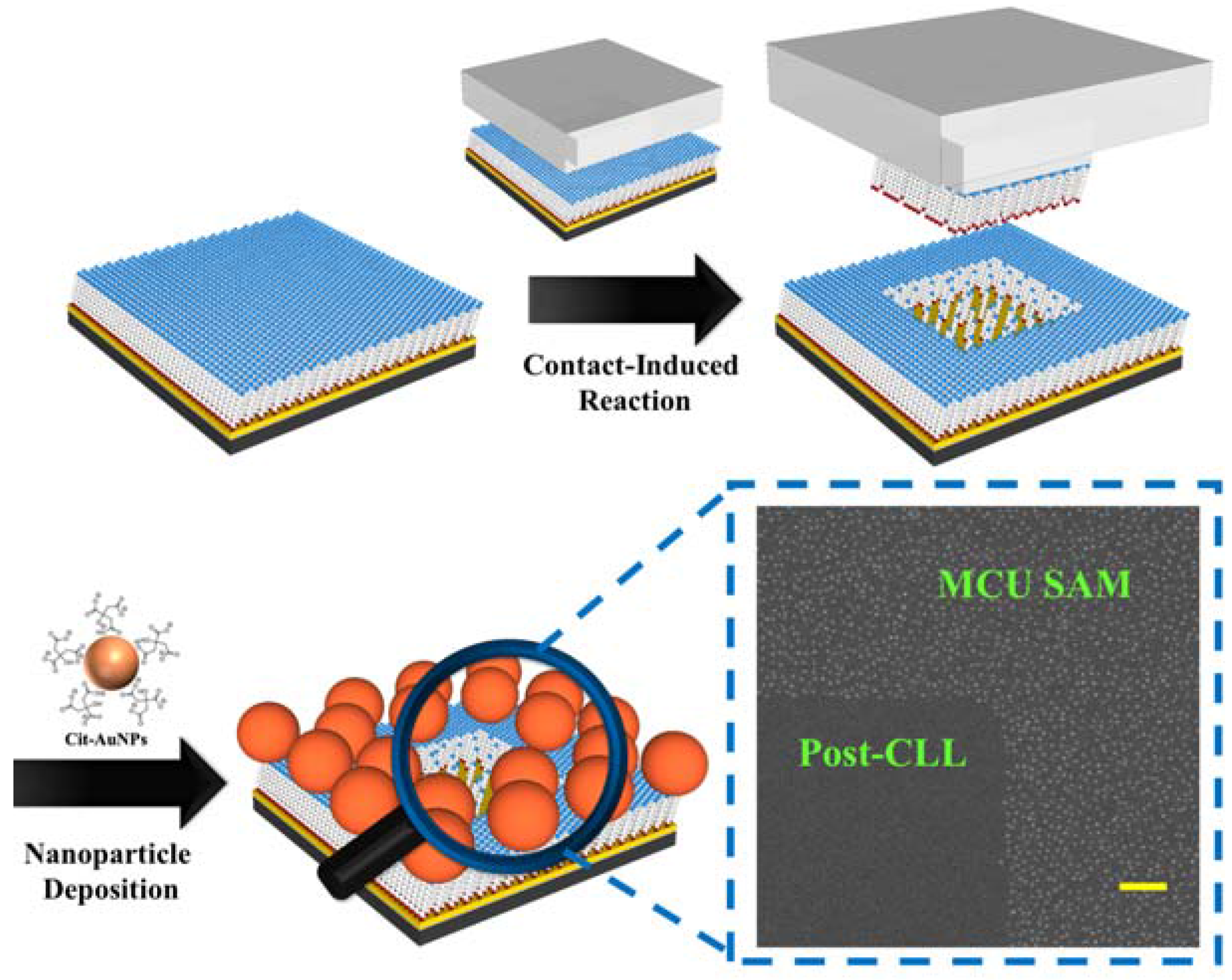
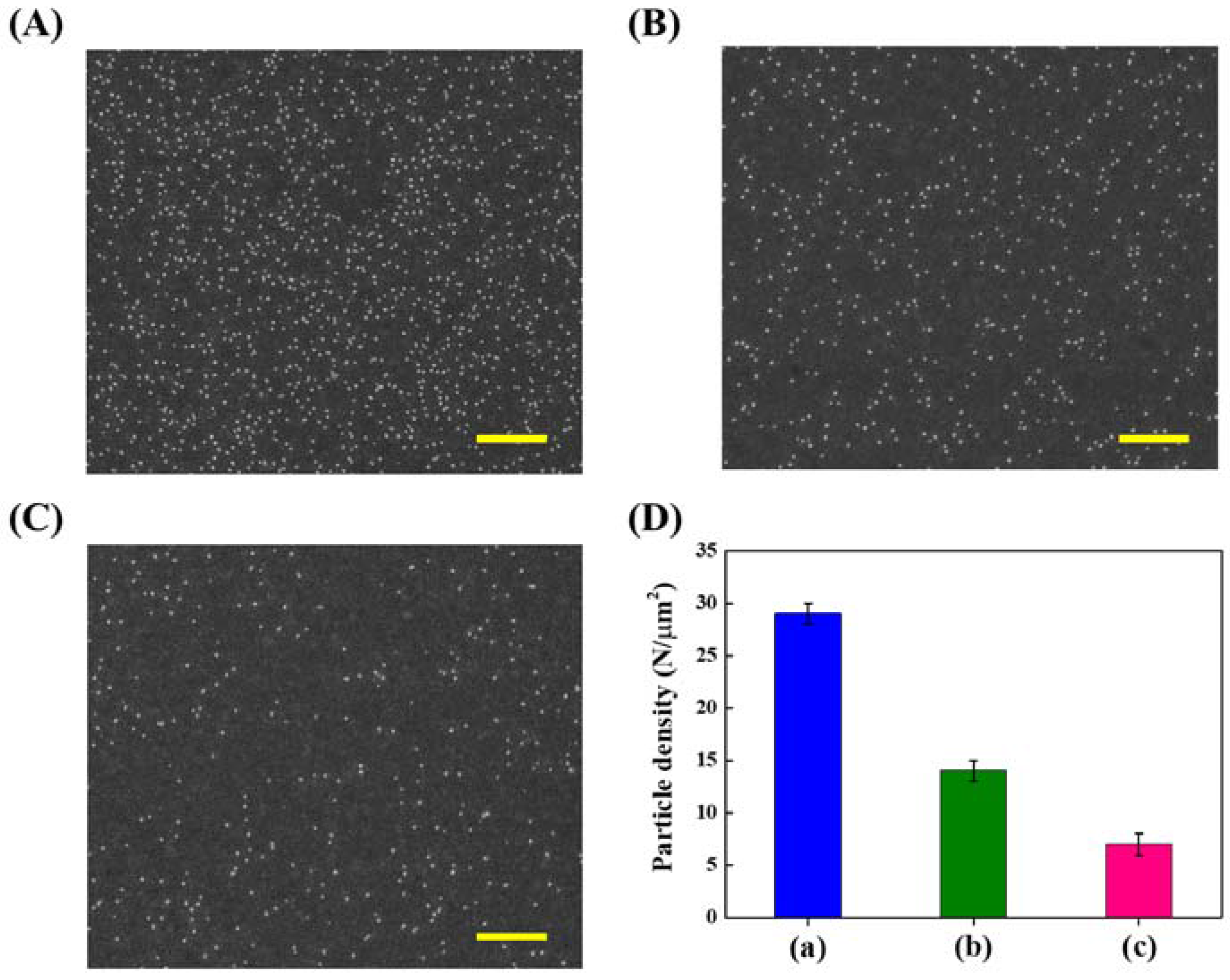
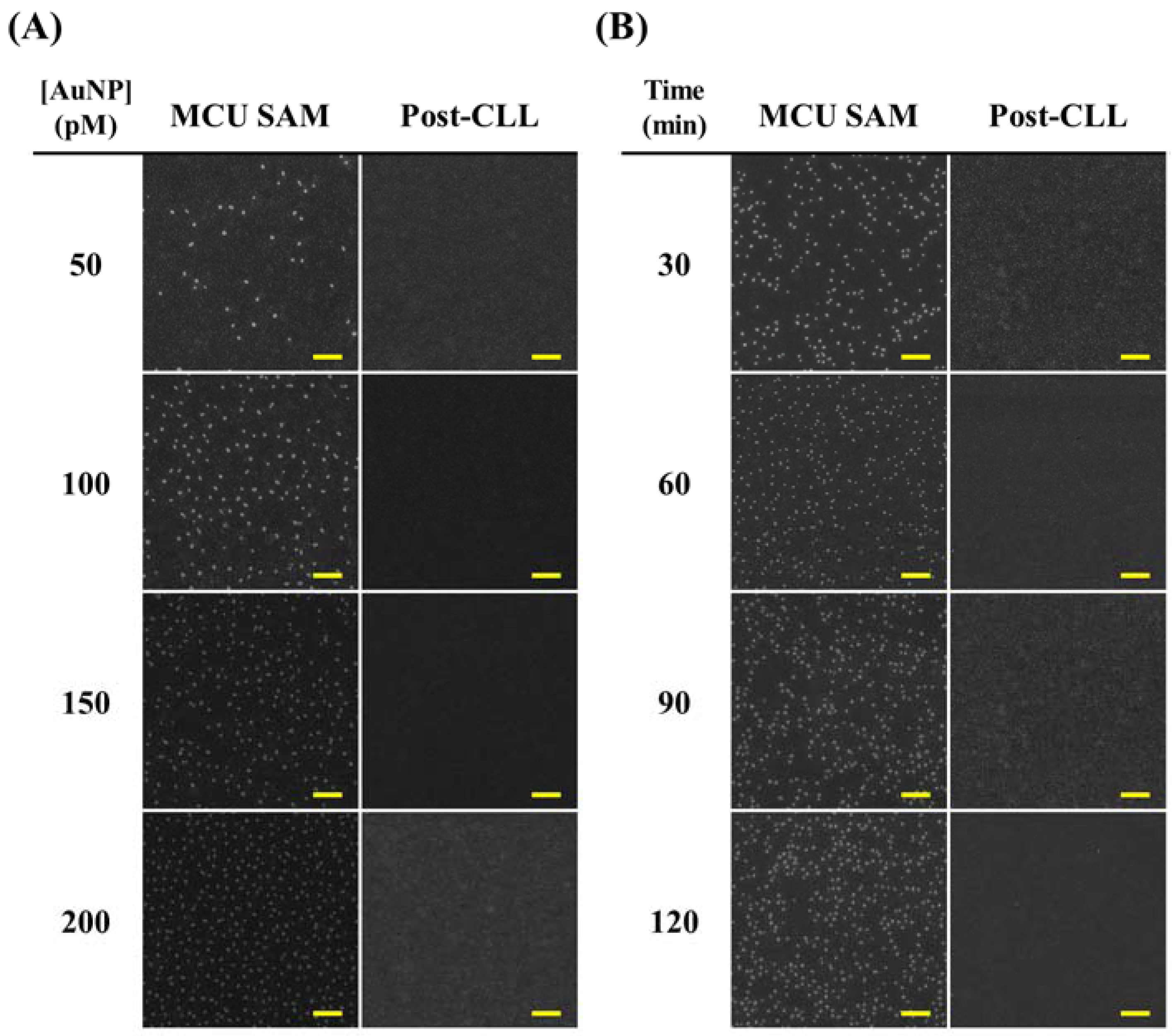
Large Area Nanoparticle Alignment by Chemical Lift-Off Lithography
Abstract
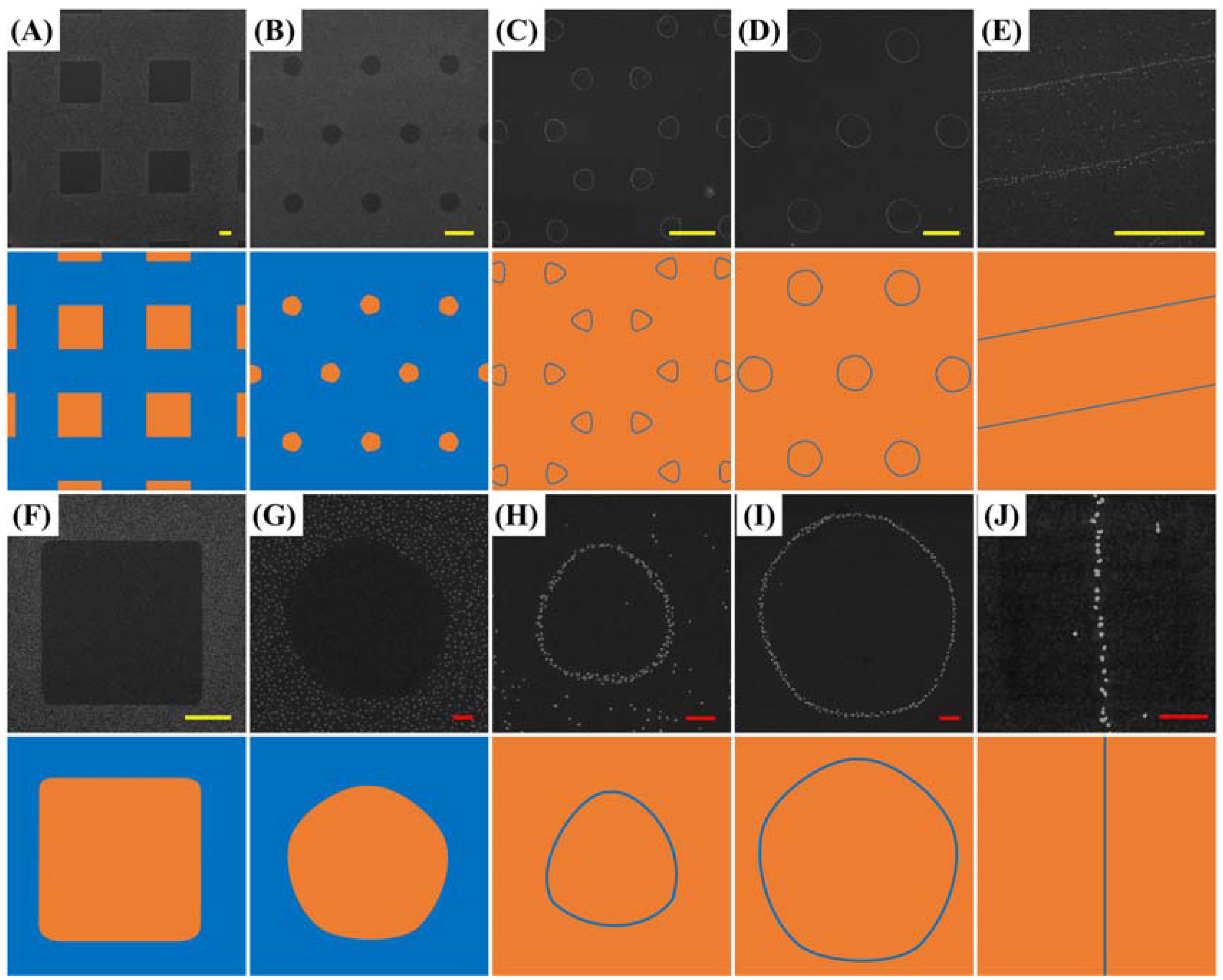
:Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kotov, N.A.; Weiss, P.S. Self-Assembly of nanoparticles: A snapshot. ACS Nano 2014, 8, 3101–3103. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ma, W.; Wang, L.; Xu, C.; Kuang, H.; Kotov, N.A. Nanoparticle assemblies: Dimensional transformation of nanomaterials and scalability. Chem. Soc. Rev. 2013, 42, 3114–3126. [Google Scholar] [CrossRef] [PubMed]
- Haryono, A.; Binder, W.H. Controlled arrangement of nanoparticle arrays in block-Copolymer domains. Small 2006, 2, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, A.J.; Reinhard, B.M.; Dal Negro, L. Engineering photonic–plasmonic coupling in metal nanoparticle necklaces. ACS Nano 2011, 5, 6578–6585. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, A.J.; Reinhard, B.M.; Dal Negro, L. Concentric necklace nanolenses for optical near-field focusing and enhancement. ACS Nano 2012, 6, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Gentili, D.; Greco, P.; Valle, F.; Biscarini, F. Micro- and nanopatterning by lithographically controlled wetting. Nat. Protoc. 2012, 7, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Cui, T.; Lvov, Y. Lithographic approach to pattern self-assembled nanoparticle multilayers. Langmuir 2002, 18, 6712–6715. [Google Scholar] [CrossRef]
- Hua, F.; Shi, J.; Lvov, Y.; Cui, T. Patterning of layer-by-layer self-assembled multiple types of nanoparticle thin films by lithographic technique. Nano Lett. 2002, 2, 1219–1222. [Google Scholar] [CrossRef]
- Porter, L.A.; Choi, H.C.; Schmeltzer, J.M.; Ribbe, A.E.; Elliott, L.C.C.; Buriak, J.M. Electroless nanoparticle film deposition compatible with photolithography, microcontact printing, and dip-pen nanolithography patterning technologies. Nano Lett. 2002, 2, 1369–1372. [Google Scholar] [CrossRef]
- Huang, J.; Kim, F.; Tao, A.R.; Connor, S.; Yang, P. Spontaneous formation of nanoparticle stripe patterns through dewetting. Nat. Mater. 2005, 4, 896. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, W.-S.; Chen, X.; Yang, T.; Wark, S.E.; Son, D.H.; Batteas, J.D.; Cremer, P.S. Evaporation-induced assembly of quantum dots into nanorings. ACS Nano 2009, 3, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, R.; Alt, M.; Rödlmeier, T.; Scharfer, P.; Lemmer, U.; Hernandez-Sosa, G. Digitally Printed dewetting patterns for self-organized microelectronics. Adv. Mater. 2016, 28, 7708–7715. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Vespini, V.; Olivieri, F.; Nasti, G.; Todino, M.; Mandracchia, B.; Pagliarulo, V.; Ferraro, P. Direct self-assembling and patterning of semiconductor quantum dots on transferable elastomer layer. Appl. Surf. Sci. 2017, 399, 160–166. [Google Scholar] [CrossRef]
- Myers, B.D.; Lin, Q.-Y.; Wu, H.; Luijten, E.; Mirkin, C.A.; Dravid, V.P. Size-selective nanoparticle assembly on substrates by dna density patterning. ACS Nano 2016, 10, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Chhabra, R.; Andersen, C.S.; Gothelf, K.V.; Yan, H.; Liu, Y. Toward Reliable gold nanoparticle patterning on self-assembled dna nanoscaffold. J. Am. Chem. Soc. 2008, 130, 7820–7821. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.M.; Jacke, S.; Critchley, K.; Plaza, J.; Chen, Y.; Nikitin, K.; Palmer, R.E.; Preece, J.A.; Evans, S.D.; Fitzmaurice, D. Gold Nanoparticle patterning of silicon wafers using chemical e-beam lithography. Langmuir 2004, 20, 3766–3768. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Simeone, F.C.; Borgatti, F.; Albonetti, C.; Morandi, V.; Sangregorio, C.; Innocenti, C.; Pineider, F.; Annese, E.; Panaccione, G.; et al. Additive nanoscale embedding of functional nanoparticles on silicon surface. Nanoscale 2010, 2, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-S.; Cheunkar, S.; Cao, H.H.; Bednar, H.R.; Weiss, P.S.; Andrews, A.M. Subtractive patterning via chemical lift-off lithography. Science 2012, 337, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Nakatsuka, N.; Liao, W.-S.; Serino, A.C.; Cheunkar, S.; Yang, H.; Weiss, P.S.; Andrews, A.M. Advancing biocapture substrates via chemical lift-off lithography. Chem. Mater. 2017, 29, 6829–6839. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Cheung, K.M.; Zhao, C.; Wattanatorn, N.; Belling, J.N.; Abendroth, J.M.; Slaughter, L.S.; Mirkin, C.A.; Andrews, A.M.; et al. Polymer-pen chemical lift-off lithography. Nano Lett. 2017, 17, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, X.; Yang, Q.; Man, T.; Jonas, S.J.; Schwartz, J.J.; Andrews, A.M.; Weiss, P.S. Self-collapse lithography. Nano Lett. 2017, 5035–5042. [Google Scholar] [CrossRef] [PubMed]
- Claridge, S.A.; Liao, W.-S.; Thomas, J.C.; Zhao, Y.; Cao, H.H.; Cheunkar, S.; Serino, A.C.; Andrews, A.M.; Weiss, P.S. From the bottom up: dimensional control and characterization in molecular monolayers. Chem. Soc. Rev. 2013, 42, 2725–2745. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.M.; Liao, W.-S.; Weiss, P.S. Double-sided opportunities using chemical lift-off lithography. Acc. Chem. Res. 2016, 49, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Nakatsuka, N.; Serino, A.C.; Liao, W.-S.; Cheunkar, S.; Yang, H.; Weiss, P.S.; Andrews, A.M. Controlled DNA patterning by chemical lift-off lithography: Matrix matters. ACS Nano 2015, 9, 11439–11454. [Google Scholar] [CrossRef] [PubMed]
- Shuster, M.J.; Vaish, A.; Cao, H.H.; Guttentag, A.I.; McManigle, J.E.; Gibb, A.L.; Martinez, M.M.; Nezarati, R.M.; Hinds, J.M.; Liao, W.S.; et al. Patterning small-molecule biocapture surfaces: Microcontact insertion printing vs. photolithography. Chem. Commun. 2011, 47, 10641–10643. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-S.; Cao, H.H.; Cheunkar, S.; Shuster, M.J.; Altieri, S.C.; Weiss, P.S.; Andrews, A.M. Small-molecule arrays for sorting G-protein-coupled receptors. J. Phys. Chem. C 2013, 117, 22362–22368. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, C.-M.; Chen, P.-S.; Liao, W.-S. Self-standing aptamers by artificial defect-rich matrix. Nanoscale 2018. [Google Scholar] [CrossRef]
- Slaughter, L.S.; Cheung, K.M.; Kaappa, S.; Cao, H.H.; Yang, Q.; Young, T.D.; Serino, A.C.; Malola, S.; Olson, J.M.; Link, S.; et al. Patterning of supported gold monolayers via chemical lift-off lithography. Beilstein J. Nanotechnol. 2017, 8, 2648–2661. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wang, C.-M.; Li, H.-H.; Chan, H.-H.; Liao, W.-S. Wafer scale bioactive substrate patterning by chemical lift-off lithography. Beilstein J. Nanotechnol. 2018, 9, 311–320. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chang, C.-H.; Wang, C.-M.; Li, Y.-J.; Chu, H.-Y.; Chan, H.-H.; Huang, Y.-W.; Liao, W.-S. Large Area Nanoparticle Alignment by Chemical Lift-Off Lithography. Nanomaterials 2018, 8, 71. https://doi.org/10.3390/nano8020071
Chen C-Y, Chang C-H, Wang C-M, Li Y-J, Chu H-Y, Chan H-H, Huang Y-W, Liao W-S. Large Area Nanoparticle Alignment by Chemical Lift-Off Lithography. Nanomaterials. 2018; 8(2):71. https://doi.org/10.3390/nano8020071
Chicago/Turabian StyleChen, Chong-You, Chia-Hsuan Chang, Chang-Ming Wang, Yi-Jing Li, Hsiao-Yuan Chu, Hong-Hseng Chan, Yu-Wei Huang, and Wei-Ssu Liao. 2018. "Large Area Nanoparticle Alignment by Chemical Lift-Off Lithography" Nanomaterials 8, no. 2: 71. https://doi.org/10.3390/nano8020071