 | Co0.16Fe2.84O4 | 49.9 | 5.98 (2) | 120.9 | 483.6 | 0.05 | 1.800 | 86.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galarreta, I.; Insausti, M.; Gil de Muro, I.; Ruiz de Larramendi, I.; Lezama, L. Exploring Reaction Conditions to Improve the Magnetic Response of Cobalt-Doped Ferrite Nanoparticles. Nanomaterials 2018, 8, 63. https://doi.org/10.3390/nano8020063
Galarreta I, Insausti M, Gil de Muro I, Ruiz de Larramendi I, Lezama L. Exploring Reaction Conditions to Improve the Magnetic Response of Cobalt-Doped Ferrite Nanoparticles. Nanomaterials. 2018; 8(2):63. https://doi.org/10.3390/nano8020063
Chicago/Turabian StyleGalarreta, Itziar, Maite Insausti, Izaskun Gil de Muro, Idoia Ruiz de Larramendi, and Luis Lezama. 2018. "Exploring Reaction Conditions to Improve the Magnetic Response of Cobalt-Doped Ferrite Nanoparticles" Nanomaterials 8, no. 2: 63. https://doi.org/10.3390/nano8020063





