Polymeric Micelle of A3B-Type Lactosome as a Vehicle for Targeting Meningeal Dissemination
Abstract
:1. Introduction
2. Results
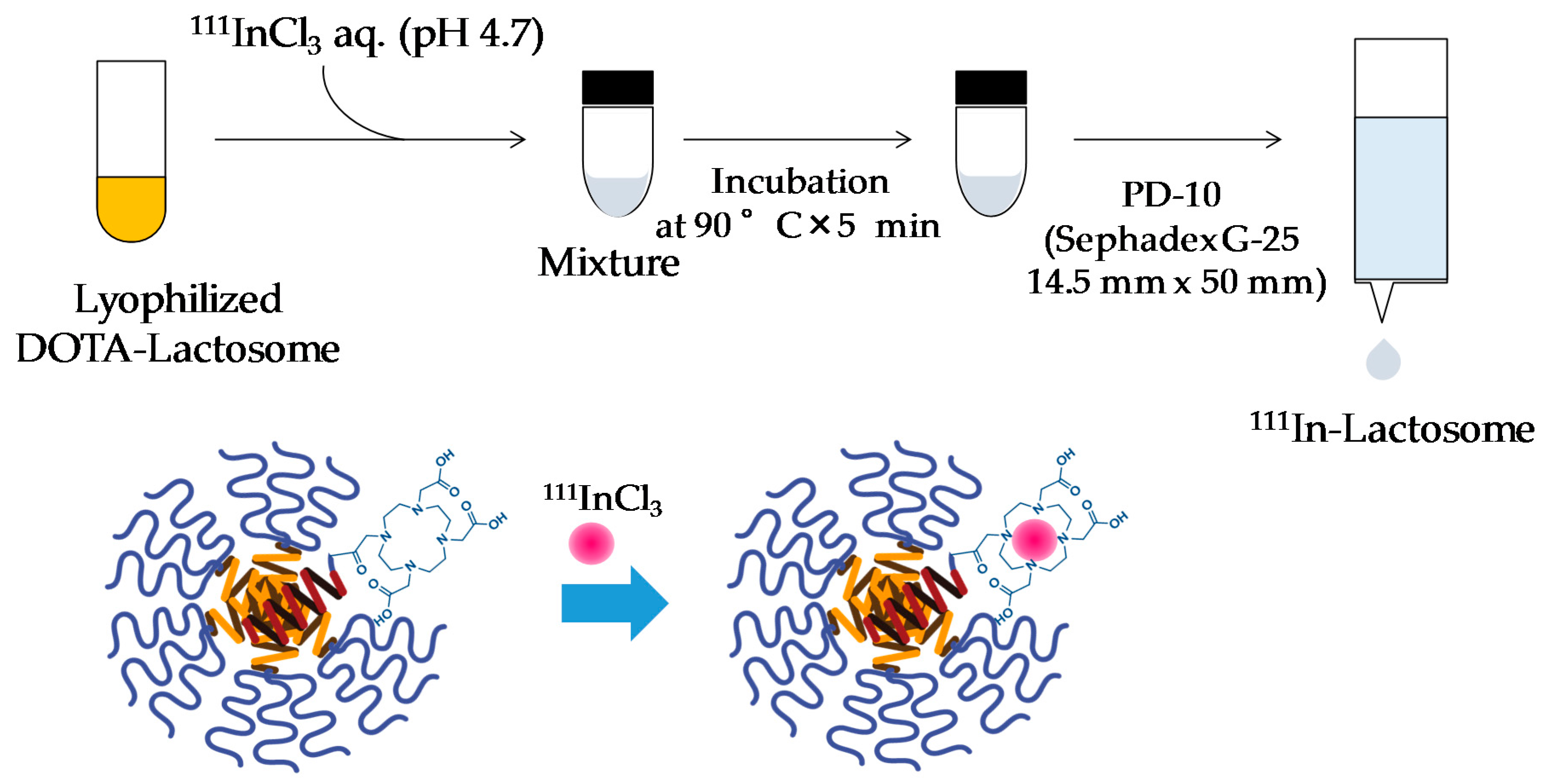
2.1. Preparation of 111In-Lactosome
2.2. Melanoma Brain Metastases
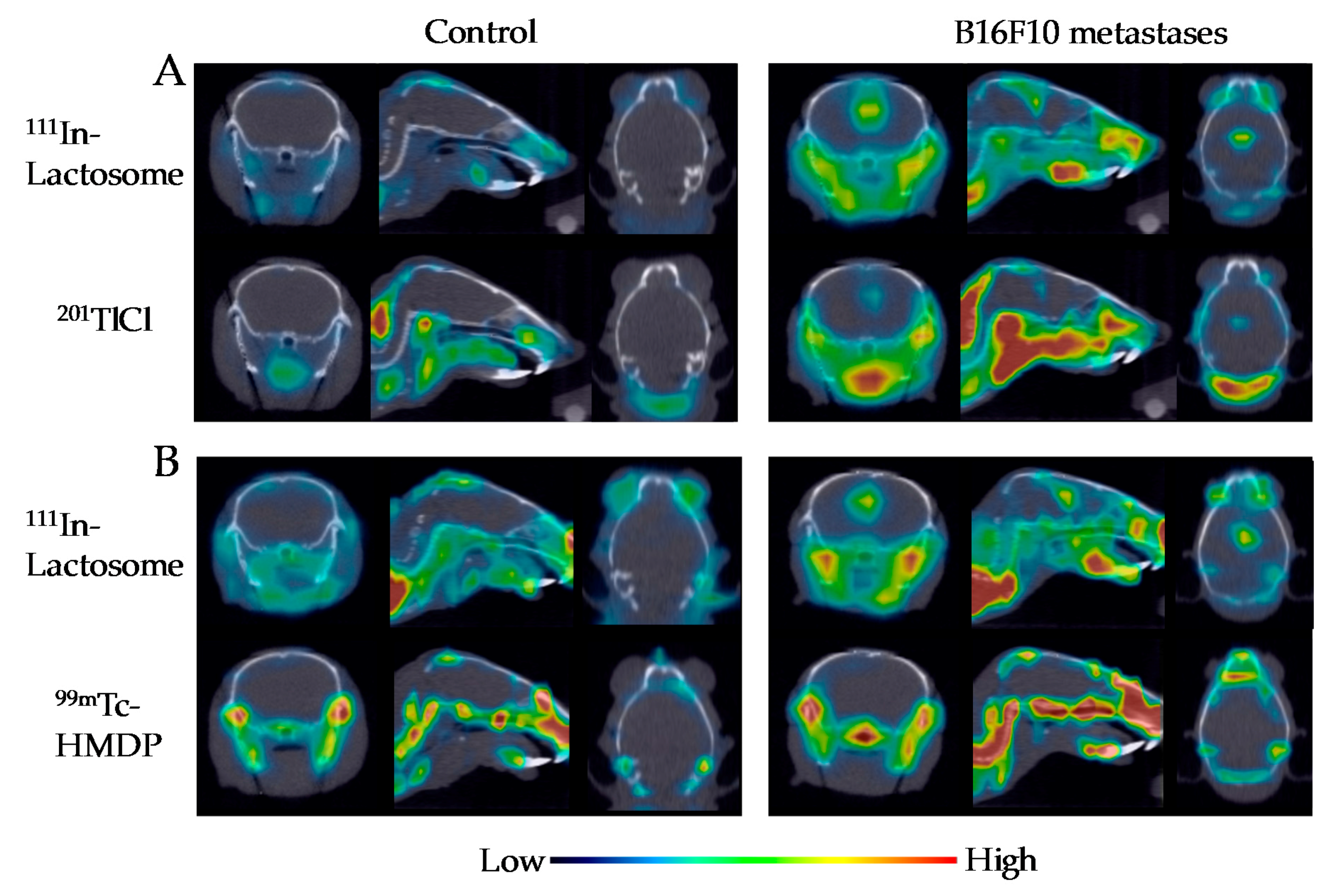
2.3. Comparison of 111In-Lactosome and 201TlCl for SPECT/CT Imaging
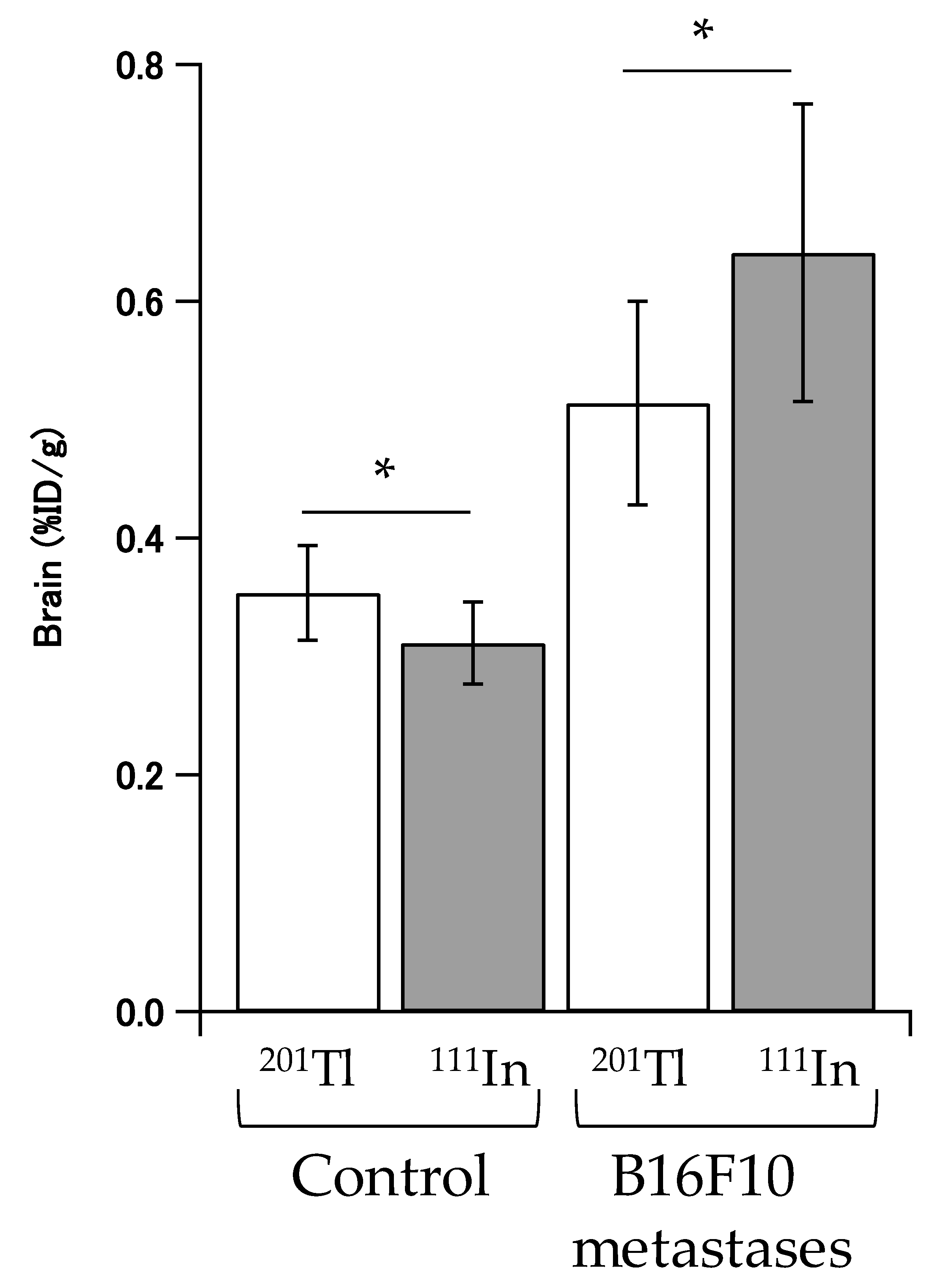
2.4. In Vivo Disposition in Brain
3. Discussion
4. Materials and Methods
4.1. Polymer Synthesis
4.2. Animal and Cell Line
4.3. Preparation of 111In-Lactosome
4.4. Imaging of Melanoma Metastasis
4.5. In Vivo Disposition in Brain
4.6. Statistical Analysis
4.7. Ethics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Rhun, E.; Galanis, E. Leptomeningeal metastases of solid cancer. Curr. Opin. Neurol. 2016, 29, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Soong, S.J.; Gershenwald, J.E.; Thompson, J.F.; Reintgen, D.S.; Cascinelli, N.; Urist, M.; McMasters, K.M.; Ross, M.I.; Kirkwood, J.M.; et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American joint committee on cancer melanoma staging system. J. Clin. Oncol. 2001, 19, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.M.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, V.; Karimi, S.; Diamond, E.L.; Lis, E.; Krol, G.; Holodny, A.I.; Young, R.J. Nonenhancing Leptomeningeal Metastases: Imaging characteristics and potential causative factors. Neurohospitalist 2016, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nose, A.; Otsuka, H.; Nose, H.; Otomi, Y.; Terazawa, K.; Harada, M. Visual and semi-quantitative assessment of brain tumors using (201)Tl-SPECT. J. Med. Investig. 2013, 60, 121–126. [Google Scholar] [CrossRef]
- Wang, L.N.; Wang, Y.B.; Li, Z.J. Nanoparticle-based tumor theranostics with molecular imaging. Curr. Pharm. Biotechnol. 2013, 14, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Y.; Jin, C.S.; Huang, H.; Ding, L.L.; Zhang, Z.H.; Chen, J.; Zheng, G. Nanoparticle-enabled, image-guided treatment planning of target specific RNAi therapeutics in an orthotopic prostate cancer model. Small 2014, 10, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Fricker, G.; Haberkorn, U.; Mier, W. Radionuclides in drug development. Drug Discov. Today 2015, 20, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Dams, E.T.M.; Laverman, P.; Oyen, W.J.G.; Storm, G.; Scherphof, G.L.; Van der Meer, J.W.M.; Corstens, F.H.M.; Boerman, O.C. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079. [Google Scholar] [PubMed]
- Ishida, T.; Harada, M.; Wang, X.Y.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J. Control. Release 2005, 105, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ichihara, M.; Hyodo, K.; Yamamoto, E.; Ishida, T.; Kiwada, H.; Ishihara, H.; Kikuchi, H. Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int. J. Pharm. 2012, 436, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ichihara, M.; Hyodo, K.; Yamamoto, E.; Ishida, T.; Kiwada, H.; Kikuchi, H.; Ishihara, H. Influence of dose and animal species on accelerated blood clearance of PEGylated liposomal doxorubicin. Int. J. Pharm. 2014, 476, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Makino, A.; Kurihara, K.; Sugai, M.; Shimizu, A.; Hara, I.; Ozeki, E.; Kimura, S. Evasion from accelerated blood clearance of nanocarrier named as “Lactosome” induced by excessive administration of Lactosome. Biochim. Biophys. Acta 2013, 1830, 4046–4052. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Makino, A.; Kurihara, K.; Ueda, M.; Hara, I.; Kawabe, T.; Yamamoto, F.; Ozeki, E.; Togashi, K.; Kimura, S. Radionuclide therapy using nanoparticle of I-131-Lactosome in combination with percutaneous ethanol injection therapy. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Hara, E.; Makino, A.; Kurihara, K.; Yamamoto, F.; Ozeki, E.; Kimura, S. Pharmacokinetic change of nanoparticulate formulation “Lactosome” on multiple administrations. Int. Immunopharmacol. 2012, 14, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Ueda, M.; Akira, M.; Hara, I.; Ozeki, E.; Kimura, S. Factors influencing in vivo disposition of polymeric micelles on multiple administrations. ACS Med. Chem. Lett. 2014, 5, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Ueda, M.; Kim, C.J.; Makino, A.; Hara, I.; Ozeki, E.; Kimura, S. Suppressive immune response of poly(sarcosine) chains in peptide-nanosheets in contrast to polymeric micelles. J. Pept. Sci. 2014, 20, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Conley, F.K. Development of a metastatic brain-tumor model in mice. Cancer Res. 1979, 39, 1001–1007. [Google Scholar] [PubMed]
- Morsi, A.; Gaziel-Sovran, A.; Cruz-Munoz, W.; Kerbel, R.S.; Golfinos, J.G.; Hernando, E.; Wadghiri, Y.Z. Development and characterization of a clinically relevant mouse model of melanoma brain metastasis. Pigment Cell Melanoma Res. 2013, 26, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Ueda, M.; Hara, I.; Hara, E.; Sano, K.; Makino, A.; Ozeki, E.; Yamamoto, F.; Saji, H.; Togashi, K.; et al. Inflammation-induced synergetic enhancement of nanoparticle treatments with DOXIL (R) and Y-90-Lactosome for orthotopic mammary tumor. J. Nanopart. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Mullins, L.J.; Moore, R.D. The movemnet of thallium ions in muscle. J. Gen. Physiol. 1960, 43, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K.; Ueda, M.; Hara, I.; Ozeki, E.; Togashi, K.; Kimura, S. Control of in vivo disposition and immunogenicity of polymeric micelles by adjusting poly(sarcosine) chain lengths on surface. J. Nanopart. Res. 2017, 19, 242. [Google Scholar] [CrossRef]
- Makino, A.; Yamahara, R.; Ozeki, E.; Kimura, S. Preparation of Novel Polymer Assemblies, “Lactosome”, Composed of Poly(l-lactic acid) and Poly(sarcosine). Chem. Lett. 2007, 36, 1220–1221. [Google Scholar] [CrossRef]
- Makino, A.; Hara, E.; Hara, I.; Ozeki, E.; Kimura, S. Size control of core-shell-type polymeric micelle with a nanometer precision. Langmuir 2014, 30, 669–674. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurihara, K.; Ueda, M.; Hara, I.; Ozeki, E.; Togashi, K.; Kimura, S. Polymeric Micelle of A3B-Type Lactosome as a Vehicle for Targeting Meningeal Dissemination. Nanomaterials 2018, 8, 79. https://doi.org/10.3390/nano8020079
Kurihara K, Ueda M, Hara I, Ozeki E, Togashi K, Kimura S. Polymeric Micelle of A3B-Type Lactosome as a Vehicle for Targeting Meningeal Dissemination. Nanomaterials. 2018; 8(2):79. https://doi.org/10.3390/nano8020079
Chicago/Turabian StyleKurihara, Kensuke, Motoki Ueda, Isao Hara, Eiichi Ozeki, Kaori Togashi, and Shunsaku Kimura. 2018. "Polymeric Micelle of A3B-Type Lactosome as a Vehicle for Targeting Meningeal Dissemination" Nanomaterials 8, no. 2: 79. https://doi.org/10.3390/nano8020079




