Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model
Abstract
:1. Introduction
2. Results and Discussion
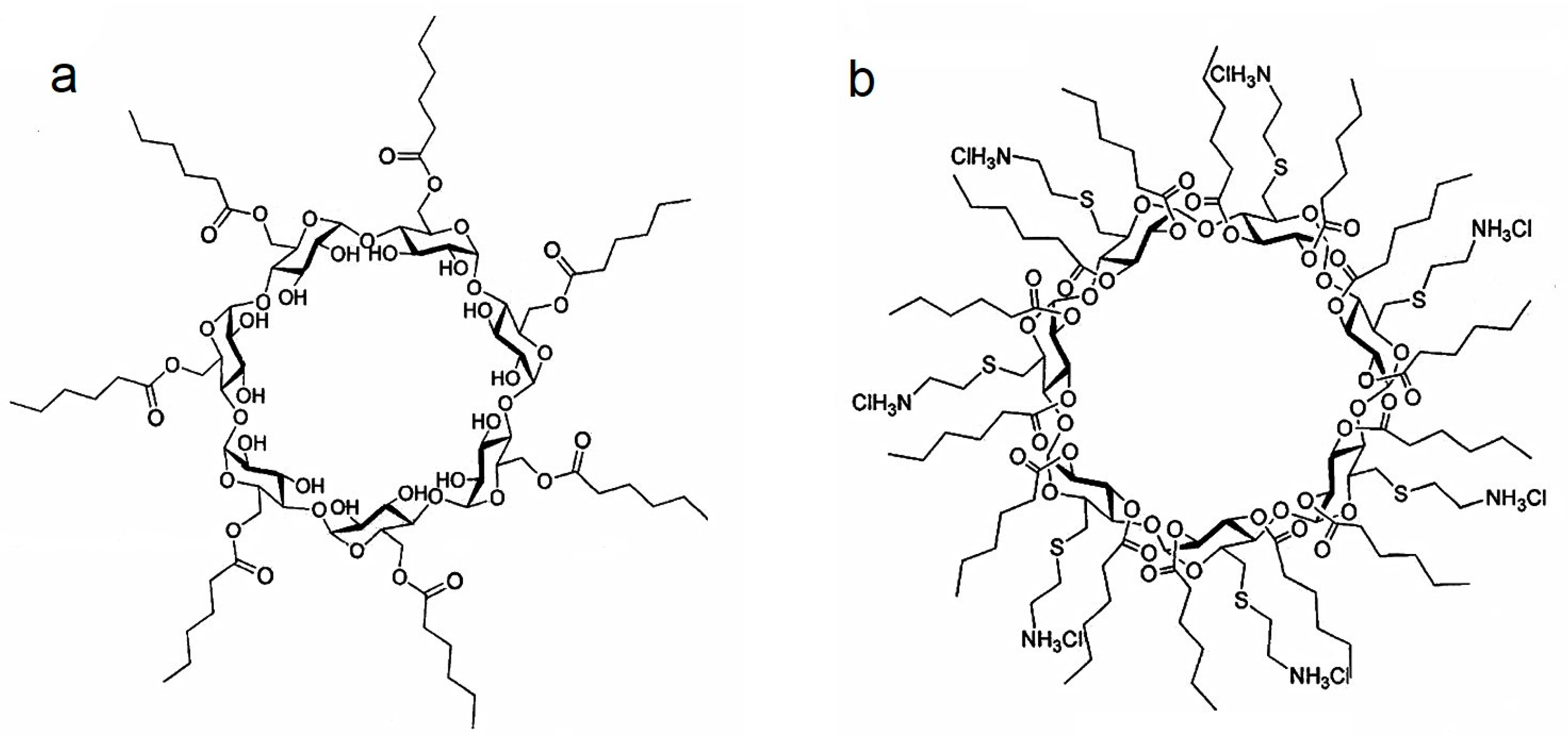
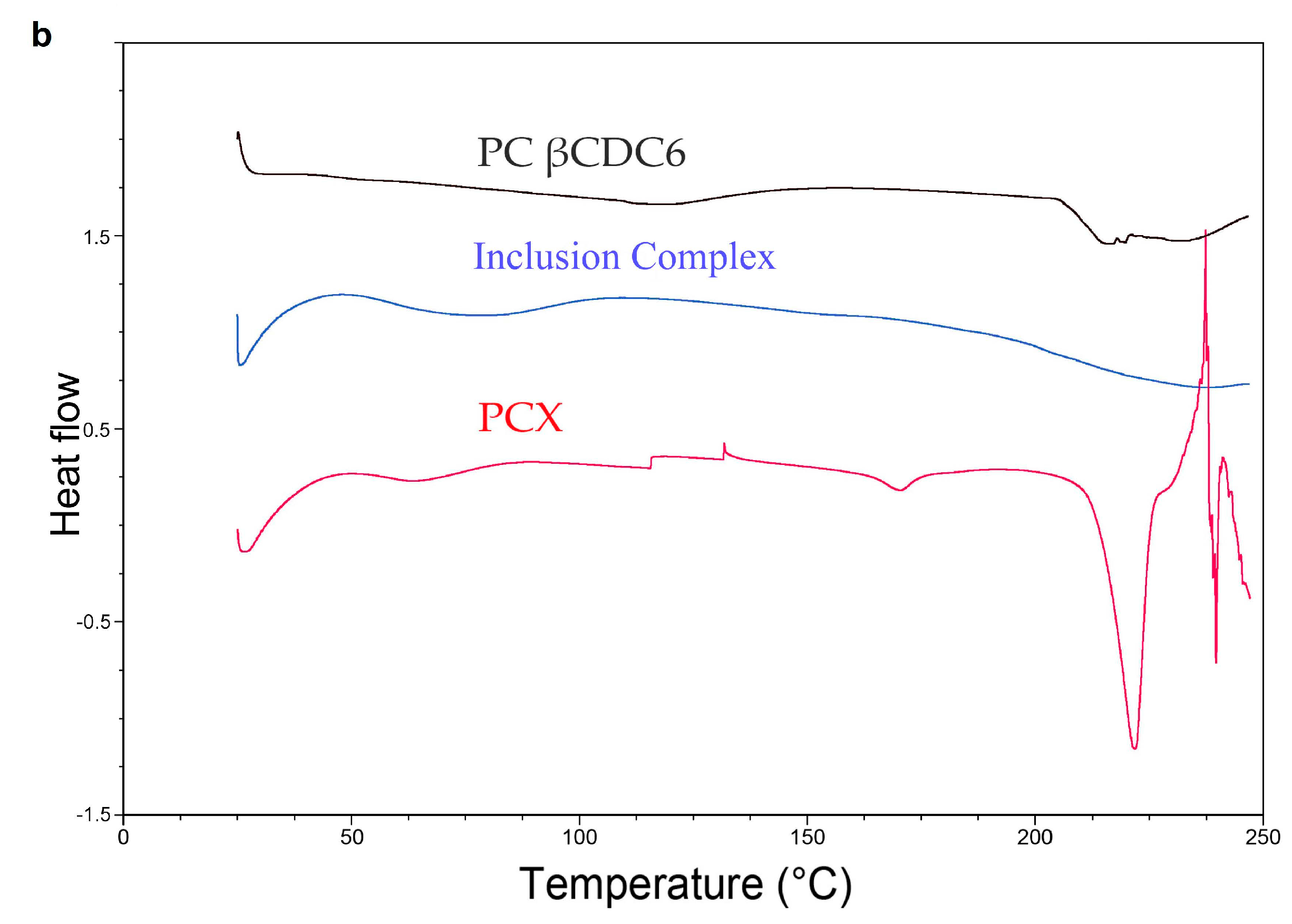
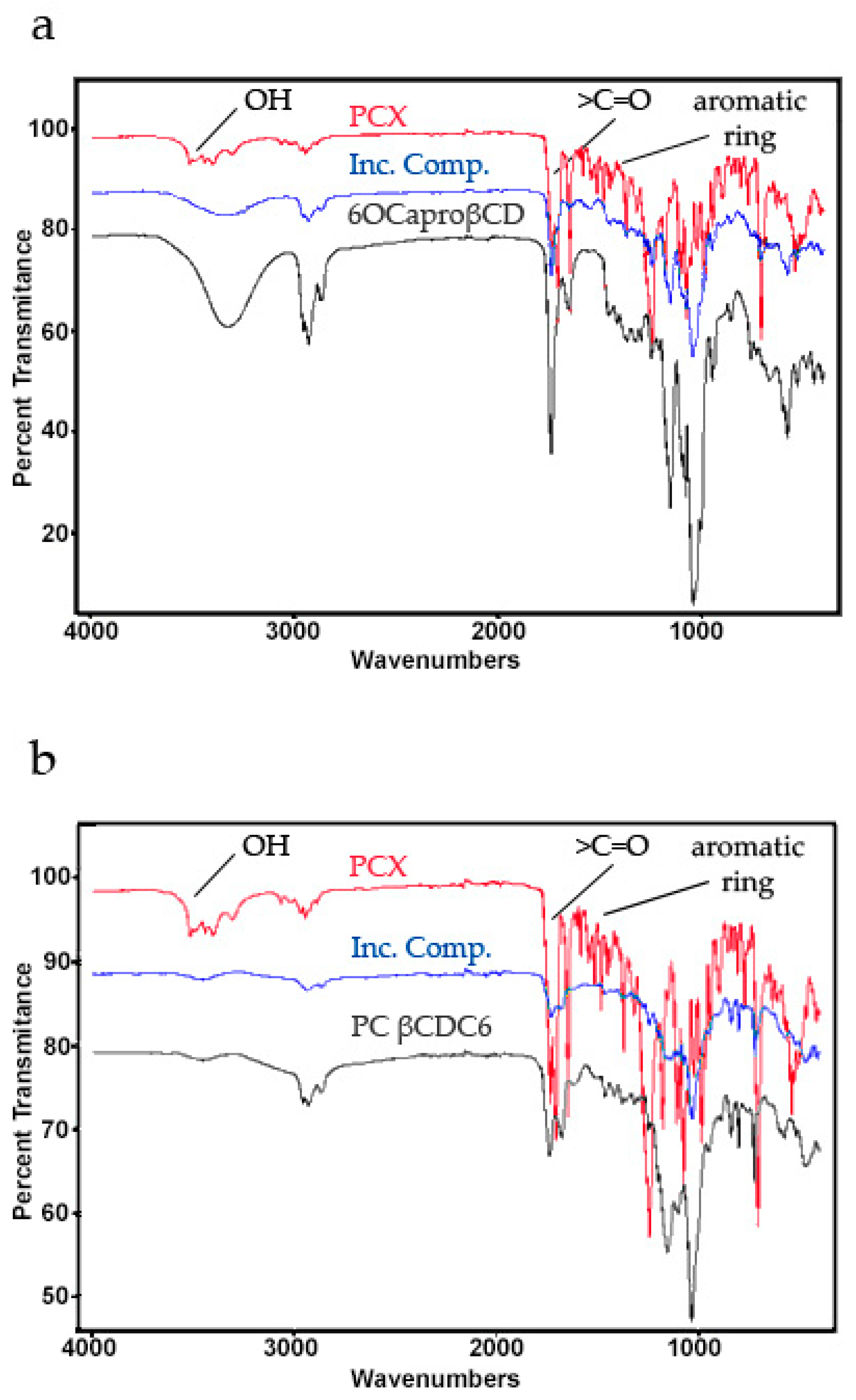
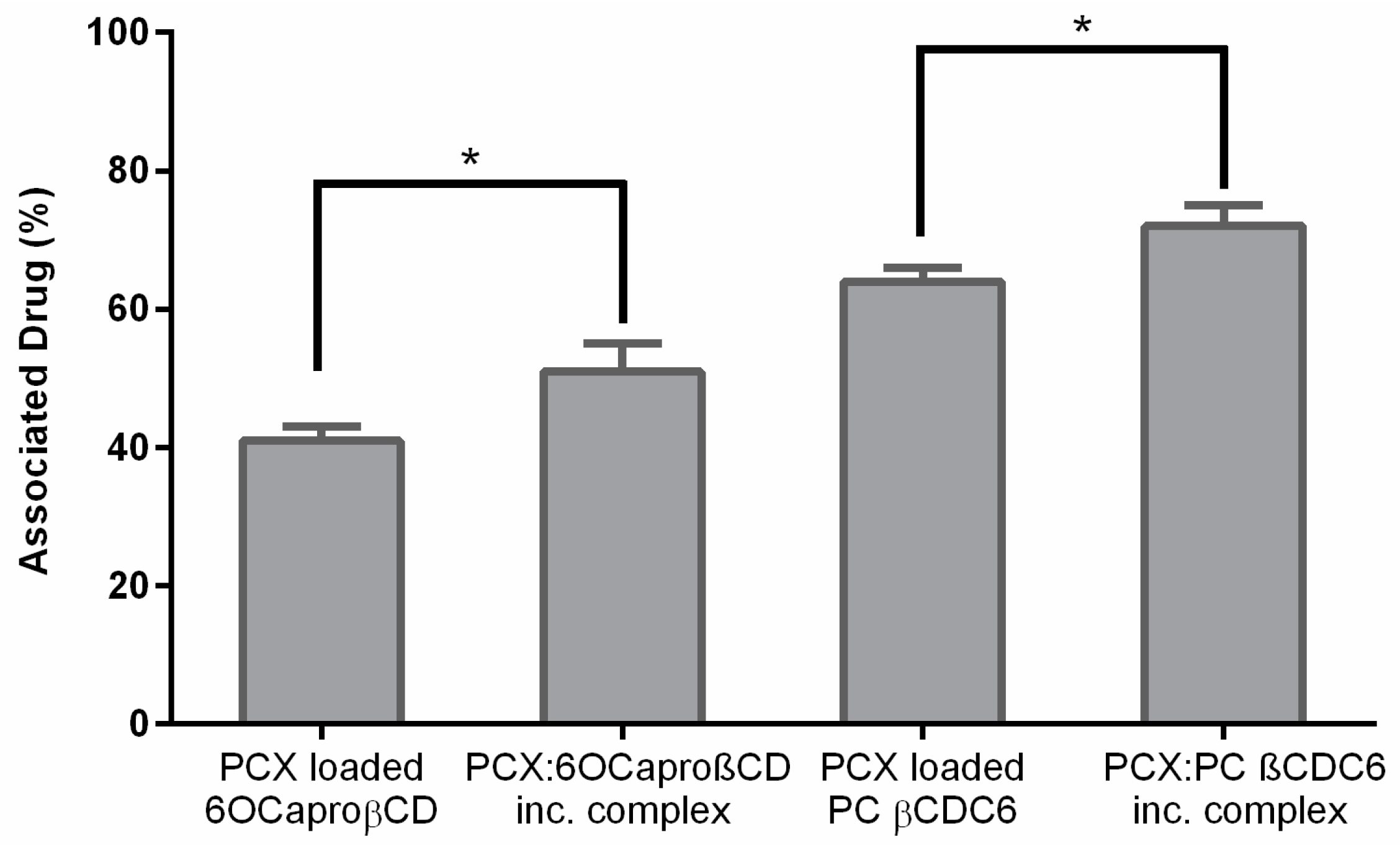
2.1. Characterization of Paclitaxel:Cyclodextrin (PCX:CD) Inclusion Complexes
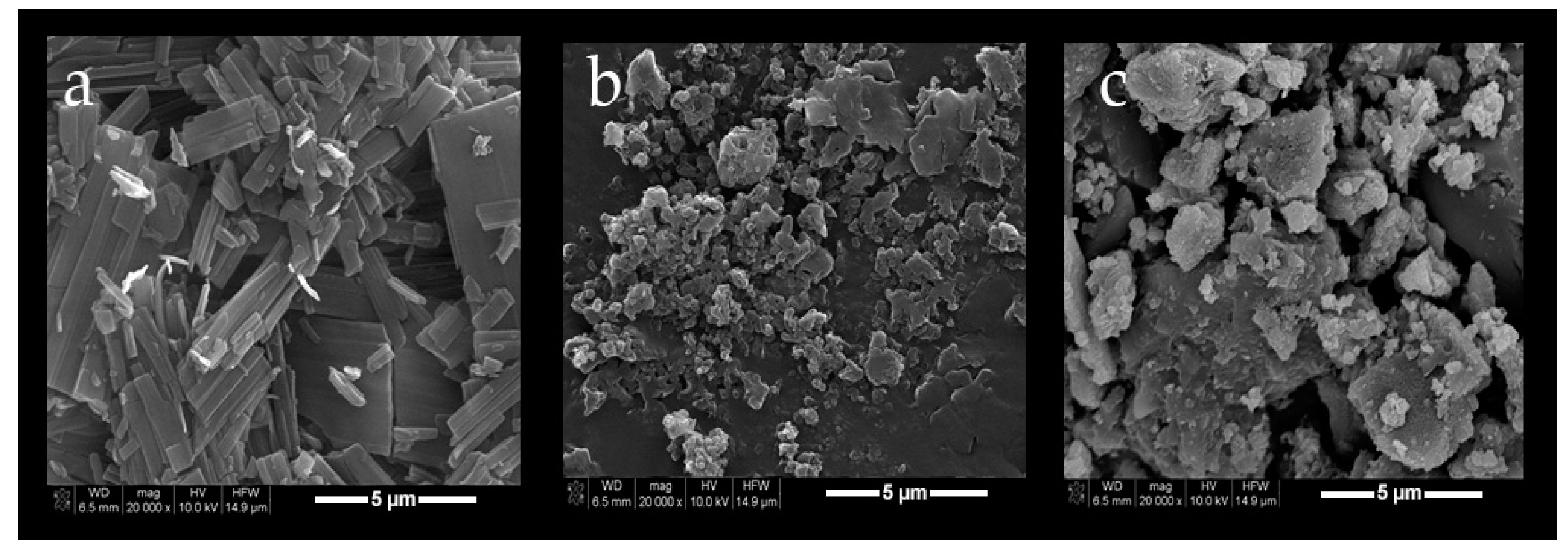
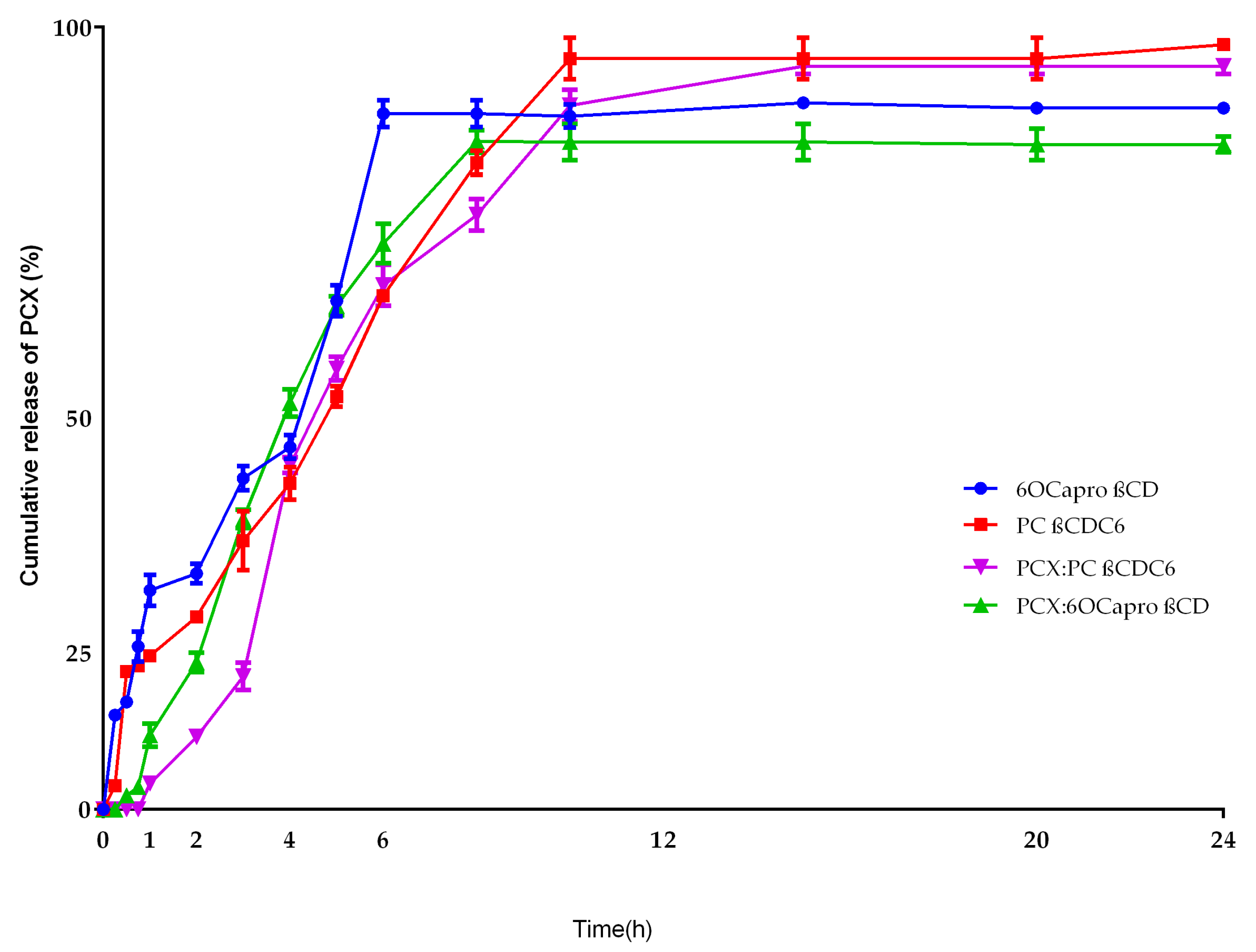
2.2. Characterization of Blank or PCX Loaded Nanoparticles
2.3. Cell Culture Studies
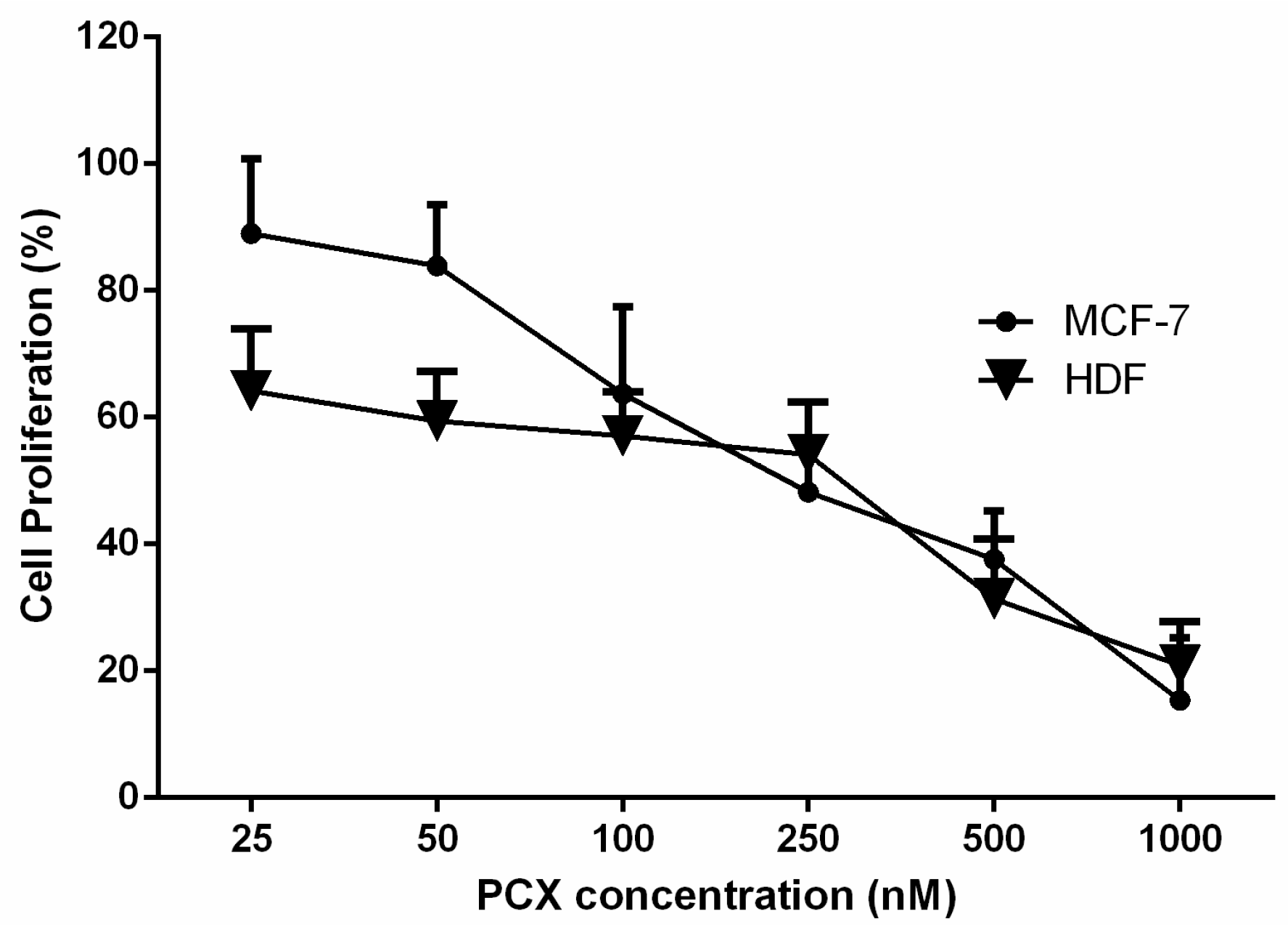
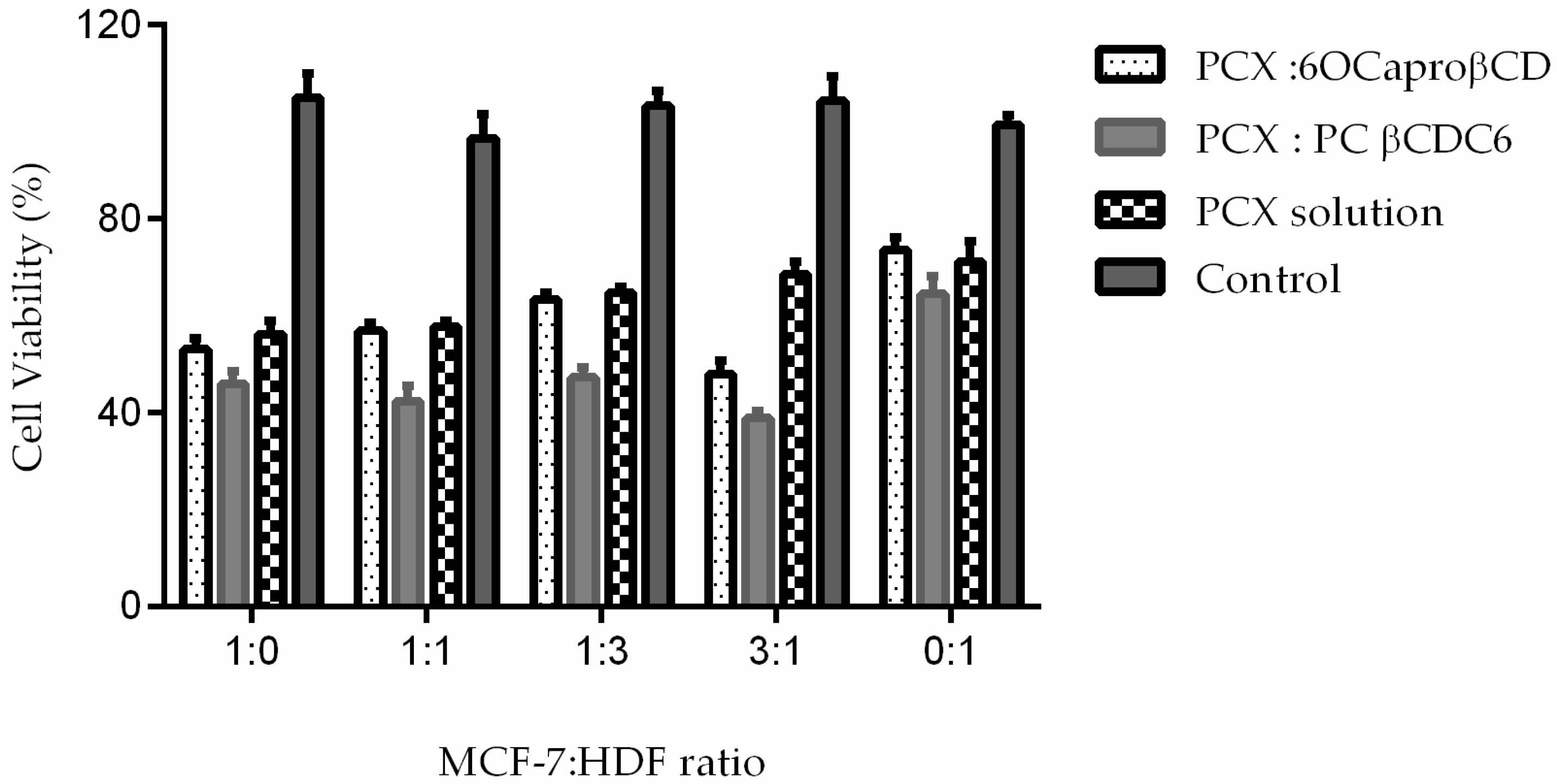
2.3.1. Determination IC50 of PCX on Co-Culture
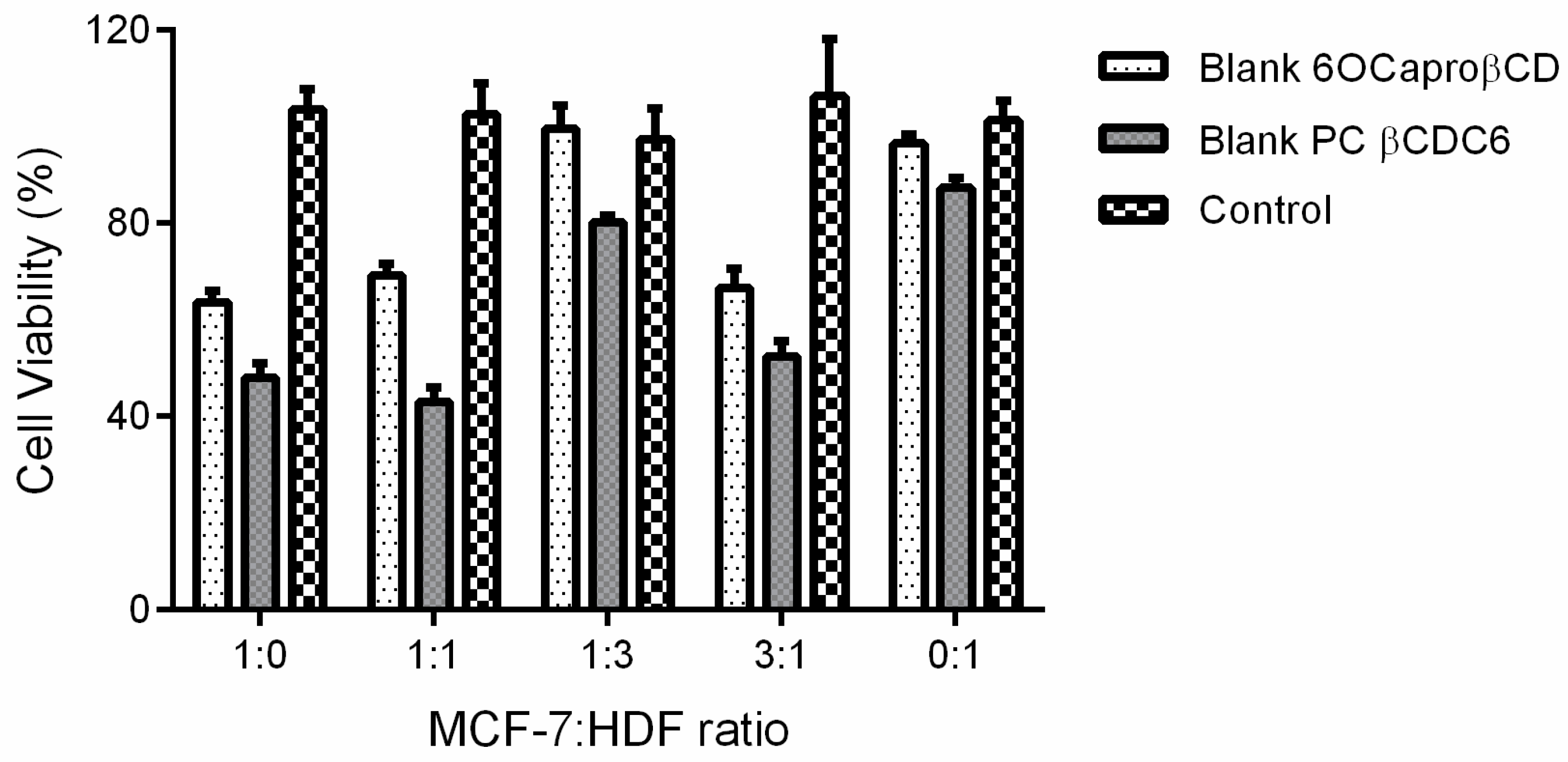
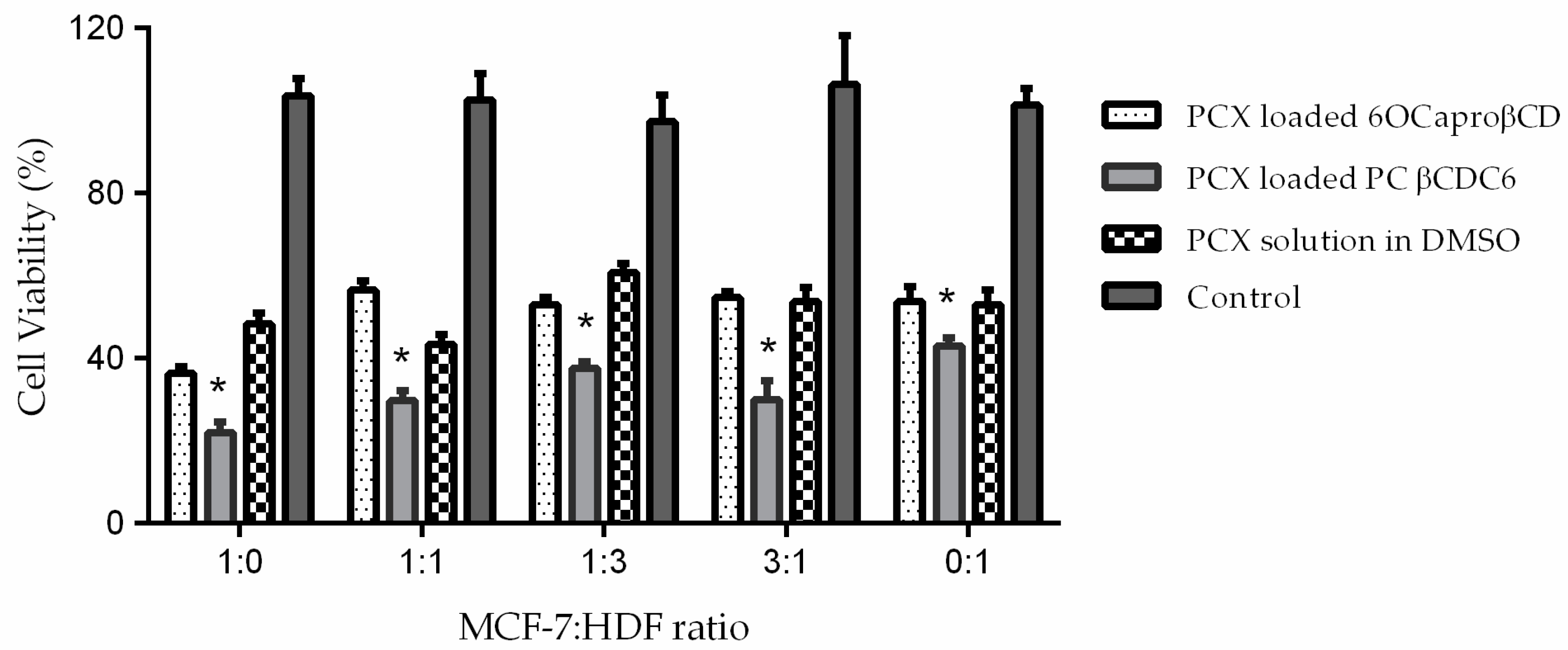
2.3.2. Determination of Anticancer Activity of Blank or PCX Loaded Nanoparticles on Co-Culture Model
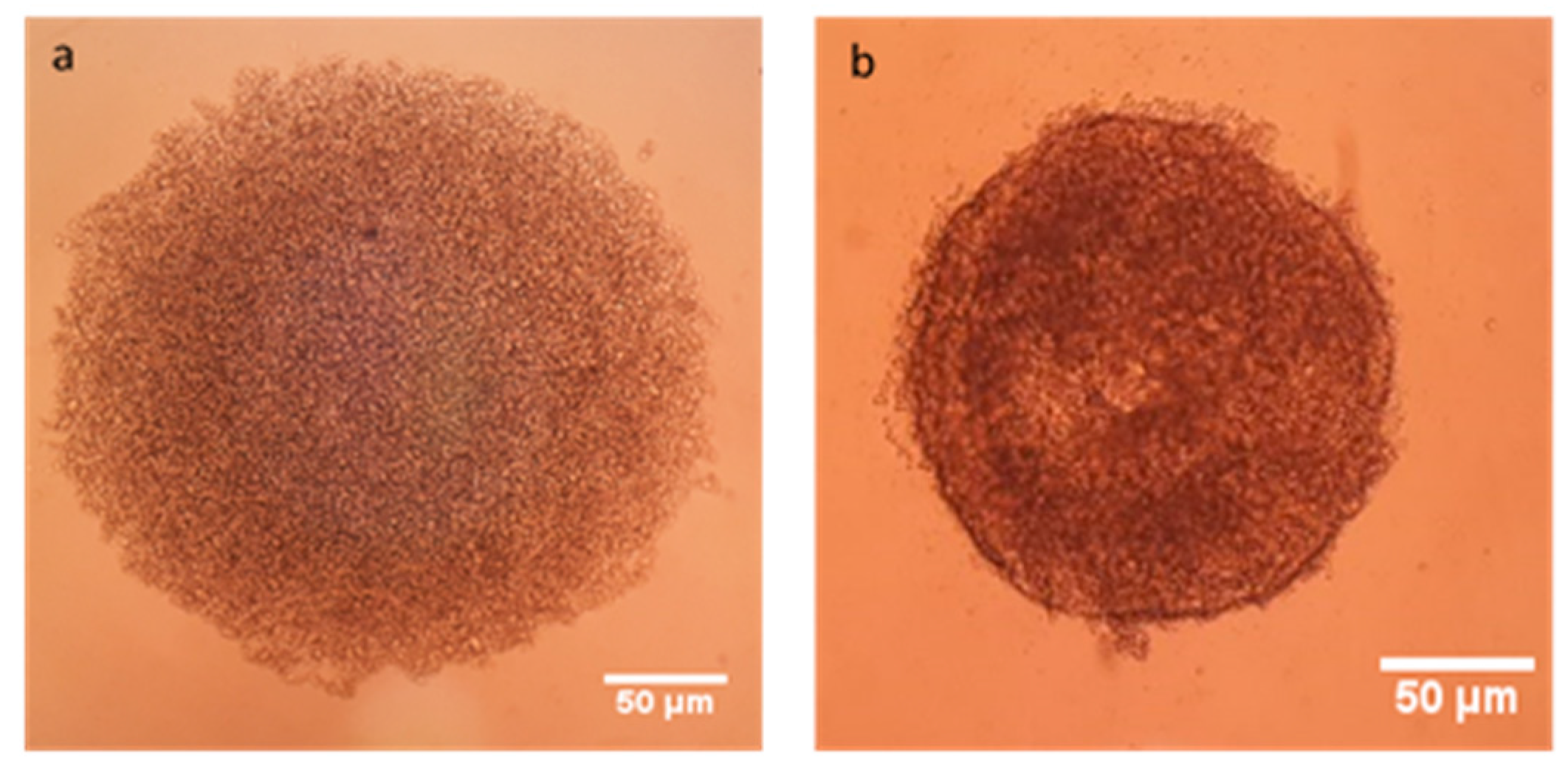
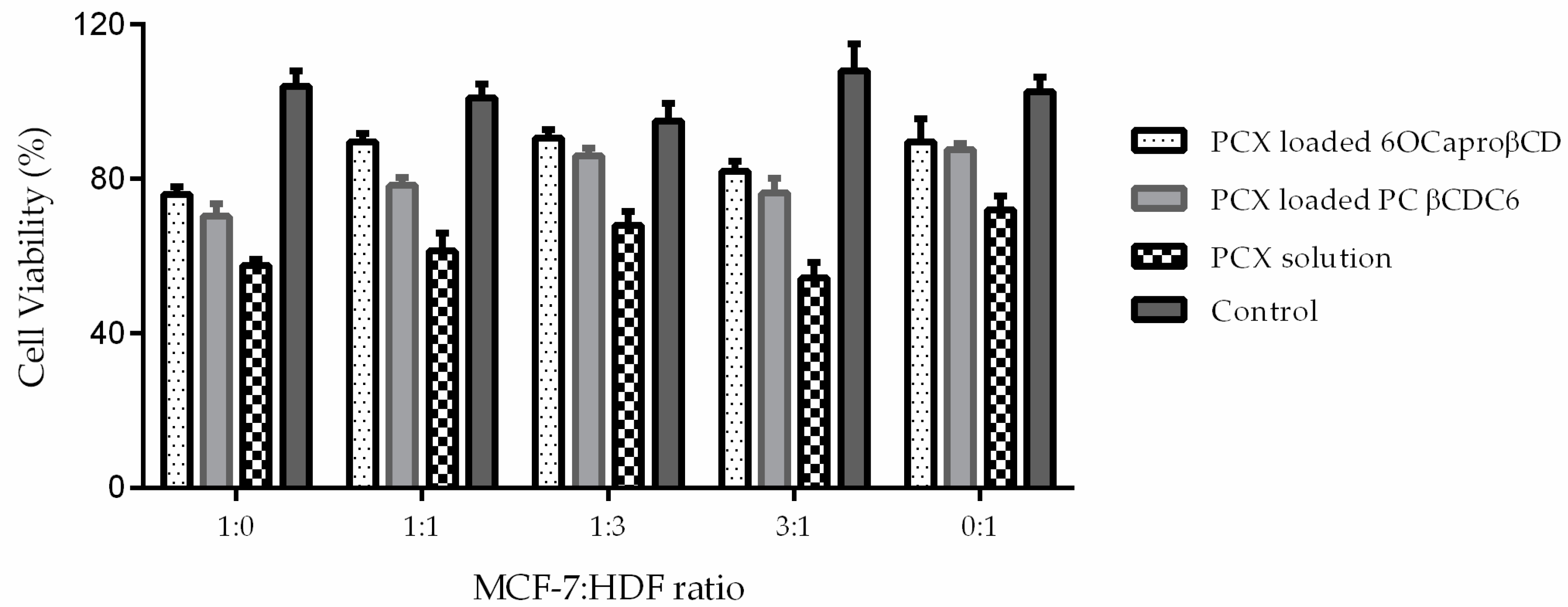
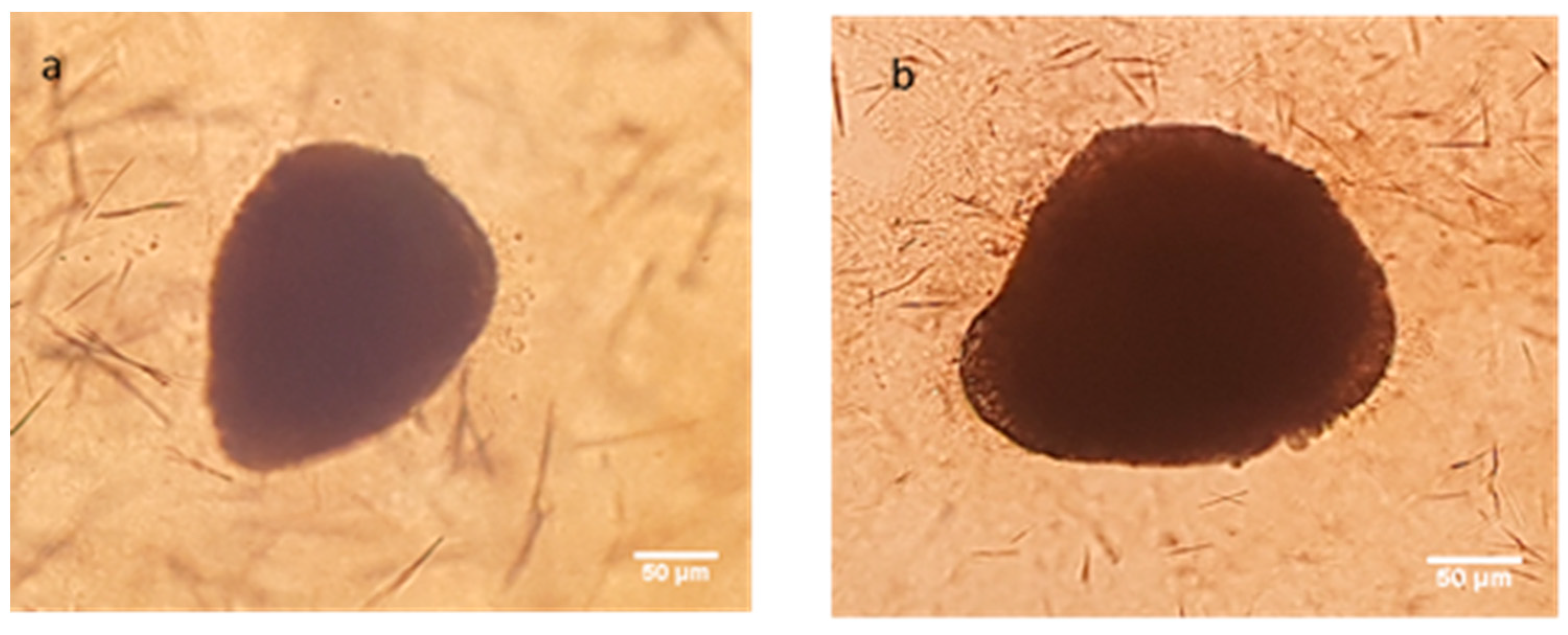
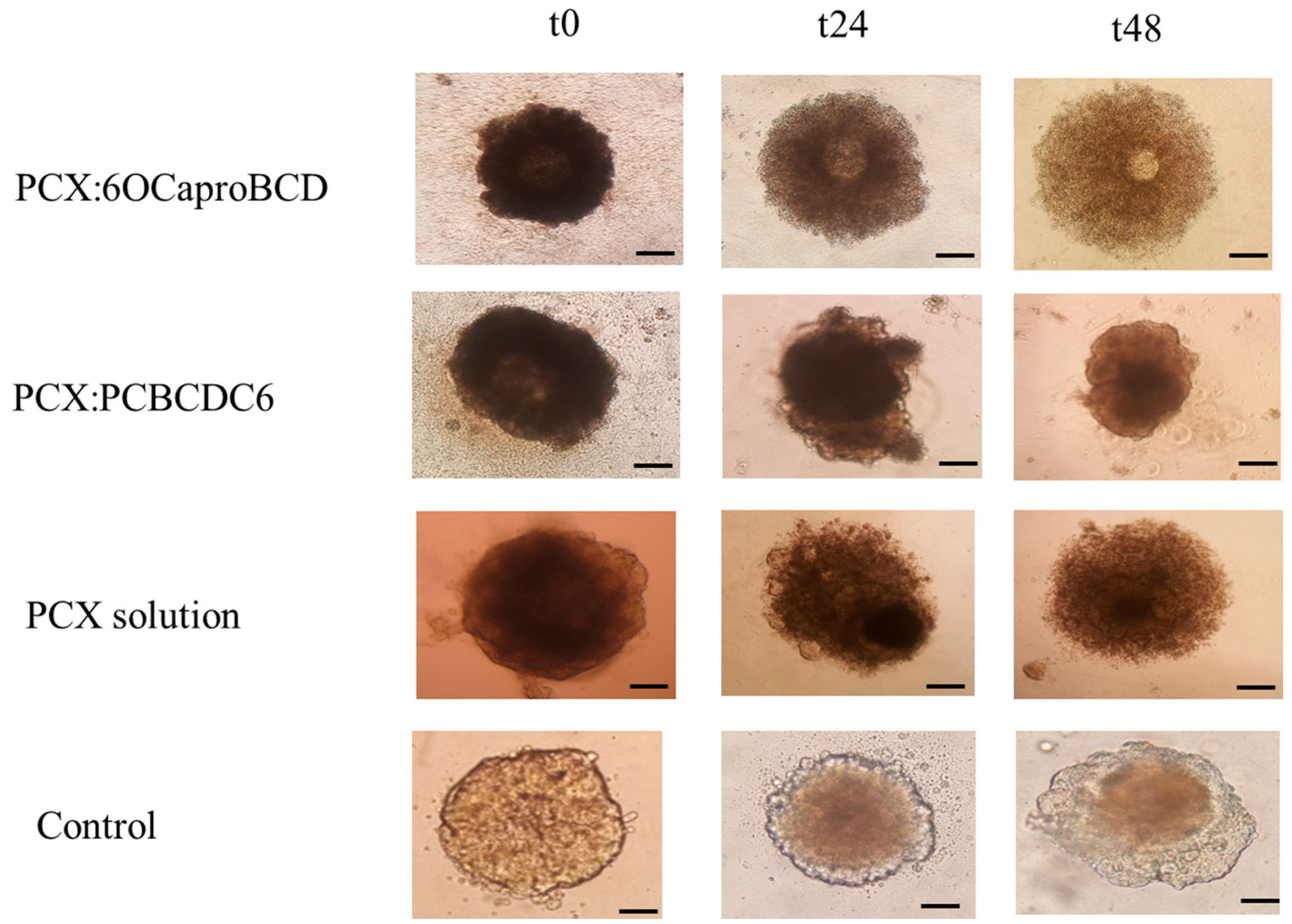
2.3.3. Determination of Antitumoral Activity of PCX Loaded Nanoparticles on 3-Dimensional (3D) Multicellular Tumor Spheroid (MCTS) Cell Culture
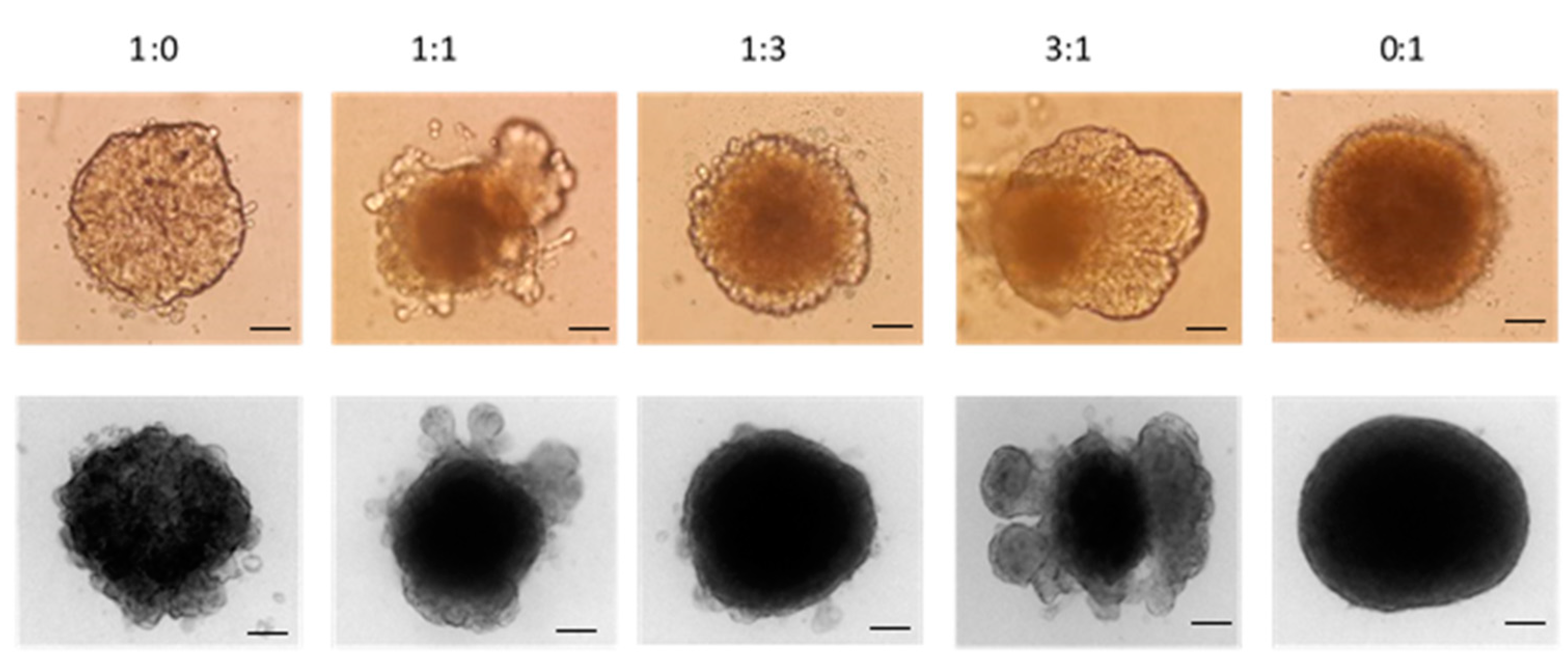
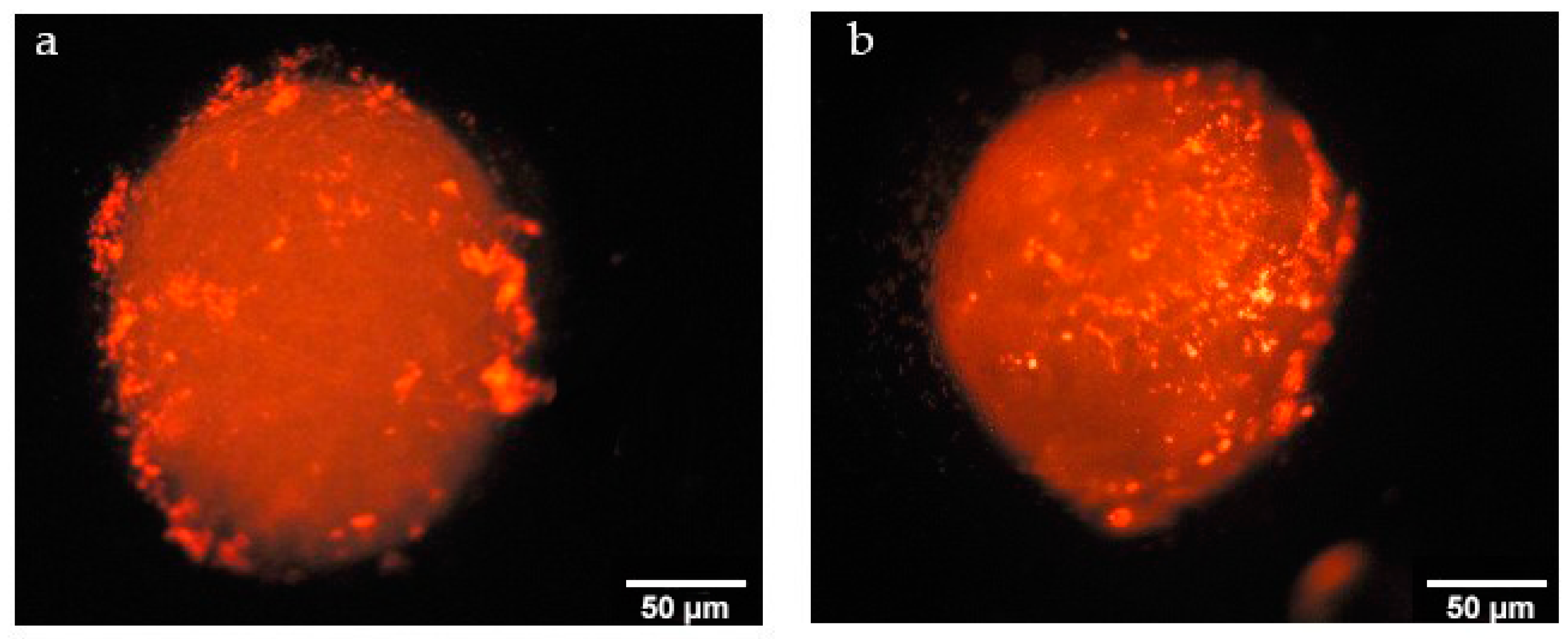
2.3.4. Determination of Penetration Properties of Amphiphilic CD Nanoparticles on 3D Cell Culture
3. Materials and Methods
3.1. Preparation and Characterization of PCX:CD Inclusion Complexes
3.2. Preparation and Characterization of Nanoparticles
3.3. Cell Culture Studies
3.1.1. Determination IC50 of PCX on Co-Culture
3.3.2. Determination of Anticancer Activity of Blank and PCX Loaded Nanoparticles on Co-Culture Model
3.3.3. Determination of Antitumoral Activity of PCX Loaded Nanoparticles on 3D Cell Culture
3.3.4. Determination of Tumoral Penetration Properties of Amphiphilic CD Nanoparticles on 3D Cell Culture
3.3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Nehate, C.; Jain, S.; Saneja, A.; Khare, V.; Alam, N.; Dubey, R.D.; Gupta, P.N. Paclitaxel formulations: Challenges and novel delivery options. Curr. Drug Deliv. 2014, 11, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Bilensoy, E.; Gurkaynak, O.; Dogan, A.L.; Hincal, A.A. Safety and efficacy of amphiphilic beta-cyclodextrin nanoparticles for paclitaxel delivery. Int. J. Pharm. 2008, 347, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E.; Gurkaynak, O.; Ertan, M.; Sen, M.; Hincal, A.A. Development of nonsurfactant cyclodextrin nanoparticles loaded with anticancer drug paclitaxel. J. Pharm. Sci. 2008, 97, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E.; Hincal, A.A. Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opin. Drug Deliv. 2009, 6, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Memişoğlu, E.; Bochot, A.; Şen, M.; Charon, D.; Duchêne, D.; Hıncal, A.A. Amphiphilic β-cyclodextrins modified on the primary face: Synthesis, characterization, and evaluation of their potential as novel excipients in the preparation of nanocapsules. J. Pharm. Sci. 2002, 91, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Duffour, M.; Chevalier, Y.; Parrot-Lopez, H. Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur. J. Pharm. Biopharm. 2013, 83, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Erdogar, N.; Varan, G.; Bilensoy, E. Amphiphilic Cyclodextrin Derivatives for Targeted Drug Delivery to Tumors. Curr. Top. Med. Chem. 2017, 17, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, E.L.; Porto, L.M.; Rambo, C.R. Nanotechnology meets 3D in vitro models: Tissue engineered tumors and cancer therapies. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Stein, R.; O’Hare, M.J. Three-dimensional in vitro tissue culture models of breast cancer—A review. Breast Cancer Res. Treat. 2004, 85, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Benito, J.M.; Mellet, C.O.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotechnol. 2017, 8, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Oncul, S.; Ercan, A.; Benito, J.M.; Ortiz Mellet, C.; Bilensoy, E. Cholesterol-Targeted Anticancer and Apoptotic Effects of Anionic and Polycationic Amphiphilic Cyclodextrin Nanoparticles. J. Pharm. Sci. 2016, 105, 3172–3182. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wen, Y.; Zhu, J.L.; Zhao, F.; Zhang, Z.X.; Li, J. Thermoresponsive Delivery of Paclitaxel by β-Cyclodextrin-Based Poly(N-isopropylacrylamide) Star Polymer via Inclusion Complexation. Biomacromolecules 2016, 17, 3957–3963. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Shah, V.; Ghosh, A.; Zhang, Z.; Minko, T. Molecular Inclusion Complexes of β-Cyclodextrin Derivatives Enhance Aqueous Solubility and Cellular Internalization of Paclitaxel: Preformulation and in vitro Assessments. J. Pharm. Pharmacol. 2015, 2, 8. [Google Scholar] [CrossRef]
- Sharma, U.S.; Balasubramanian, S.V.; Straubinger, R.M. Pharmaceutical and physical properties of paclitaxel (taxol) complexes with cyclodextrins. J. Pharm. Sci. 1995, 84, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Lee, S.E.; Kang, B.S.; Ng, C.L.; Davaa, E.; Park, J.S. Thermosensitive and Mucoadhesive Sol-Gel Composites of Paclitaxel/Dimethyl-β-Cyclodextrin for Buccal Delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef] [PubMed]
- Varan, C.; Wickstrom, H.; Sandler, N.; Aktas, Y.; Bilensoy, E. Inkjet printing of antiviral PCL nanoparticles and anticancer cyclodextrin inclusion complexes on bioadhesive film for cervical administration. Int. J. Pharm. 2017, 531, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Verheul, R.; Defrees, K.; Collins, C.J.; Schuldt, R.A.; Vlahu, A.; Thompson, D.H. Microfluidic assembly of cationic-β-cyclodextrin:hyaluronic acid-adamantane host:guest pDNA nanoparticles. Biomater. Sci. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Sadlonova, A.; Novak, Z.; Johnson, M.R.; Bowe, D.B.; Gault, S.R.; Page, G.P.; Thottassery, J.V.; Welch, D.R.; Frost, A.R. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 2005, 7, R46–R59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Rimann, M.; Graf-Hausner, U. Synthetic 3D multicellular systems for drug development. Curr. Opin. Biotechnol. 2012, 23, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Ghajar, C.M.; Bissell, M.J. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv. Drug Deliv. Rev. 2014, 69–70, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Fryknas, M.; Larsson, R.; Nygren, P. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp. Cell Res. 2012, 318, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Gowan, S.; Boxall, F.; Patterson, L.; Zimmermann, M.; Court, W.; Lomas, C.; Mendiola, M.; Hardisson, D.; Eccles, S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Herceg, V.; Ateb, I.; Allemann, E.; Lange, N. Tunable phosphatase-sensitive stable prodrugs of 5-aminolevulinic acid for tumor fluorescence photodetection. J. Control. Release 2016, 235, 155–164. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticle Formulations | Particle Size (nm) ± SD | PDI ± SD | Zeta Potential (mV) ± SD |
|---|---|---|---|
| Blank 6OCaproβCD | 103 ± 1 | 0.13 ± 0.02 | −24 ± 0.3 |
| Blank PC βCDC6 | 75 ± 2 | 0.16 ± 0.02 | +61 ± 1.4 |
| PCX loaded 6OCaproβCD | 113 ± 4 | 0.22 ± 1 | −29 ± 2 |
| PCX loaded PC βCDC6 | 82 ± 2 | 0.24 ± 5 | +62 ± 1 |
| PCX:6OCaproβCD inclusion complex | 135 ± 2 | 0.13 ± 0.04 | −31 ± 3 |
| PCX:PC βCDC6 inclusion complex | 120 ± 4 | 0.15 ± 0.2 | +59 ± 2 |
| MCF-7: HDF Ratio | Cell Viability % |
|---|---|
| 1:0 (MCF-7 alone) | 48.4 ± 0.8 |
| 1:1 | 40.2 ± 0.9 * |
| 1:3 | 62.1 ± 1.5 * |
| 3:1 | 54.8 ± 1.4 * |
| 0:1 (HDF alone) | 51.3 ± 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varan, G.; Patrulea, V.; Borchard, G.; Bilensoy, E. Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model. Nanomaterials 2018, 8, 67. https://doi.org/10.3390/nano8020067
Varan G, Patrulea V, Borchard G, Bilensoy E. Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model. Nanomaterials. 2018; 8(2):67. https://doi.org/10.3390/nano8020067
Chicago/Turabian StyleVaran, Gamze, Viorica Patrulea, Gerrit Borchard, and Erem Bilensoy. 2018. "Cellular Interaction and Tumoral Penetration Properties of Cyclodextrin Nanoparticles on 3D Breast Tumor Model" Nanomaterials 8, no. 2: 67. https://doi.org/10.3390/nano8020067






