Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for Caryl–N Cross Coupling
2.3. General Procedure for Carbonylation of Haloarenes
2.4. General Procedure for Reduction of Nitroarenes
3. Results and Discussion
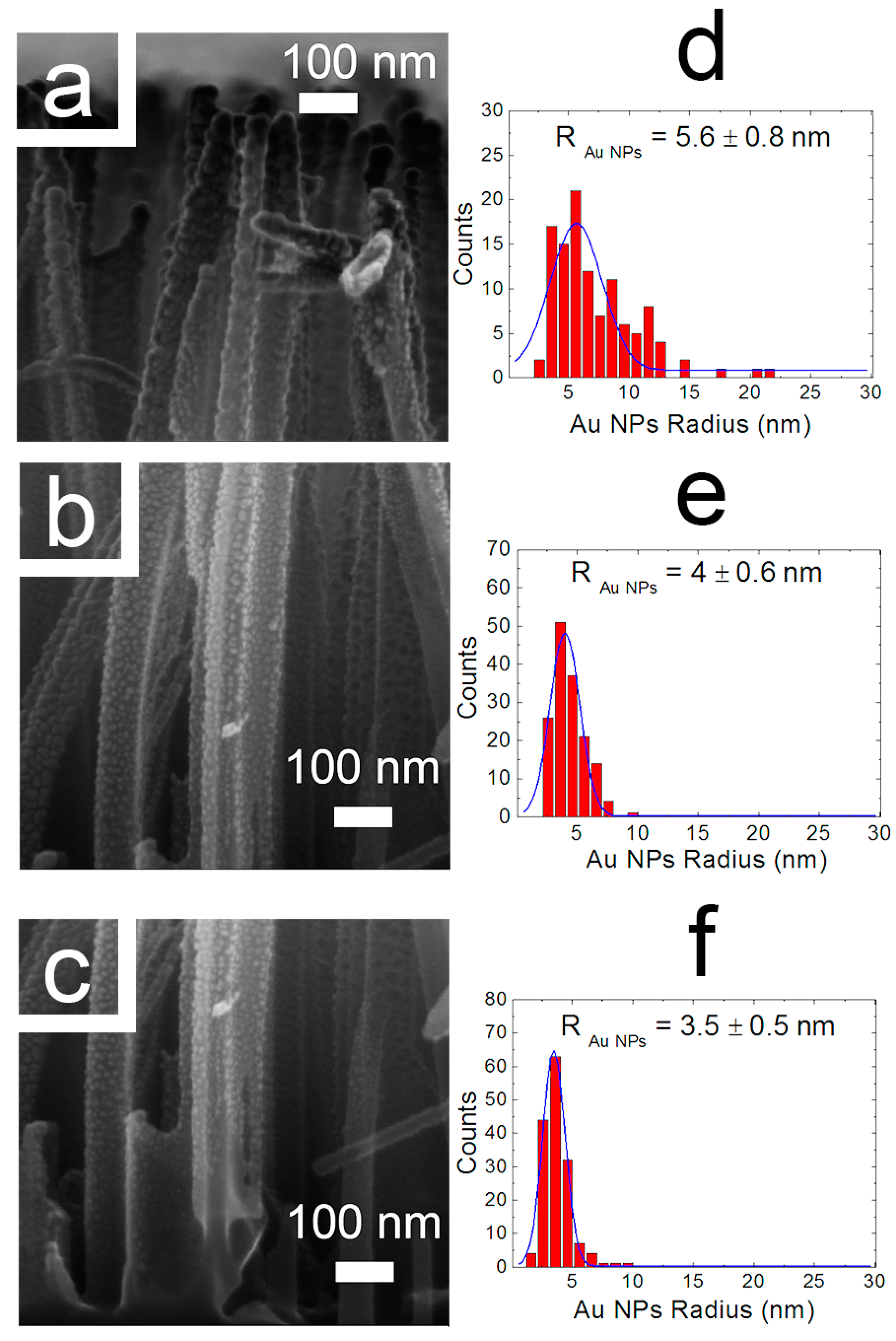
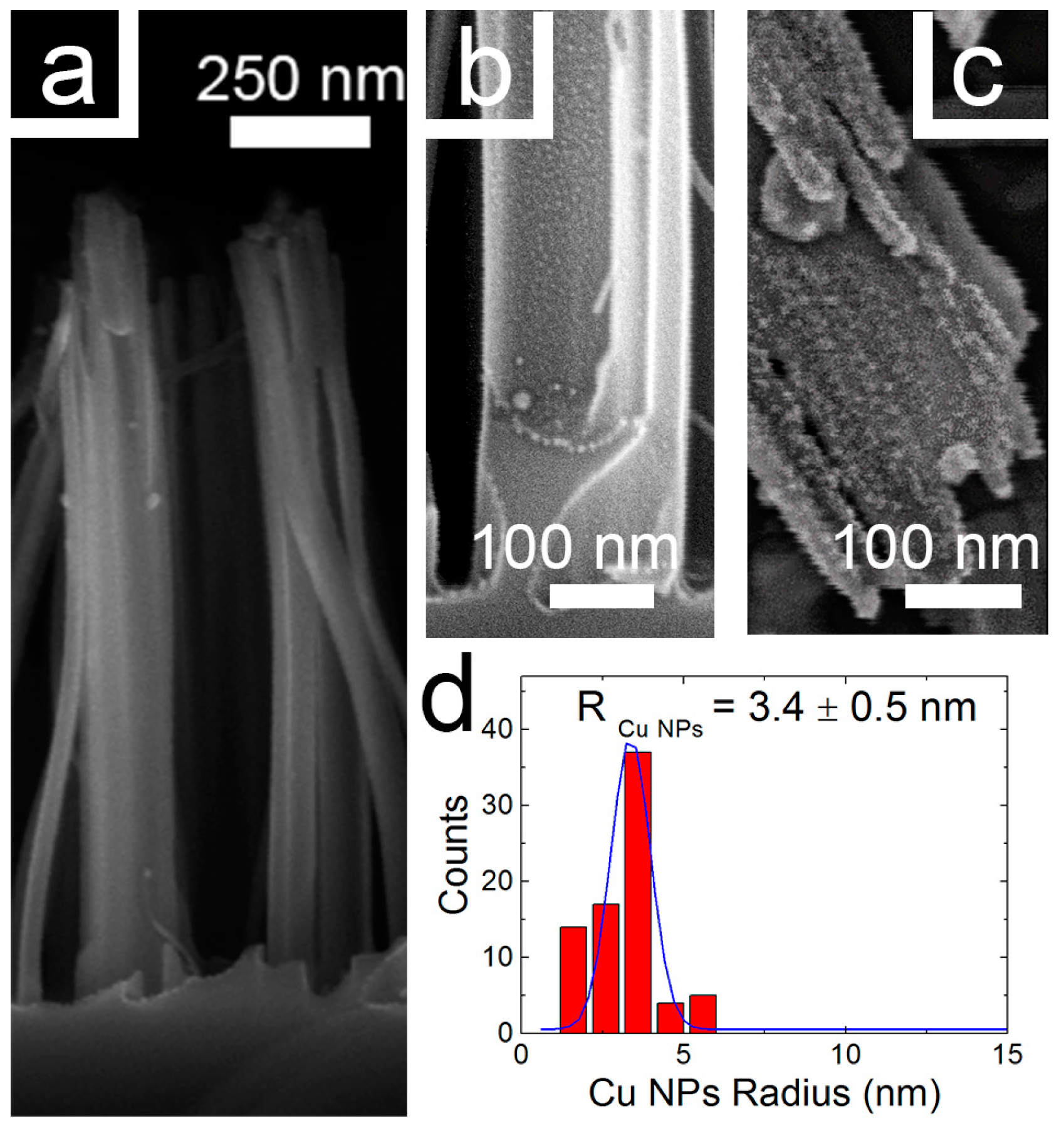
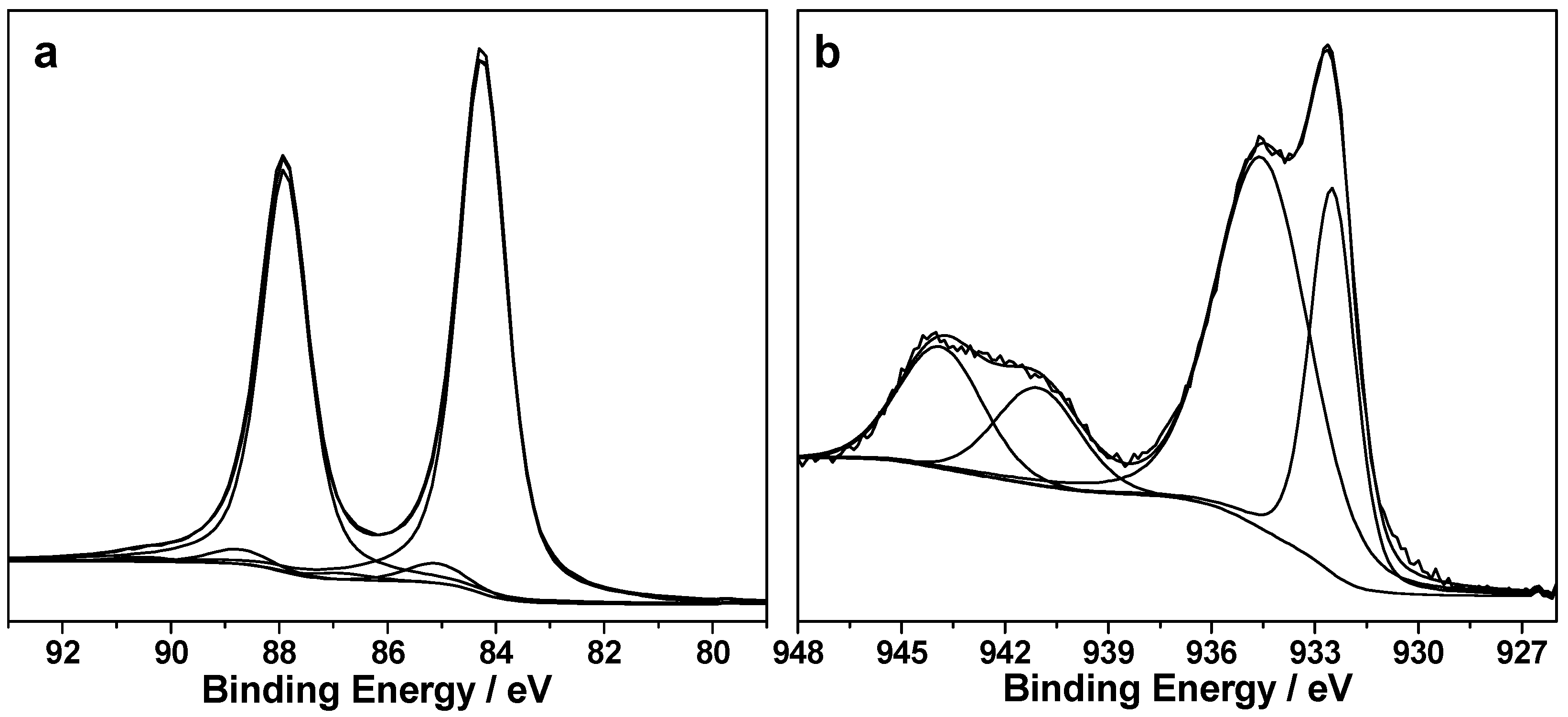
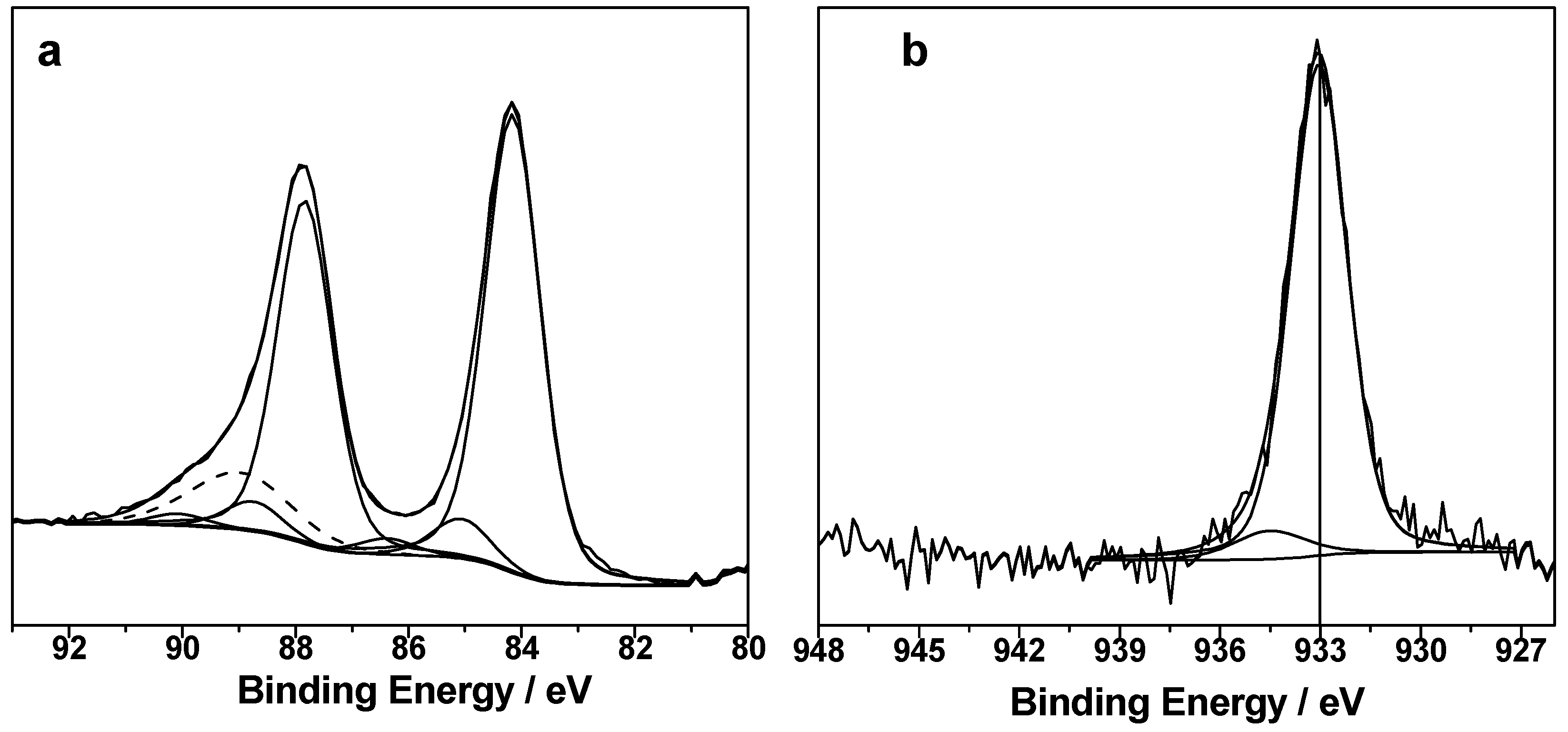
3.1. Structural Properties of Catalysts Nanocomposites
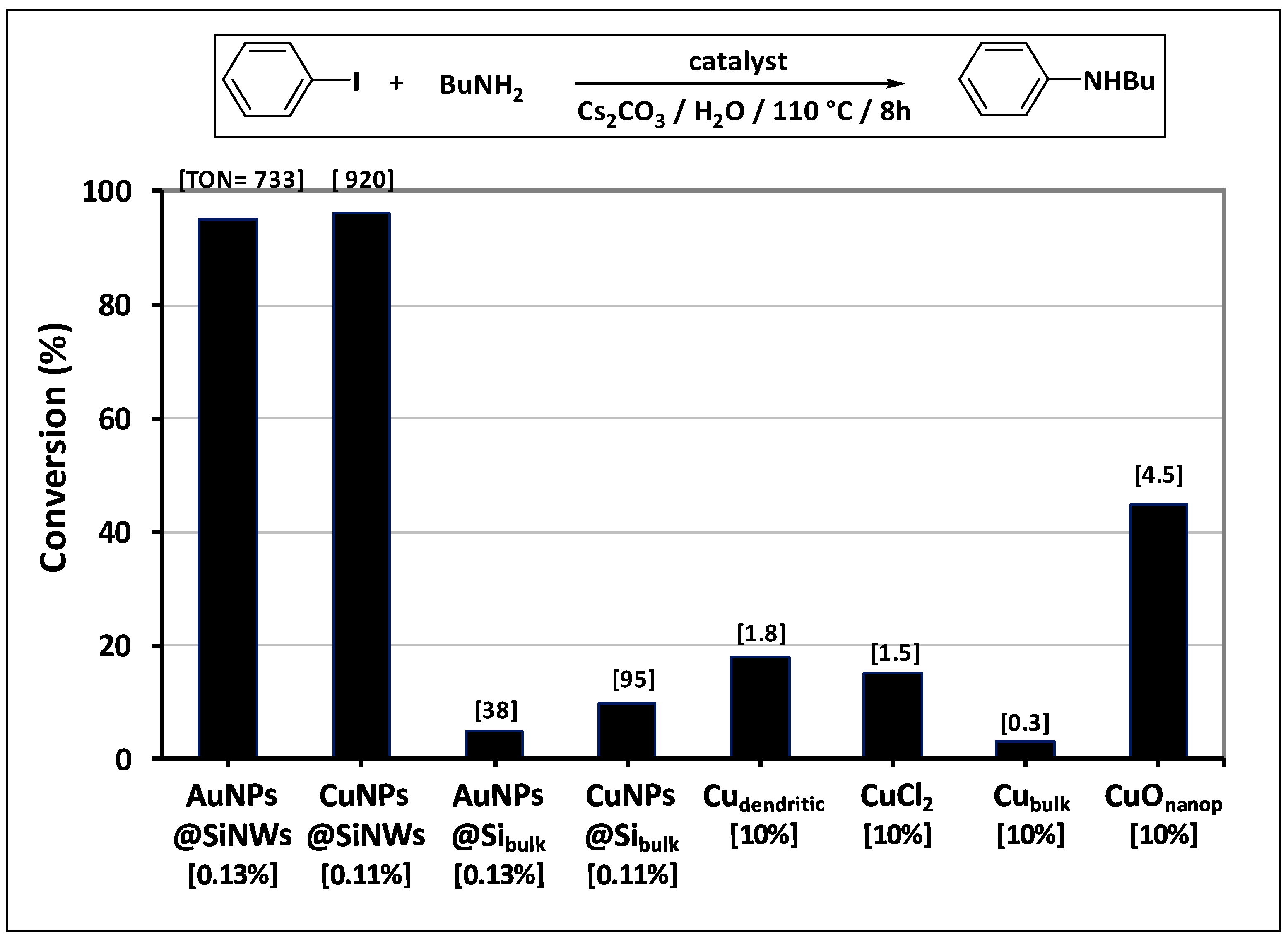
3.2. Catalytic Activity in Caryl–N Coupling Reactions
3.3. Carbonylation of Haloarenes
3.4. Reduction of Nitroarenes
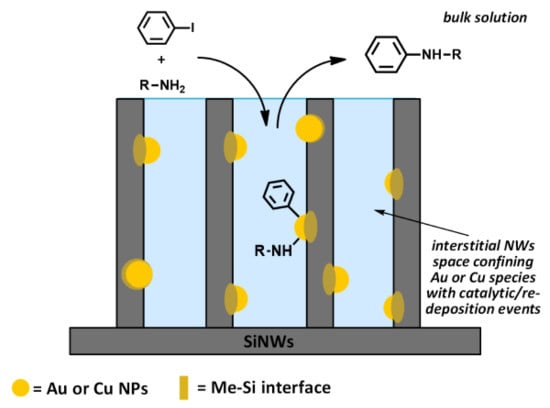
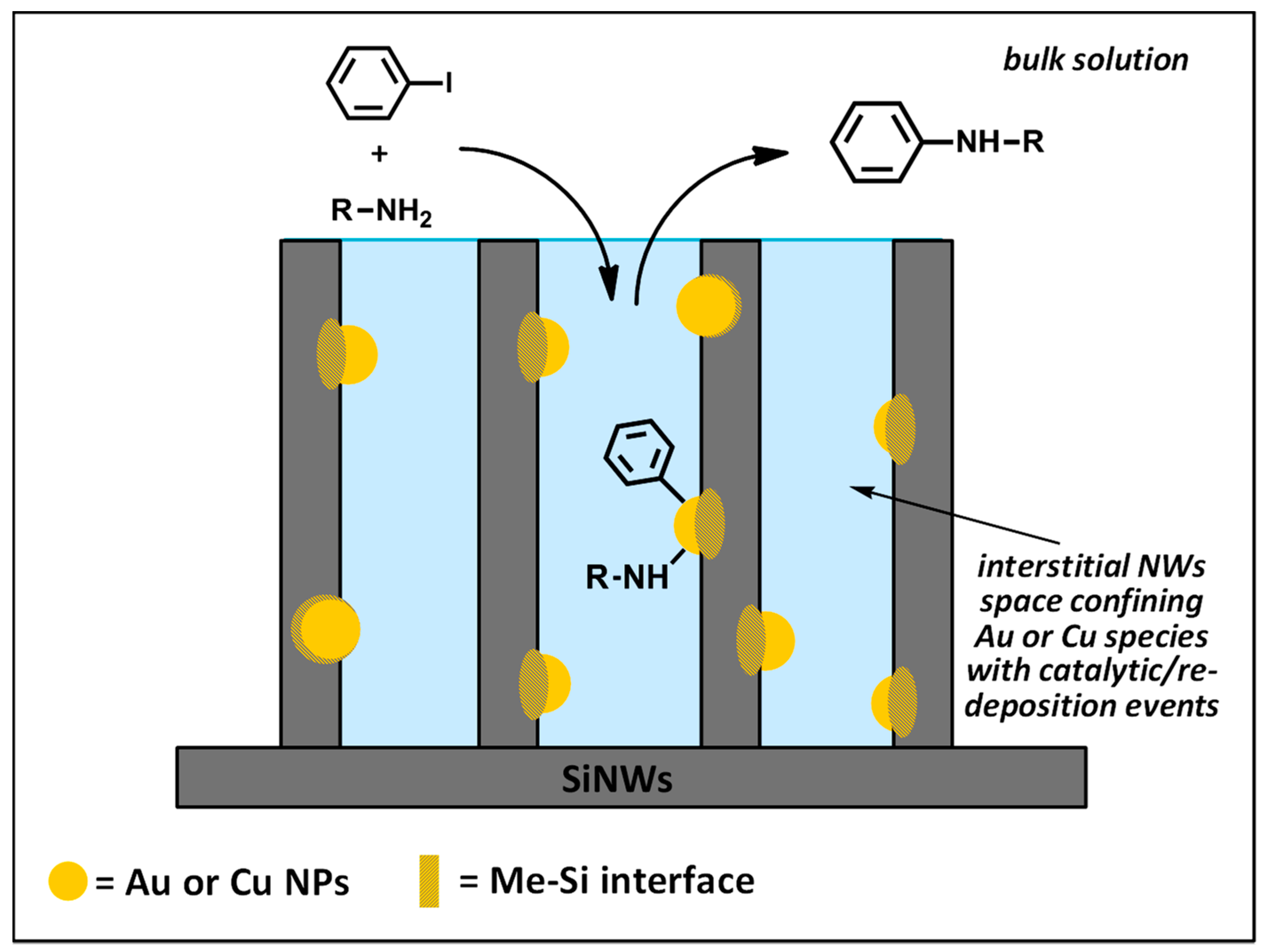
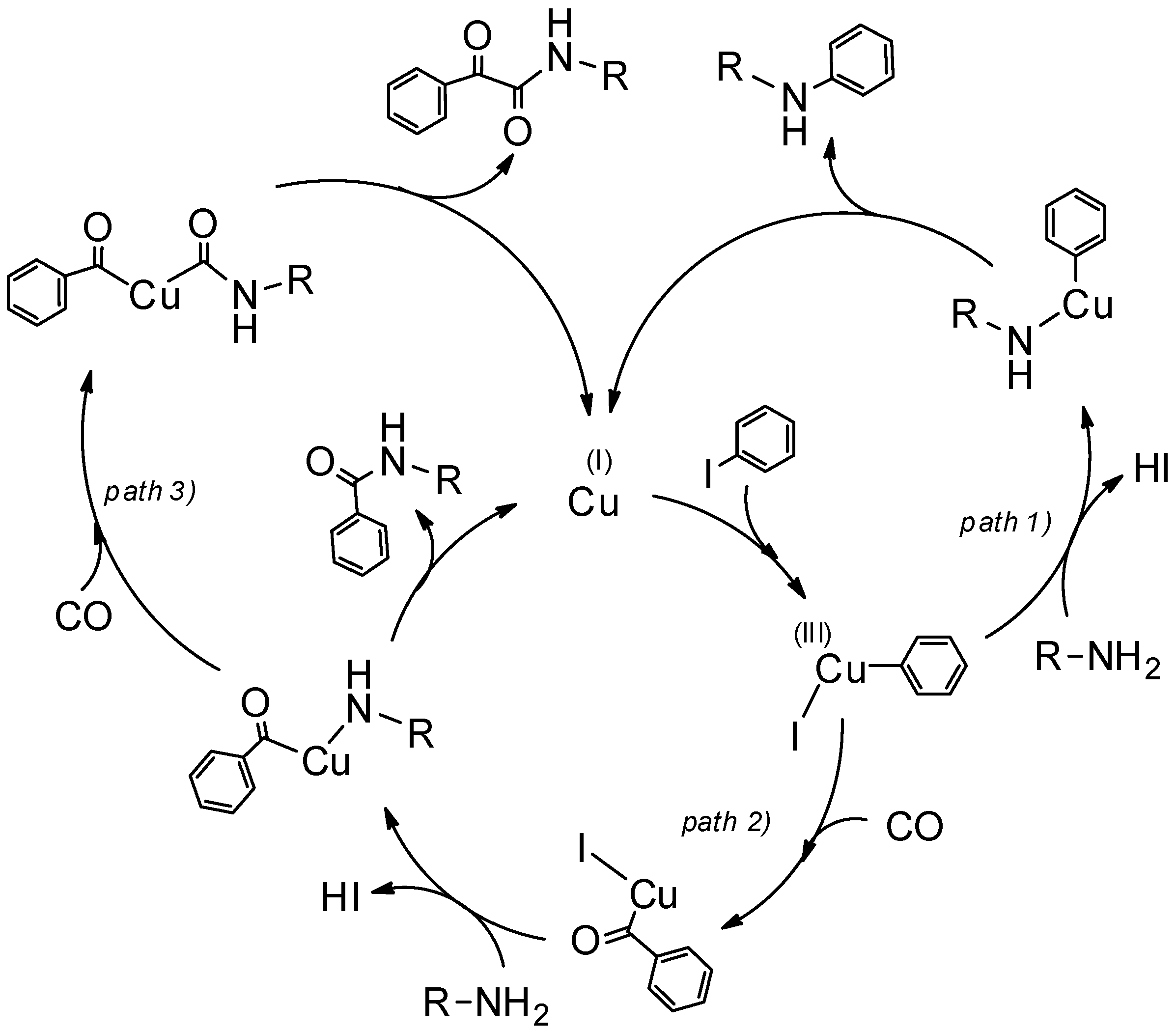
3.5. Mechanistic Insights
4. Conclusions
- (i)
- an unexpected activity for some chemical transformations like Caryl–N couplings and subsequent carbonylations that have no precedents in the literature for gold and copper catalysts, respectively;
- (ii)
- an extraordinary activity, attested by the very high TON values (about ten up to hundred times higher than those of the most commonly used catalysts (Figure 5)), surely due to the uniform coverage along the NW length and absence of the chemical shell surrounding the MeNPs;
- (iii)
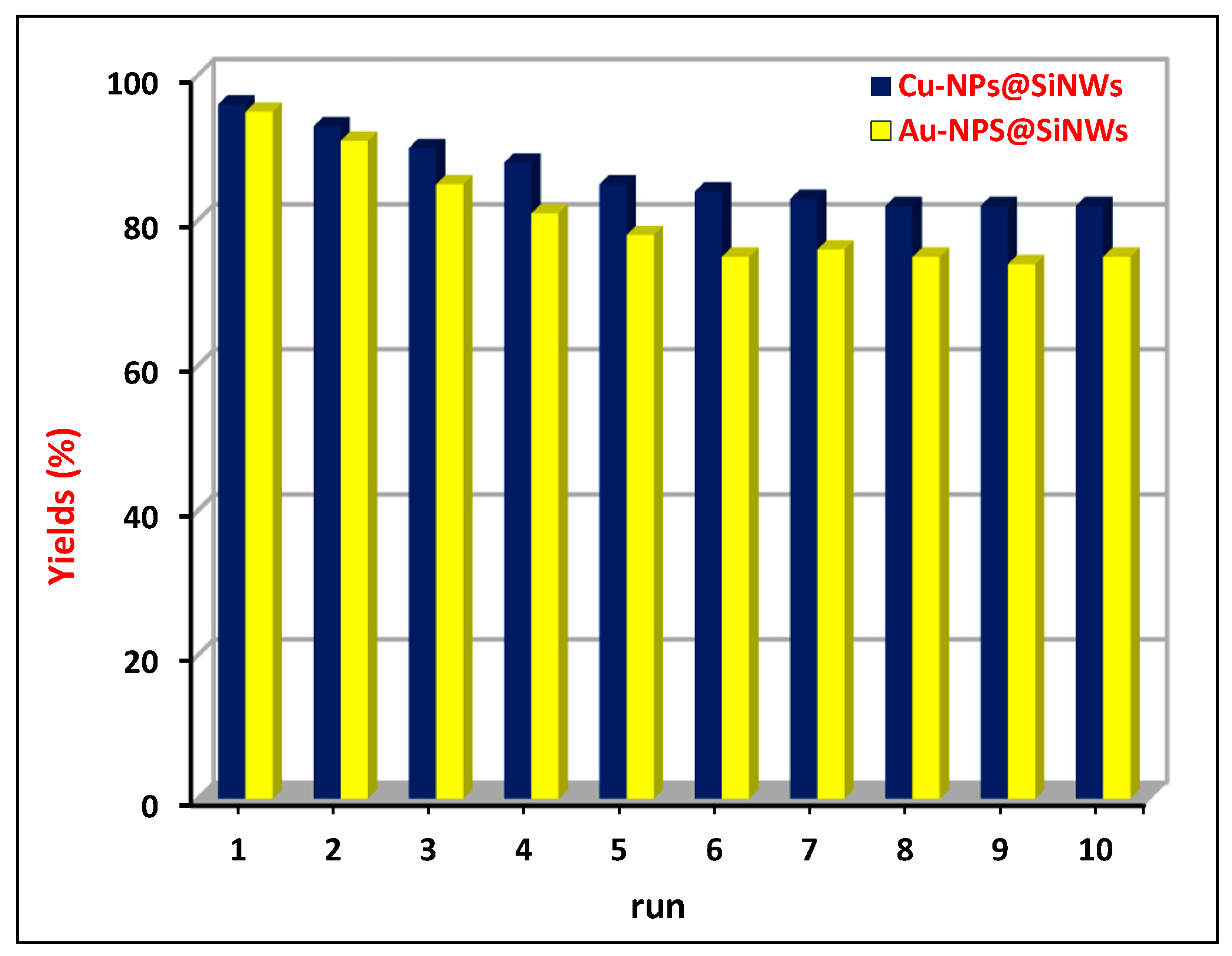
- a high recyclability, with more than ten recycling runs occurring with limited loss of activity, which can be ascribed to two main effects: (a) the high availability of MeNPs all over the length of NWs, notwithstanding the initial depletion in the upper layer of wires, and (b) the strong covalent interaction at the Me–Si interface by virtue of metal “silicides” formation. This confines the metal catalyst to the voids among the wires, transforming them into “micro-reactors” (Figure 9).
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, R.; Giri, P.K. Silicon nanowire heterostructures for advanced energy and environmental applications: A review. Nanotechnology 2017, 28, 012001. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Wang, T.; Shao, M. Silicon nanowires: Applications in catalysis with distinctive surface property. J. Mater. Sci. Mater. Electron. 2015, 26, 4722–4729. [Google Scholar] [CrossRef]
- Picca, R.A.; Calvano, C.D.; Lo Faro, M.J.; Fazio, B.; Trusso, S.; Ossi, P.M.; Neri, F.; D’Andrea, C.; Irrera, A.; Cioffi, N. Functionalization of silicon nanowire arrays by silver nanoparticles for the laser desorption ionization mass spectrometry analysis of vegetable oils. J. Mass Spectrom. 2016, 51, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzo, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Da, P.; Xu, M.; Wu, H.; Zheng, G. Silicon Nanowires for Biosensing, Energy Storage, and Conversion. Adv. Mater. 2013, 25, 5177–5195. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Wittemann, J.V.; Gösele, U. Growth, Thermodynamics, and Electrical Properties of Silicon Nanowires. Chem. Rev. 2010, 110, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, P. Direct Observation of Vapor-Liquid-Solid Nanowire Growth. J. Am. Chem. Soc. 2001, 123, 3165–3166. [Google Scholar] [CrossRef]
- Swain, B.S.; Park, J.-W.; Yang, S.-M.; Mahmood, K.; Swain, B.P.; Lee, J.-G.; Hwang, N.-M. Alignment of nanoparticles, nanorods, and nanowires during chemical vapor deposition of silicon. Appl. Phys. A Mater. Sci. Process. 2015, 120, 889–895. [Google Scholar] [CrossRef]
- Morales, A.M.; Lieber, C.M. A Laser Ablation Method for the Synthesis of Crystalline Semiconductor Nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Lifshitz, Y.; Lee, S.T. Oxide-Assisted Growth of Semiconducting Nanowires. Adv. Mater. 2003, 15, 635–640. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Fazio, B.; Irrera, A.; Pirotta, S.; D’Andrea, C.; Del Sorbo, S.; Lo Faro, M.J.; Gucciardi, P.G.; Iatì, M.A.; Saija, R.; Patrini, M.; et al. Coherent backscattering of Raman light. Nat. Photonics 2017, 11, 170–176. [Google Scholar] [CrossRef]
- Irrera, A.; Lo Faro, M.J.; D’Andrea, C.; Leonardi, A.A.; Artoni, P.; Fazio, B.; Picca, R.A.; Cioffi, N.; Trusso, S.; Franzo, G.; et al. Light-emitting silicon nanowires obtained by metal-assisted chemical etching. Semicond. Sci. Technol. 2017, 32, 043004. [Google Scholar] [CrossRef]
- Toor, F.; Miller, J.B.; Davidson, L.M.; Nichols, L.; Duan, W.; Jura, M.P.; Yim, J.; Forziati, J.; Black, M.R. Nanostructured silicon via metal assisted catalyzed etch (MACE): chemistry fundamentals and pattern engineering. Nanotechnology 2016, 27, 412003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Peng, K.Q.; Fan, X.; Jie, J.S.; Zhang, R.Q.; Lee, S.T.; Wong, N.B. Preparation of Large-Area Uniform Silicon Nanowires Arrays through Metal-Assisted Chemical Etching. J. Phys. Chem. C 2008, 112, 4444–4450. [Google Scholar] [CrossRef]
- Elnathan, R.; Kwiat, M.; Patolsky, F.; Voelcker, N.A.H. Engineering vertically aligned semiconductor nanowire arrays for applications in the life sciences. Nano Today 2014, 9, 172–196. [Google Scholar] [CrossRef]
- Galopin, E.; Barbillat, J.; Coffinier, Y.; Szunerits, S.; Patriarche, G.; Boukherroub, R. Silicon Nanowires Coated with Silver Nanostructures as Ultrasensitive Interfaces for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.W.; Zhang, M.L.; Wong, N.B.; Ma, D.D.; Wang, H.; Chen, W.; Lee, S.T. Ag-modified silicon nanowires substrate for ultrasensitive surface-enhanced raman spectroscopy. Appl. Phys. Lett. 2008, 93, 233118. [Google Scholar] [CrossRef]
- Zhang, M.L.; Fan, X.; Zhou, H.W.; Shao, M.W.; Zapien, J.A.; Wong, N.B.; Lee, S.T. A High-Efficiency Surface-Enhanced Raman Scattering Substrate Based on Silicon Nanowires Array Decorated with Silver Nanoparticles. J. Phys. Chem. C 2010, 114, 1969–1975. [Google Scholar] [CrossRef]
- Naama, S.; Hadjersi, T.; Menari, H.; Nezzal, G.; Ahmed, L.B.; Lamrani, S. Enhancement of the tartrazine photodegradation by modification of silicon nanowires with metal nanoparticles. Mater. Res. Bull. 2016, 76, 317–326. [Google Scholar] [CrossRef]
- Shao, M.; Wang, H.; Zhang, M.; Duo Duo Ma, D.; Lee, S.-T. The mutual promotional effect of Au–Pd bimetallic nanoparticles on silicon nanowires: A study of preparation and catalytic activity. Appl. Phys. Lett. 2008, 93, 243110–243113. [Google Scholar] [CrossRef]
- Amdouni, S.; Coffinier, Y.; Szunerits, S.; Zaïbi, M.A.; Oueslati, M.; Boukherroub, R. Catalytic activity of silicon nanowires decorated with silver and copper nanoparticles. Semicond. Sci. Technol. 2016, 31, 014011. [Google Scholar] [CrossRef]
- Shao, Y.; Wei, Y.; Wang, Z. Synthesis of silicon nanowires supported Ag nanoparticles and their catalytic activity in photo-degradation of Rhodamine B. Front. Optoelectron. China 2011, 4, 171–175. [Google Scholar] [CrossRef]
- Hua, J.; Shao, M.; Cheng, L.; Wang, X.; Fu, Y.; Ma, D.D.D. The fabrication of silver-modified silicon nanowires and their excellent catalysis in the decomposition of fluorescein sodium. J. Phys. Chem. Solids 2009, 70, 192–196. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Yuyama, Y.; Sato, T.; Fujikawa, S.; Uozumi, Y. A Palladium-Nanoparticle and Silicon-Nanowire-Array Hybrid: A Platform for Catalytic Heterogeneous Reactions. Angew. Chem. Int. Ed. 2014, 53, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, M.; Wang, X. Silicon Nanowires Supported Palladium Nanoparticles: An Efficient and Recyclable Heterogeneous Catalyst for Heck Reaction. Asian J. Chem. 2011, 23, 3059–3062. [Google Scholar]
- Wang, F.; Shao, M.; Cheng, L.; Chen, D.; Fu, Y.; Ma, D.D.D. Si/Pd nanostructure with high catalytic activity in degradation of eosin Y. Mater. Res. Bull. 2009, 44, 126–129. [Google Scholar] [CrossRef]
- Hu, H.; Shao, M.; Zhang, W.; Lu, L.; Wang, H.; Wang, S. Synthesis of Layer-Deposited Silicon Nanowires, Modification with Pd Nanoparticles, and Their Excellent Catalytic Activity and Stability in the Reduction of Methylene Blue. J. Phys. Chem. C 2007, 111, 3467–3470. [Google Scholar] [CrossRef]
- Brahiti, N.; Hadjersi, T.; Menari, H.; Amirouche, S.; El Kechai, O. Enhanced photocatalytic degradation of methylene blue by metal-modified silicon nanowires. Mater. Res. Bull. 2015, 62, 30–36. [Google Scholar] [CrossRef]
- Qu, Y.; Zhong, X.; Li, Y.; Liao, L.; Huang, Y.; Duan, X. Photocatalytic properties of porous silicon nanowires. J. Mater. Chem. 2010, 20, 3590–3594. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhong, H.; Zhu, Y.; Jiang, H.; Shen, J.; Huang, J.; Li, C. Highly efficient reusable catalyst based on silicon nanowire arrays decorated with copper nanoparticles. J. Mater. Chem. A 2014, 2, 9040–9047. [Google Scholar] [CrossRef]
- Pan, K.; Ming, H.; Yu, H.; Huang, H.; Liu, Y.; Kang, Z. Copper nanoparticles modified silicon nanowires with enhanced cross-coupling catalytic ability. Dalton Trans. 2012, 41, 2564–2566. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, C.; Neri, F.; Ossi, P.M.; Santo, N.; Trusso, S. The controlled pulsed laser deposition of Ag nanoparticle arrays for surface enhanced Raman scattering. Nanotechnology 2009, 20, 245606. [Google Scholar] [CrossRef] [PubMed]
- Mikac, L.; Ivanda, M.; Gotic, M.; Maksimovic, A.; Trusso, S.; D’Andrea, C.; Foti, A.; Irrera, A.; Fazio, B.; Gucciardi, P.G. Metal Nanoparticles Deposited on Porous Silicon Templates as Novel Substrates for SERS. Croat. Chem. Acta 2015, 88, 437–444. [Google Scholar] [CrossRef]
- Mollica Nardo, V.; Aliotta, F.; Mastelloni, M.A.; Ponterio, R.C.; Saija, F.; Trusso, S.; Vasi, C.S. A Spectroscopic Approach to The Study of Organic Pigments In The Field of Cultural Heritage. Atti Accad. Pelorit. Pericol. Cl. Sci. Fis. Mat. Nat. 2017, 95, A5. [Google Scholar]
- D’Andrea, C.; Lo Faro, M.J.; Bertino, G.; Ossi, P.M.; Neri, F.; Trusso, S.; Musumeci, P.; Galli, M.; Cioffi, N.; Irrera, A.; et al. Decoration of silicon nanowires with silver nanoparticles for ultrasensitive surface enhanced Raman scattering. Nanotechnology 2016, 27, 375603. [Google Scholar] [CrossRef] [PubMed]
- Bailini, A.; Ossi, P.M. Expansion of an ablation plume in a buffer gas and cluster growth. EPL Europhys. Lett. 2007, 79, 35002. [Google Scholar] [CrossRef]
- Spadaro, M.C.; Fazio, E.; Neri, F.; Trusso, S.; Ossi, P.M. On the role of the ablated mass on the propagation of a laser-generated plasma in an ambient gas. EPL Europhys. Lett. 2015, 109, 25002. [Google Scholar] [CrossRef]
- Li, Y.; Shi, W.; Gupta, A.; Chopra, N. Morphological evolution of gold nanoparticles on silicon nanowires and their plasmonics. RSC Adv. 2015, 5, 49708–49718. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology, “XPS Database”. 2012. Available online: http://srdata.nist.gov/xps (accessed on 15 November 2017).
- Cioffi, N.; Ditaranto, N.; Torsi, L.; Picca, R.A.; De Giglio, E.; Sabbatini, L.; Novello, L.; Tantillo, G.; Bleve-Zacheo, T.; Zambonin, P.G. Synthesis, analytical characterization and bioactivity of Ag and Cu nanoparticles embedded in poly-vinyl-methyl-ketone films. Anal. Bioanal. Chem. 2005, 382, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Dey, R.; Chatterjee, T.; Ahammed, S. Copper Nanoparticle-Catalyzed Carbon-Carbon and Carbon-Heteroatom Bond Formation with a Greener Perspective. ChemSusChem 2012, 5, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Priecel, P.; Salami, H.A.; Padilla, R.H.; Zhong, Z.; Lopez-Sanchez, J.A. Anisotropic gold nanoparticles: Preparation and applications in catalysis. Chin. J. Catal. 2016, 37, 1619–1650. [Google Scholar] [CrossRef]
- Lukosi, M.; Zhu, H.; Dai, S. Recent advances in gold-metal oxide core-shell nanoparticles: Synthesis, characterization, and their application for heterogeneous catalysis. Front. Chem. Sci. Eng. 2016, 10, 39–56. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber Acridinsynthesen aus Aldehyden und aromatischen Basen. Ber. Dtsch. Chem. Ges. 1903, 36, 2389–2391. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)-O, C(aryl-N, and C(aryl)-S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; Abbrescia, D.I.; Catucci, L.; Denora, N.; Fanizza, I.; Fusco, C.; Lapiana, G. Site-dependent biological activity of valinomycin analogs bearing derivatizable hydroxyl sites. J. Pept. Sci. 2013, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; D’Accolti, L.; Filardi, R.; Tommasi, I.; Fusco, C. Oxidative cleavage of lactams in water using dioxiranes: An expedient and environmentally-safe route to ω-nitro acids. Tetrahedron Lett. 2013, 54, 515–517. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules. Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO Nanoparticle Catalyzed C−N Cross Coupling of Amines with Iodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef] [PubMed]
- Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, T.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. CuO Nanoparticles Catalyzed C−N, C−O, and C−S Cross-Coupling Reactions: Scope and Mechanism. J. Org. Chem. 2009, 74, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Firouzabadi, H.; Etemadi-Davan, E.; Nematollahi, A.; Firouzi, H.R. A novel nickel-catalyzed synthesis of thioesters, esters and amides from aryl iodides in the presence of chromium hexacarbonyl. New J. Chem. 2015, 39, 6445–6452. [Google Scholar] [CrossRef]
- Hajipour, A.-R.; Tavangar-Rizia, Z.; Iranpoor, N. Palladium-catalyzed carbonylation of aryl halides: An efficient, heterogeneous and phosphine-free catalytic system for aminocarbonylation and alkoxycarbonylation employing Mo(CO)6 as a solid carbon monoxide source. RSC Adv. 2016, 6, 78468–78476. [Google Scholar] [CrossRef]
- Prasada, A.S.; Satyanarayana, B. Fe3O4 supported Pd(0) nanoparticles catalyzed alkoxycarbonylation of aryl halides. J. Mol. Catal. A Chem. 2013, 370, 205–209. [Google Scholar] [CrossRef]
- Seo, Y.S.; Ahn, E.-Y.; Park, J.; Kim, T.Y.; Hong, J.E.; Kim, K.; Park, Y.; Park, Y. Catalytic reduction of 4-nitrophenol with gold nanoparticles synthesized by caffeic acid. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ciganda, R.; Li, N.; Deraedt, C.; Gatard, S.; Zhao, P.; Salmon, L.; Hernández, R.; Ruiza, J.; Astruc, D. Gold nanoparticles as electron reservoir redox catalysts for 4-nitrophenol reduction: A strong stereoelectronic ligand influence. Chem. Commun. 2014, 50, 10126–10129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Zou, P.; Zhang, P.; Zhang, M.; Mu, J.; Guo, Z.; Li, X.; Wang, C.; Liu, Y. In situ assembly of well-dispersed gold nanoparticles on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. Chem. Commun. 2011, 47, 3906–3908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-L.; Jiang, T.-T.; Lu, Y.; Liu, H.-J.; Chen, Y. Gold nanoparticles immobilized in hyperbranched polyethylenimine modified polyacrylonitrile fiber as highly efficient and recyclable heterogeneous catalysts for the reduction of 4-nitrophenol. J. Mater. Chem. A 2013, 1, 5923–5933. [Google Scholar] [CrossRef]
- Tang, R.; Liao, X.-P.; Shi, B. Heterogeneous Gold Nanoparticles Stabilized by Collagen and Their Application in Catalytic Reduction of 4-Nitrophenol. Chem. Lett. 2008, 37, 834–835. [Google Scholar] [CrossRef]
- Liu, B.C.; Yu, S.L.; Wang, Q.; Hu, W.T.; Jing, P.; Liu, Y.; Jia, W.J.; Liu, Y.X.; Liu, L.X.; Zhang, J. Hollow mesoporous ceria nanoreactors with enhanced activity and stability for catalytic application. Chem. Commun. 2013, 49, 3757–3759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.H.; Sui, M.Y.; Xiao, G.J.; Wang, Y.N.; Wang, C.Z.; Liu, B.B.; Zou, G.T.; Zou, B. Facile fabrication of faceted copper nanocrystals with high catalytic activity for p-nitrophenol reduction. J. Mater. Chem. A 2013, 1, 1632–1638. [Google Scholar] [CrossRef]


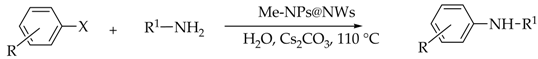
| Run | X | R | Amine (R1NH2) | Yield (%) (b) | |
|---|---|---|---|---|---|
| CuNPs@SiNWs | AuNPs@SiNWs | ||||
| 1 | I | H | BuNH2 | 96 | 95 |
| 2 | I | H | AllylNH2 | 80 | 85 |
| 3 | I | H | BnNH2 | 99 | 98 |
| 4 | I | H | HO(CH2)4NH2 | 75 (c) | 80 (c) |
| 5 | I | H | H2N(CH2)4NH2 | 99 (d) | 95 (d) |
| 6 | I | H | CyNH2 | 98 | 97 |
| 7 | I | H | 2-NH2CyNH2 | 99 (e) | 99 (e) |
| 8 | I | H | n-C12H25NH2 | 82 | 79 |
| 9 | I | I | Bu2NH | 20 | 19 |
| 10 | I | H | PhNH2 | 15 | 18 |
| 11 | I | 4-NO2 | BuNH2 | 99 | 96 |
| 12 | I | 4-Br | BuNH2 | 50 | 55 |
| 13 | I | 4-MeO | BuNH2 | 40 | 49 |
| 14 | I | 4-CH3 | BuNH2 | 94 | 98 |
| 15 | I | 2-CH3 | BuNH2 | 72 | 73 |
| 16 | Br | H | BuNH2 | 15 | 10 |
| 17 | Br | 4-CN | BuNH2 | 19 | 20 |
| 18 | I | H | HO(CH2)4SH (f) | 90 | 95 |
| 19 | I | H | HO(CH2)4OH | <1 | 3 |
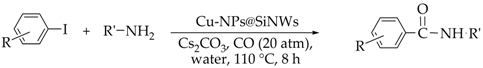
| Run | R | R′-NH2 | Product | Yields (b) (%) | Sel. (b) (%) |
|---|---|---|---|---|---|
| 1 | H | BuNH2 |  | 97 | 84 |
| 2 | H | AllylNH2 |  | 89 | 85 |
| 3 | H | BnNH2 |  | 90 | 80 |
| 4 | H(c) | 4-AB | 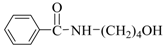 | 95 | 83 |
| 5 | H | CyNH2 | 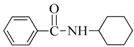 | 92 | 82 |
| 6 | 4-NO2 | BuNH2 | 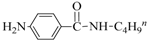 | 99 | 55 (d) |
| 7 | 4-Br | BuNH2 |  | 98 | 64 |
| 8 | 4-MeO | BuNH2 | 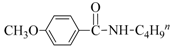 | 85 | 88 |
| 9 | 4-Me | BuNH2 |  | 88 | 86 |
| 10 | 2-Me | BuNH2 | 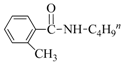 | 75 | 85 |
| 11 | H (e) | BuNH2 |  | <5 | - |
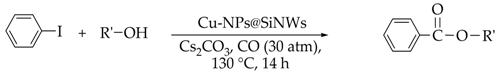
| Run | R′-OH | Product | Yields (b) (%) | Sel. (b) (%) |
|---|---|---|---|---|
| 1 | Me-OH | 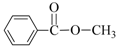 | 89 | 90 |
| 2 | Bu-OH | 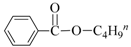 | 82 | 95 |
| 3 | iPr-OH | 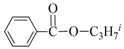 | 62 | 93 |
| 4 | Allyl-OH | 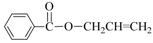 | 72 | 92 |
| 5 | Cy-OH |  | 68 | 91 |
| 6 | Bn-OH |  | 90 | 65 |
| 7 | 1,3-BD (c) | 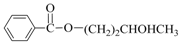 | 66 | 55 |
| 8 | Ph-OH (c) |  | <5 | - |
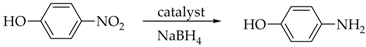
| Run | Catalyst | TOF (b) | Ref. |
|---|---|---|---|
| 1 | AuNPs@SiNWs | 123 | This work |
| 2 | CuNPs@SiNWs | 255 | This work |
| 3 | CuNPs@SiNWs | 220 | [33] |
| 5 | Au/CeO2 | 240 | [64] |
| 6 | Cu cubes | 136 | [65] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiello, M.; Picca, R.A.; Fusco, C.; D’Accolti, L.; Leonardi, A.A.; Lo Faro, M.J.; Irrera, A.; Trusso, S.; Cotugno, P.; Sportelli, M.C.; et al. Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation. Nanomaterials 2018, 8, 78. https://doi.org/10.3390/nano8020078
Casiello M, Picca RA, Fusco C, D’Accolti L, Leonardi AA, Lo Faro MJ, Irrera A, Trusso S, Cotugno P, Sportelli MC, et al. Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation. Nanomaterials. 2018; 8(2):78. https://doi.org/10.3390/nano8020078
Chicago/Turabian StyleCasiello, Michele, Rosaria Anna Picca, Caterina Fusco, Lucia D’Accolti, Antonio Alessio Leonardi, Maria Josè Lo Faro, Alessia Irrera, Sebastiano Trusso, Pietro Cotugno, Maria Chiara Sportelli, and et al. 2018. "Catalytic Activity of Silicon Nanowires Decorated with Gold and Copper Nanoparticles Deposited by Pulsed Laser Ablation" Nanomaterials 8, no. 2: 78. https://doi.org/10.3390/nano8020078