Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers
Abstract
:1. Introduction
2. The Basics of Polymer-Based Nanocarriers
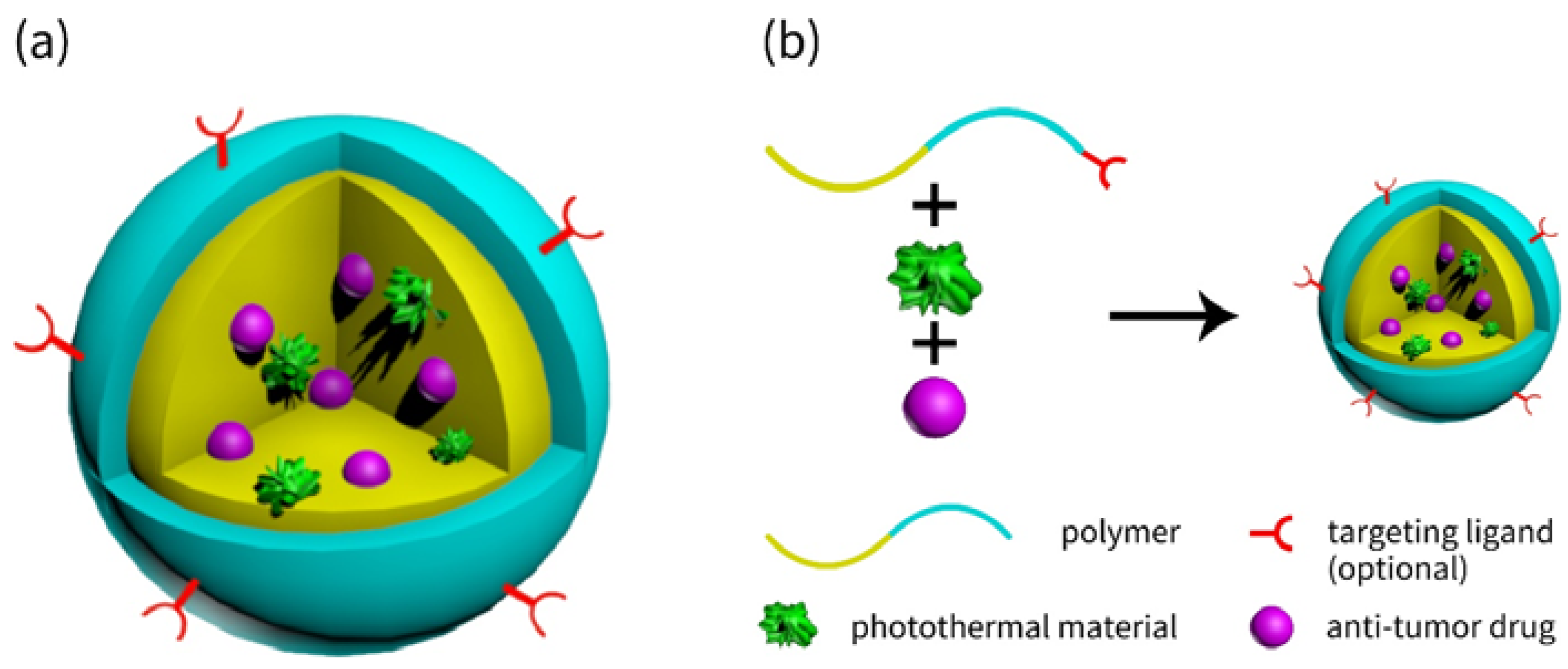
2.1. Ingredients of Nanocarriers
2.2. Core-Shell Structured Nanocarriers
2.2.1. Typical Core-Shell Structured Nanocarriers
2.2.2. Recent Advances of Novel Core-Shell Structured Nanocarriers
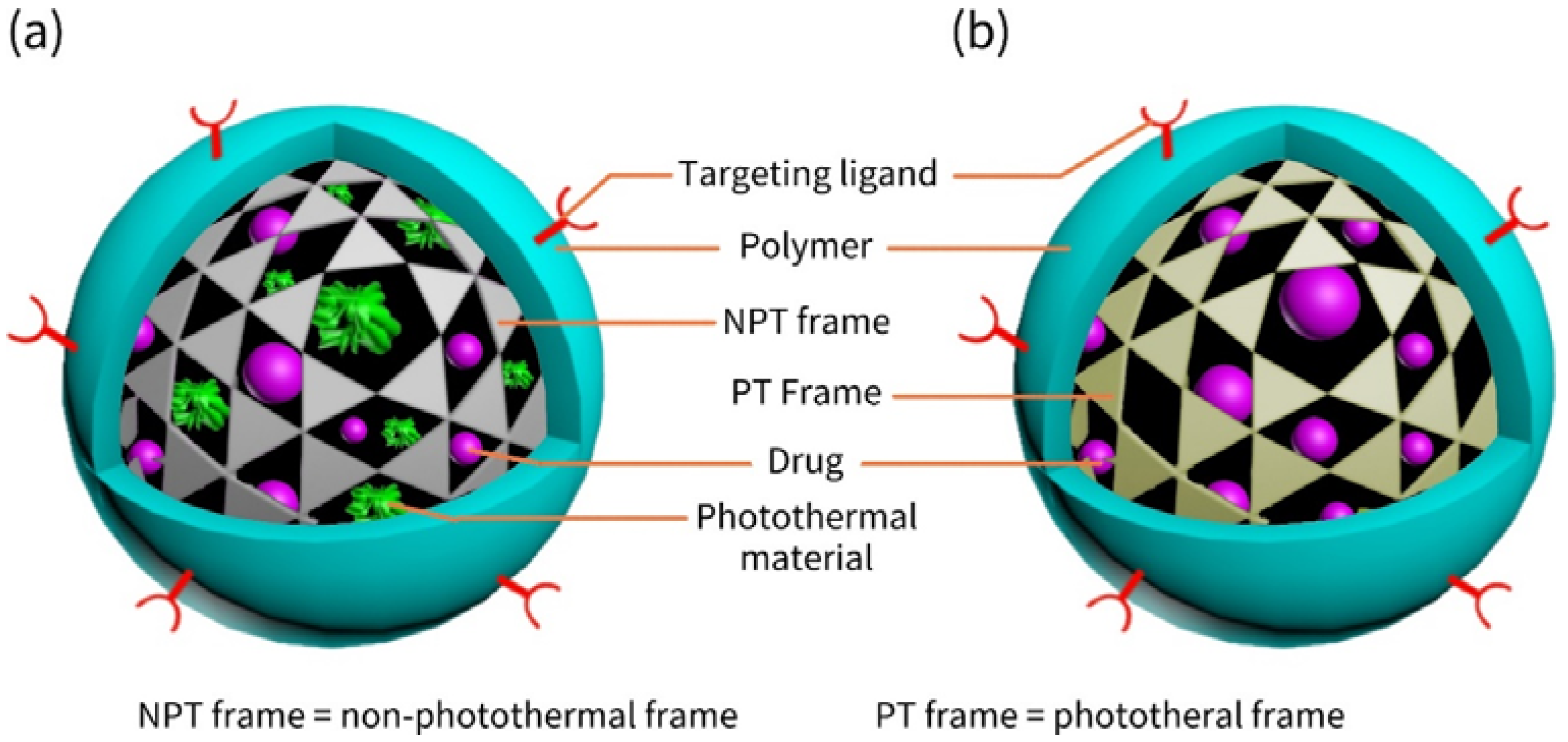
2.3. Frame-Coat Structured Nanocarriers
2.3.1. Various Frames
2.3.2. Preparation of Frame-Coat Structured Nanocarriers
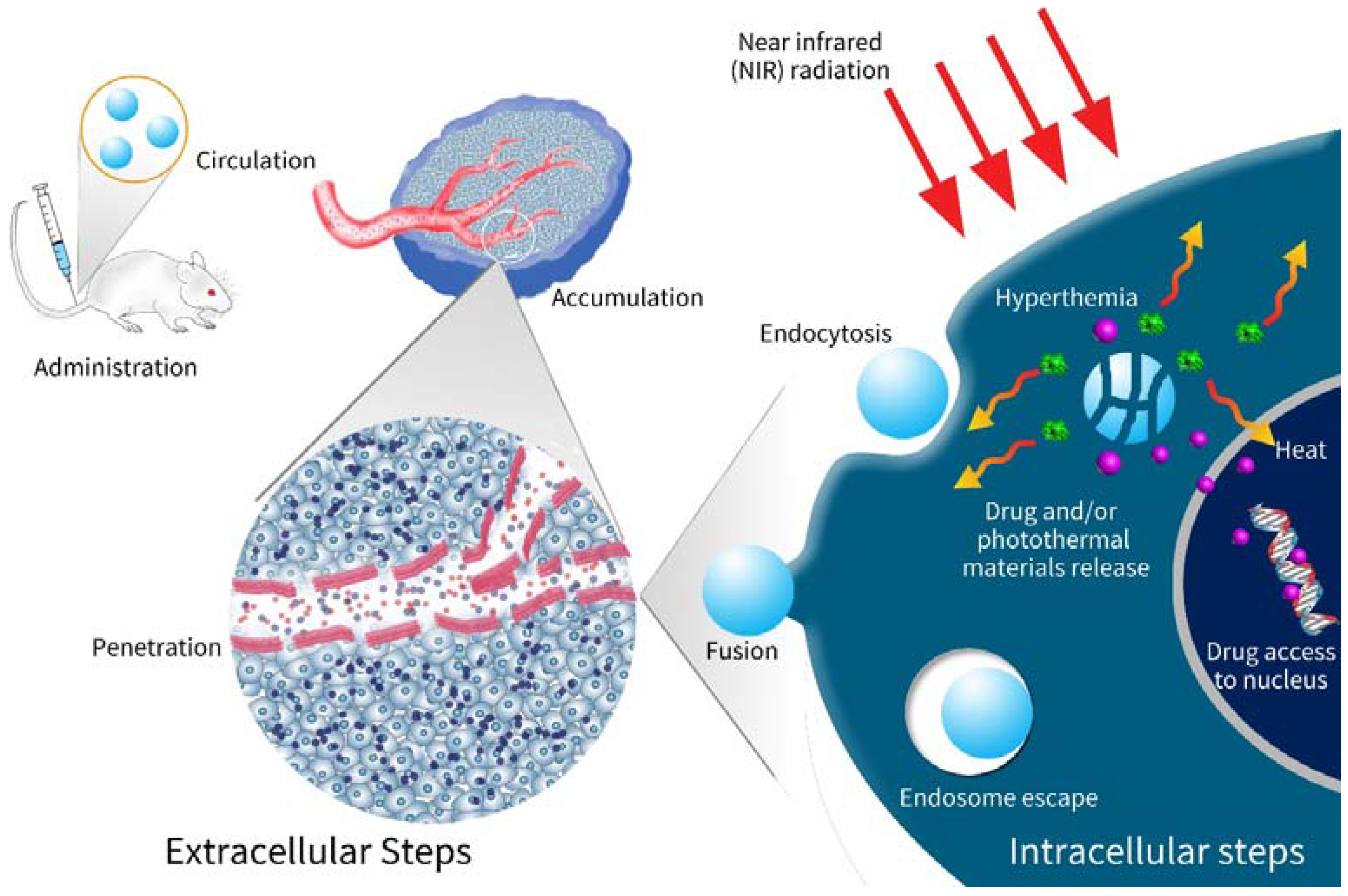
2.4. Navigation of Nanocarriers to Tumors
2.5. A New Trend: Preventions to Premature Leak of Drug
3. Combination of Chemotherapy with Photothermal Therapy via Nanocarriers
3.1. The Basics of Photothermal Therapy
3.2. The Interactions of Chemotherapy and Photothermal Therapy
3.3. The Ways to Fight Against Multi-Drug Resistance
3.4. Light-Controlled Drug Release
4. The Therapeutic Effects of Combinational Therapy with Nanocarriers
4.1. In Vitro Cell Experiments
4.2. In Vivo Experiments
4.3. Fighting Against Recurrence and Metastasis
4.4. Triple-Modality Therapies
4.5. Pending Clinical Trials
5. Problems, Hurdles and Challenges
6. Discussions and Suggestions
7. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Watanabe, K.; Timerman, D.; Schoenfeld, D.; Hasan, T. Prediction of Tumor Recurrence and Therapy Monitoring Using Ultrasound-Guided Photoacoustic Imaging. Theranostics 2015, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-Function Nanostructured Lipid Carriers to Deliver IR780 for Breast Cancer Treatment: Anti-Metastatic and Photothermal Anti-Tumor Therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Boisgerault, N.; Kottke, T.; Pulido, J.; Thompson, J.; Diaz, R.M.; Rommelfanger-Konkol, D.; Embry, A.; Saenz, D.; Poeschla, E.; Pandha, H.; et al. Functional Cloning of Recurrence-Specific Antigens Identifies Molecular Targets to Treat Tumor Relapse. Mol. Ther. 2013, 21, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Nakanishi, K.; Yokoo, H.; Kamachi, H.; Tahara, M.; Kakisaka, T.; Tsuruga, Y.; Todo, S.; Taketomi, A. Analysis of the Risk Factors for Early Death Due to Disease Recurrence or Progression within 1 Year After Hepatectomy in Patients with Hepatocellular Carcinoma. World J. Surg. Oncol. 2012, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Tansık, G.; Yakar, A.; Gündüz, U. Tailoring Magnetic PLGA Nanoparticles Suitable for Doxorubicin Delivery. J. Nanopart. Res. 2014, 16, 2171. [Google Scholar] [CrossRef]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, J.; Liu, Y.; Saw, P.E.; Tao, W.; Yu, M.; Zope, H.; Si, M.; Victorious, A.; Rasmussen, J. Multifunctional Envelope-Type siRNA Delivery Nanoparticle Platform for Prostate Cancer Therapy. ACS Nano 2017, 11, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Ma, Y.T. Synthesis, Characterization, and Biological Verification of anti-HER2 Indocyanine Green-Doxorubicin-Loaded Polyethyleneimine-Coated Perfluorocarbon Double Nanoemulsions for Targeted Photochemotherapy of Breast Cancer Cells. J. Nanobiotechnol. 2017, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Wang, L.; Cao, Y.; Ma, Y.; Shen, H.; Zhang, M.; Zhang, Z. Efficient Cancer Ablation by Combined Photothermal and Enhanced Chemo-Therapy Based on Carbon Nanoparticles/Doxorubicin@SiO2 Nanocomposites. Carbon 2016, 97, 35–44. [Google Scholar] [CrossRef]
- Thapa, R.K.; Youn, Y.S.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Graphene Oxide-Wrapped PEGylated Liquid Crystalline Nanoparticles for Effective Chemo-Photothermal Therapy of Metastatic Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2016, 143, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Brousell, S.C.; Liu, Y.; Maccarini, P.F.; Palmer, G.M.; Etienne, W.; Zhao, Y.; Lee, C.; Tuan, V.; Inman, B.A. Synergistic Immuno-Photothermal Nanotherapy (Symphony): A Novel Treatment for Localized and Metastatic Bladder Cancer. Sci. Rep. 2017, 7, 8606. [Google Scholar] [CrossRef]
- Brown, C. Targeted Therapy: An Elusive Cancer Target. Nature 2016, 537, S106–S108. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Fragogeorgi, E.; Palamaris, L.; Karampelas, T.; Lelovas, P.; Loudos, G.; Tamvakopoulos, C.; Kostomitsopoulos, N.; Kordas, G. Versatile Quarto Stimuli Nanostructure Based on Trojan Horse Approach for Cancer Therapy: Synthesis, Characterization, in Vitro and in vivo Studies. Mater. Sci. Eng. C 2017, 79, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, X.; Hou, M.; Xue, P.; Gao, Y.; Liu, S.; Kang, Y. Disassembly of Amphiphilic Small Molecular Prodrug with Fluorescence Switch Induced by pH and Folic Acid Receptors for Targeted Delivery and Controlled Release. Colloids Surf. B Biointerfaces 2017, 150, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Chen, Q.; Xu, L.; Wei, H.; Ma, C.; Sun, Y. Preparation and Evaluation of Liver-Targeting Micelles Loaded with Oxaliplatin. Artif. Cells Nanomed. Biotechnol. 2014, 44, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Han, X.; Peng, Z.; Li, A.; Liu, J. Improvement of the Hydrophilicity of 7,8-Dihydroxyflavone by in Situ Grafting of PEG-A Via RAFT Polymerization and the Drug Efficacy Tests. Eur. Polym. J. 2016, 81, 327–336. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Jia, Y.; Liu, S.; Tian, C.; Pan, W.; Liu, X.; Wang, H. Co-Delivery of Docetaxel and Curcumin Prodrug Via Dual-Targeted Nanoparticles with Synergistic Antitumor Activity Against Prostate Cancer. Biomed. Pharmacother. 2017, 88, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Shi, H.; Qiao, M.; Zhang, Z.; Yang, W.; Dong, L.; Xie, F.; Zhao, C.; Kang, L. PH-sensitive Micelles for the Intracellular Co-Delivery of Curcumin and Pluronic L61 Unimers for Synergistic Reversal Effect of Multidrug Resistance. Sci. Rep. 2017, 7, 42465. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regi, M. Recent Advances in Porous Nanoparticles for Drug Delivery in Antitumoral Applications: Inorganic Nanoparticles and Nanoscale Metal-Organic Frameworks. Expert Opin. Drug Deliv. 2017, 14, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; He, D.; He, X.; Wang, K.; Yang, X.; Liu, J.; Cheng, H.; Huang, X.; Shangguan, J. Facile Fabrication of a Resveratrol Loaded Phospholipid@Reduced Graphene Oxide Nanoassembly for Targeted and Near-Infrared Laser-Triggered Chemo/Photothermal Synergistic Therapy of Cancer in vivo. J. Mater. Chem. B 2017, 5, 5783–5792. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; He, D.; Hai, L.; Tang, J.; Li, H.; He, X.; Wang, K. A Metal-Organic Framework Based Nanocomposite with Co-Encapsulation of Pd@Au Nanoparticles and Doxorubicin for pH- and NIR-triggered Synergistic Chemo-Photothermal Treatment of Cancer Cells. J. Mater. Chem. B 2017, 5, 4648–4659. [Google Scholar] [CrossRef]
- Moreira, A.F.; Dias, D.R.; Costa, E.C.; Correia, I.J. Thermo- and pH-responsive Nano-In-Micro Particles for Combinatorial Drug Delivery to Cancer Cells. Eur. J. Pharm. Sci. 2017, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold Nanoshell Coated thermo-pH Dual Responsive Liposomes for Resveratrol Delivery and Chemo-Photothermal Synergistic Cancer Therapy. J. Mater. Chem. B 2017, 5, 2161–2171. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Da, L.; Yang, C.; Chen, R.; Gao, L.; Fan, L.; Han, J. Mn2+-Coordinated PDA@DOX/PLGA Nanoparticles as a Smart Theranostic Agent for Synergistic Chemo-Photothermal Tumor Therapy. Int. J. Nanomed. 2017, 12, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, Y.; Wang, X.; Wu, X.; Zha, Z. Facile Synthesis of Prussian Blue Nanoparticles as pH-responsive Drug Carriers for Combined Photothermal-Chemo Treatment of Cancer. RSC Adv. 2017, 7, 248–255. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-Responsive Photothermal-Chemotherapy with Drug-Loaded Melanin-Like Nanoparticles for Synergetic Tumor Ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Jin, J.O.; Oh, J. Photothermal-Triggered Control of Sub-Cellular Drug Accumulation Using Doxorubicin-Loaded Single-Walled Carbon Nanotubes for the Effective Killing of Human Breast Cancer Cells. Nanotechnology 2017, 28, 125101. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wang, B.; Shi, J.; Zhang, Y.; Zhang, Q.; Chen, Z.; Li, J. Photothermal and Biodegradable Polyaniline/Porous Silicon Hybrid Nanocomposites as Drug Carriers for Combined Chemo-Photothermal Therapy of Cancer. Acta Biomater. 2017, 51, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yuan, W.; Tham, H.P.; Chen, H.; Xing, P.; Xiang, H.; Yao, X.; Qiu, X.; Dai, Y.; Zhu, L.; et al. Fast-Clearable Nanocarriers Conducting Chemo/Photothermal Combination Therapy to Inhibit Recurrence of Malignant Tumors. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Cheng, X.; Bao, T.; Zu, M.; Yan, L.; Yin, W.; Ge, C.; Wang, D.; Gu, Z.; Zhao, Y. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for in vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano 2015, 9, 12451–12463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Liu, Z.; Wang, L.; Luo, L.; Wang, M.; Wang, Q.; Gao, D. Gold Nanoshell-Based Betulinic Acid Liposomes for Synergistic Chemo-Photothermal Therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, H.T.; Le, N.V.; Tran, T.; Lee, J.S.; Ku, S.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Engineering of Multifunctional Temperature-Sensitive Liposomes for Synergistic Photothermal, Photodynamic and Chemotherapeutic Effects. Int. J. Pharm. 2017, 528, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Shanthi, K.; Sundarraj, S.; Kannan, S. Synergistic Effect of Chemo-Photothermal for Breast Cancer Therapy Using Folic Acid (FA) Modified Zinc Oxide Nanosheet. J. Colloid Interface Sci. 2017, 488, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, T.; Tao, J.; Wan, G.; Zhao, H. Co-Delivery of Paclitaxel and Indocyanine Green by PEGylated Graphene Oxide: A Potential Integrated Nanoplatform for Tumor Theranostics. RSC Adv. 2016, 6, 15460–15468. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Zhang, Z.; Shen, Y.; Guo, S. A Chemo/Photo- Co-Therapeutic System for Enhanced Multidrug Resistant Cancer Treatment Using Multifunctional Mesoporous Carbon Nanoparticles Coated with Poly (Curcumin-Dithiodipropionic Acid). Carbon 2017, 122, 524–537. [Google Scholar] [CrossRef]
- Wan, Z.; Mao, H.; Guo, M.; Li, Y.; Zhu, A.; Yang, H.; He, H.; Shen, J.; Zhou, L.; Jiang, Z.; et al. Highly Efficient Hierarchical Micelles Integrating Photothermal Therapy and Singlet Oxygen-Synergized Chemotherapy for Cancer Eradication. Theranostics 2014, 4, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Li, W.; Chen, R.; Zhang, Z.; Zhang, W.; Tang, Y.; Chen, X.; Liu, G.; Lee, C.S. Degradable Hollow Mesoporous Silicon/Carbon Nanoparticles for Photoacoustic Imaging-Guided Highly Effective Chemo-Thermal Tumor Therapy in Vitro and in vivo. Theranostics 2017, 7, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, D.; Han, S.; Yan, G.; Ma, C.; Wei, C.; Yu, M.; Li, D.; Sun, Y. Preparation and Evaluation of Reduction-Responsive Nano-Micelles for Miriplatin Delivery. Exp. Biol. Med. 2016, 241, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An Innovative MWCNTs/DOX/TC Nanosystem for Chemo-Photothermal Combination Therapy of Cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.F.; Zhao, Y.; Zhang, H.; et al. Biodegradable Black Phosphorus-Based Nanospheres for in vivo Photothermal Cancer Therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.M.; Gao, Y.Q.; Li, D.; Zhou, S.B. Controllably Switched Drug Release from Successively Dual-Targeted Nanoreservoirs. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Long, M.; Qiu, L.; Zhu, M.; Li, Z. Decoration of pH-sensitive Copolymer Micelles with Tumor-Specific Peptide for Enhanced Cellular Uptake of Doxorubicin. Int. J. Nanomed. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Peng, C.L.; Yang, S.J.; Shieh, M.J. Photothermal, Targeting, Theranostic Near-Infrared Nanoagent with SN38 against Colorectal Cancer for Chemothermal Therapy. Mol Pharm 2017, 14, 2766–2780. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ramasamy, T.; Choi, J.Y.; Kim, S.T.; Youn, Y.S.; Choi, H.; Yong, C.S.; Kim, J.O. PEGylated Polypeptide Lipid Nanocapsules to Enhance the Anticancer Efficacy of Erlotinib in Non-Small Cell Lung Cancer. Colloids Surf. B Biointerfaces 2017, 150, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yong, Y.; Dong, X.; Du, J.; Guo, Z.; Gong, L.; Zhu, S.; Tian, G.; Yu, S.; Gu, Z.; et al. Therapeutic Nanoparticles Based on Curcumin and Bamboo Charcoal Nanoparticles for Chemo-Photothermal Synergistic Treatment of Cancer and Radioprotection of Normal Cells. ACS Appl. Mater. Interfaces 2017, 9, 14281–14291. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Tran, T.H.; Thapa, R.K.; Phung, C.D.; Shin, B.S.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Targeted Co-Delivery of Polypyrrole and Rapamycin by Trastuzumab-Conjugated Liposomes for Combined Chemo-Photothermal Therapy. Int. J. Pharm. 2017, 527, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Liu, Y.; Ji, B.; Wang, Q.; Wang, M.; Dai, K.; Gao, D. On-Demand Drug Release of ICG-liposomal Wedelolactone Combined Photothermal Therapy for Tumor. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Byeon, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. PEGylated Lipid Bilayer-Wrapped Nano-Graphene Oxides for Synergistic Co-Delivery of Doxorubicin and Rapamycin to Prevent Drug Resistance in Cancers. Nanotechnology 2017, 28, 295101. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Xing, L.; Zhou, B.; Du, L.; Shi, X. Dendrimer-Modified MoS2 Nanoflakes as a Platform for Combinational Gene Silencing and Photothermal Therapy of Tumors. ACS Appl. Mater. Interfaces 2017, 9, 15995–16005. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.; Lu, F.; Liang, Y.; Li, Z. SiRNA Delivery with PEGylated Graphene Oxide Nan osheets for Combined Photothermal and Genetherapy for Pancreatic Cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Teng, Z.; Dang, M.; Tian, Y.; Zhang, Y.; Huang, P.; Su, X.; Lu, N.; Yang, Z.; Tian, W.; et al. Gold Nanorod Embedded Large-Pore Mesoporous Organosilica Nanospheres for Gene and Photothermal Cooperative Therapy of Triple Negative Breast Cancer. Nanoscale 2017, 9, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Chen, J.; Hu, Y.; Li, X.; Wang, H.; Shen, M.; Shi, X. Dendrimer-Stabilized Gold Nanostars as a Multifunctional Theranostic Nanoplatform for CT Imaging, Photothermal Therapy and Gene Silencing of Tumors. Adv. Healthc. Mater. 2016, 5, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yong, Y.; Song, L.; Dong, X.; Zhang, X.; Liu, X.; Gu, Z.; Zhao, Y.; Hu, Z. Multifunctional WS2@Poly(Ethylene imine) Nanoplatforms for Imaging Guided Gene-Photothermal Synergistic Therapy of Cancer. Adv. Healthc. Mater. 2016, 5, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; He, X.; Luo, T.Y.; Zhang, J.; Liu, Y.H.; Yu, X.Q. Amphiphilic Carbon Dots as Versatile Vectors for Nucleic Acid and Drug Delivery. Nanoscale 2017, 9, 5935–5947. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wu, P.; Wang, M.; Li, P.; Chen, W.; Liu, L. Anti-Body Guided Au-Ag Nanoframes with Highly Targeted Photothermal Properties for Cancer Cell Therapy. Sci. Adv. Mater. 2017, 9, 1334–1339. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Gong, P.; Tian, M.; Sun, L.; Ji, S.; Zhang, L.; Liu, Z. Construction of a Novel Fluorinated Graphene-Based Magnetic Nanocomposite and its Application in Cancer Photo-Chemotherapy. Mater. Lett. 2017, 196, 165–167. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Z.; Cao, Y.; Dai, W.; Zhang, K.; Dong, H.; Feng, X.; Zhang, X. Fabricating Aptamer-Conjugated PEGylated-MoS2/Cu1.8S Theranostic Nanoplatform for Multiplexed Imaging Diagnosis and Chemo-Photothermal Therapy of Cancer. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, D.S. Fabrication, Characterization and Biological Evaluation of anti-HER2 Indocyanine Green-Doxorubicin-Encapsulated PEG-b-PLGA Copolymeric Nanoparticles for Targeted Photochemotherapy of Breast Cancer Cells. Sci. Rep. 2017, 7, 46688. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, D.R.; Mei, L.; Lu, B.G.; Chen, L.B.; Li, Q.H.; Zhu, H.Z.; Wang, T.H. Encapsulating Gold Nanoparticles or Nanorods in Graphene Oxide Shells as a Novel Gene Vector. ACS Appl. Mater. Interfaces 2013, 5, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Feng, Q.; Wang, Y.; Yang, X.; Ren, J.; Shi, Y.; Shan, X.; Yuan, Y.; Wang, Y.; Zhang, Z. Multifunctional Hyaluronic Acid Modified Graphene Oxide Loaded with Mitoxantrone for Overcoming Drug Resistance in Cancer. Nanotechnology 2016, 27, 015701. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lin, I.C.; Sung, S.Y.; Su, Y.L.; Huang, Y.F.; Chiang, C.S.; Hu, S.H. Dual-Targeted Photopenetrative Delivery of Multiple Micelles/Hydrophobic Drugs by a Nanopea for Enhanced Tumor Therapy. Adv. Funct. Mater. 2016, 26, 4169–4179. [Google Scholar] [CrossRef]
- Kim, S.H.; In, I.; Park, S.Y. PH-Responsive NIR-Absorbing Fluorescent Polydopamine with Hyaluronic Acid for Dual Targeting and Synergistic Effects of Photothermal and Chemotherapy. Biomacromolecules 2017, 18, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, Q.; Feng, B.; Xu, Z.; Zhang, J.; Xu, J.; Lu, L.; Yu, H.; Wang, M.; Li, Y.; et al. Polydopamine-Functionalized Graphene Oxide Loaded with Gold Nanostars and Doxorubicin for Combined Photothermal and Chemotherapy of Metastatic Breast Cancer. Adv. Healthc. Mater. 2016, 5, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous Polydopamine Nanoparticles with Co-Delivery Function for Overcoming Multidrug Resistance Via Synergistic Chemo-Photothermal Therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, X.; Shi, W.; Sun, Y.; Su, H.; He, Y.; Liu, X.; Zhang, Z.; Ge, D. Polydopamine-Functionalized Nanographene Oxide: A Versatile Nanocarrier for Chemotherapy and Photothermal Therapy. Nanotechnology 2017, 28, 295102. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liu, T.; Fu, C.; Wang, S.; Fu, S.; Ren, J.; Meng, X. Hollow ZrO2/PPy Nanoplatform for Improved Drug Delivery and Real-Time CT Monitoring in Synergistic Photothermal-Chemo Cancer Therapy. J. Mater. Chem. B 2016, 4, 859–866. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, X.; Liu, Z.; Chen, S.; Yu, N.; Wei, P.; Chen, Z.; Zhu, M. NIR-laser-triggered Smart Full-Polymer Nanogels for Synergic Photothermal-/Chemo-Therapy of Tumors. RSC Adv. 2016, 6, 90111–90119. [Google Scholar] [CrossRef]
- Chen, R.; Yang, F.; Xue, Y.; Wei, X.; Song, L.; Liu, X. Polypyrrole Confined in Dendrimer-Like Silica Nanoparticles for Combined Photothermal and Chemotherapy of Cancer. RSC Adv. 2016, 6, 38931–38942. [Google Scholar] [CrossRef]
- Park, D.; Ahn, K.O.; Jeong, K.C.; Choi, Y. Polypyrrole-Based Nanotheranostics for Activatable Fluorescence Imaging and Chemo/Photothermal Dual Therapy of Triple-Negative Breast Cancer. Nanotechnology 2016, 27, 185102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Chen, S.P.; Zhao, H.; Yang, Y.; Chen, X.Q.; Sun, J.; Fan, H.S.; Zhang, X.D. PPy@MIL-100 Nanoparticles as a pH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Xu, S.; Wang, F.; Shen, Y.; Huang, S.; Guo, S. PH, Redox and Photothermal Tri-Responsive DNA/Polyethylenimine Conjugated Gold Nanorod as Nanocarrier for Specific Intracellular Co-Release of Doxorubicin and Chemosensitizer Pyronaridine to Combat Multidrug Resistant Cancer. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Brann, T.; Patel, D.; Chauhan, R.; James, K.T.; Bates, P.J.; Malik, M.T.; Keynton, R.S.; O’Toole, A.G. Gold Nanoplates as Cancer-Targeted Photothermal Actuators for Drug Delivery and Triggered Release. J. Nanomater. 2016, 2016, 2036029. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, K.; Liu, H.; Ouyang, J.; Wang, L.; Liu, Y.; Wang, H.; Deng, L.; Liu, Y.N. Cell Membrane Camouflaged Hollow Prussian Blue Nanoparticles for Synergistic Photothermal-/Chemotherapy of Cancer. Adv. Funct. Mater. 2017, 27, 1605795. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Xu, S.; Yu, Y.; Hussain, A.; Shen, Y.; Guo, S. Photothermal Gold Nanocages Filled with Temperature Sensitive Tetradecanol and Encapsulated with Glutathione Responsive Polycurcumin for Controlled DOX Delivery to Maximize anti-MDR Tumor Effects. J. Mater. Chem. B 2017, 5, 5464–5472. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A New NIR-triggered Doxorubicin and Photosensitizer Indocyanine Green Co-Delivery System for Enhanced Multidrug Resistant Cancer Treatment through Simultaneous Chemo/Photothermal/Photodynamic Therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, B.; Chen, Z.; Liu, W.; Pan, J.; Hou, L.; Zhang, Z. A Multi-Functional Tumor Theranostic Nanoplatform for MRI Guided Photothermal-Chemotherapy. Pharm. Res. 2016, 33, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, Y.; Wang, G.; Yi, J.; Yu, C.; Huang, Y.; Sun, L.; Bao, Y.; Li, Y. Near-Infrared Conjugated Polymers for Photoacoustic Imaging-Guided Photothermal/Chemo Combination Therapy. J. Mater. Chem. B 2017, 5, 5479–5487. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Huang, H.; Zhang, H.; Hou, L.; Zhang, Z. Folic Acid-Modified and Functionalized CuS Nanocrystal-Based Nanoparticles for Combined Tumor Chemo- and Photothermal Therapy. J. Drug Target. 2017, 25, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Fu, J.; Huang, R.; Tan, L. Core-Shell Structured Nanospheres for Photothermal Ablation and pH-triggered Drug Delivery Toward Synergistic Cancer Therapy. RSC Adv. 2017, 7, 26640–26649. [Google Scholar] [CrossRef]
- Hung, C.C.; Huang, W.C.; Lin, Y.W.; Yu, T.W.; Chen, H.H.; Lin, S.C.; Chiang, W.H.; Chiu, H.C. Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy. Theranostics 2016, 6, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Chen, K.T.; Sheu, Y.C.; Sung, S.Y.; Hsu, R.S.; Chiang, C.S.; Hu, S.H. The Penetrated Delivery of Drug and Energy to Tumors by Lipo-Graphene Nanosponges for Photolytic Therapy. ACS Nano 2016. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yin, W.; Yu, J.; Dou, R.; Bao, T.; Zhang, X.; Yan, L.; Yong, Y.; Su, C.; Wang, Q.; et al. Mesoporous Bamboo Charcoal Nanoparticles as a New Near-Infrared Responsive Drug Carrier for Imaging-Guided Chemotherapy/Photothermal Synergistic Therapy of Tumor. Adv. Healthc. Mater. 2016, 5, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Syu, W.; Huang, T.; Lee, Y.; Hsiao, J.; Huang, K.; Yu, H.; Liao, M.; Lai, P. Encapsulation of Au/Fe3O4 Nanoparticles into a Polymer Nanoarchitecture with Combined Near Infrared-Triggered Chemo-Photothermal Therapy Based On Intracellular Secondary Protein Understanding. J. Mater. Chem. B 2017, 5, 5774–5782. [Google Scholar] [CrossRef]
- Shen, S.; Ding, B.; Zhang, S.; Qi, X.; Wang, K.; Tian, J.; Yan, Y.; Ge, Y.; Wu, L. Near-Infrared Light-Responsive Nanoparticles with Thermosensitive Yolk-Shell Structure for Multimodal Imaging and Chemo-Photothermal Therapy of Tumor. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Deng, Y.; Sun, H.; Ke, X.; Ci, T. Near-Infrared Light Triggered Drug Delivery System for Higher Efficacy of Combined Chemo-Photothermal Treatment. Acta Biomater. 2017, 51, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Geng, J.; Li, N.; Carter, K.A.; Shao, S.; Atilla-Gokcumen, G.E.; Lovell, J.F. Vessel-Targeted Chemophototherapy with Cationic Porphyrin-Phospholipid Liposomes. Mol. Cancer Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. PH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [PubMed]
- Nehate, C.; Alex, M.R.A.; Kumar, A.; Koul, V. Combinatorial Delivery of Superparamagnetic Iron Oxide Nanoparticles (Gamma Fe2O3) and Doxorubicin Using Folate Conjugated Redox Sensitive Multiblock Polymeric Nanocarriers for Enhancing the Chemotherapeutic Efficacy in Cancer Cells. Mater. Sci. Eng. C 2017, 75, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, C.; Li, B.; Shan, M.; Wu, G. A Magnetic Polypeptide Nanocomposite with pH and Near-Infrared Dual Responsiveness for Cancer Therapy. J. Polym. Res. 2017, 24, 122. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Tang, Y.; Wang, S.; Wang, C.; Li, Y.; Su, X.; Tian, J.; Tian, Y.; Pan, J.; et al. Synergistic Chemo-Photothermal Therapy of Breast Cancer by Mesenchymal Stem Cell-Encapsulated Yolk-Shell GNR@HPMO-PTX Nanospheres. ACS Appl. Mater. Interfaces 2016, 8, 17927–17935. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, C.; Meng, L.; Long, J.; Jing, J.; Dang, D.; Fei, Z.; Dyson, P.J. Stepwise Growth of Gold Coated Cancer Targeting Carbon Nanotubes for the Precise Delivery of Doxorubicin Combined with Photothermal Therapy. J. Mater. Chem. B 2017, 5, 1380–1387. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhou, J.; Huo, M. Somatostatin Receptor-Mediated Tumor-Targeting Nanocarriers Based on Octreotide-PEG Conjugated Nanographene Oxide for Combined Chemo and Photothermal Therapy. Small 2016, 12, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bi, Y.; Chen, J.; Peng, L.; Wen, K.; Ji, P.; Ren, W.; Li, X.; Zhang, N.; Gao, J.; et al. Near-Infrared Light-Triggered Switchable Nanoparticles for Targeted Chemo/Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 15103–15112. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Du, Z.; Bao, T.; Dong, X.; Zheng, X.; Yu, M.; Yin, W.; Dong, B.; Yan, L.; Gu, Z. The Polyvinylpyrrolidone Functionalized rGO/Bi2S3 Nanocomposite as a Near-Infrared Light-Responsive Nanovehicle for Chemo-Photothermal Therapy of Cancer. Nanoscale 2016, 8, 11531–11542. [Google Scholar] [CrossRef] [PubMed]
- Bani, F.; Adeli, M.; Movahedi, S.; Sadeghizadeh, M. Graphene-Polyglycerol-Curcumin Hybrid as a Near-Infrared (NIR) Laser Stimuli-Responsive System for Chemo-Photothermal Cancer Therapy. RSC Adv. 2016, 6, 61141–61149. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Hu, N.; Zhang, L.; Zhang, Y.; Yin, L. Docetaxel-Loaded SiO2@Au@GO Core-Shell Nanoparticles for Chemo-Photothermal Therapy of Cancer Cells. RSC Adv. 2016, 6, 48379–48386. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, H.; Wang, X.; Liu, Q.; Ye, M.; Tan, W. A Smart, Photocontrollable Drug Release Nanosystem for Multifunctional Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, C.; Wang, W.; Li, H.; Sun, Z.; Song, C.; Li, B.; Duan, S.; Hu, Y. A 54 Peptide-Mediated Functionalized Gold Nanocages for Targeted Delivery of DOX as a Combinational Photothermal-Chemotherapy for Liver Cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, Y.; Cheng, Z.; Deng, K.; Ma, P.; Hou, Z.; Liu, B.; Huang, S.; Jin, D.; Lin, J. Rational Design of a Comprehensive Cancer Therapy Platform Using Temperature-Sensitive Polymer Grafted Hollow Gold Nanospheres: Simultaneous Chemo/Photothermal/Photodynamic Therapy Triggered by a 650 Nm Laser with Enhanced Anti-Tumor Efficacy. Nanoscale 2016, 8, 6837–6850. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Qiu, W.X.; Liu, L.H.; Zheng, D.W.; Fan, J.X.; Rong, L.; Sun, Y.X.; Zhang, X.Z. Mitochondria-Targeting “Nanoheater” for Enhanced Photothermal/Chemo-Therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, M.; Li, S.; Li, L.; Zhang, L.; Wang, T.; Yu, M.; Mou, Z.; Wang, C. Facile Synthesis of Polypyrrole@Metal-Organic Framework Core-Shell Nanocomposites for Dual-Mode Imaging and Synergistic Chemo-Photothermal Therapy of Cancer Cells. J. Mater. Chem. B 2017, 5, 1772–1778. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Chen, H.; Wu, X.; Qian, H.; Yang, X.; Zha, Z. Novel Doxorubicin Loaded PEGylated Cuprous Telluride Nanocrystals for Combined Photothermal-Chemo Cancer Treatment. Colloids Surf. B Biointerfaces 2017, 152, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Yin, W.; Zheng, X.; Zhang, X.; Yu, J.; Dong, X.; Yong, Y.; Gao, F.; Yan, L.; Gu, Z.; et al. One-Pot Synthesis of PEGylated Plasmonic MoO3–X Hollow Nanospheres for Photoacoustic Imaging Guided Chemo-Photothermal Combinational Therapy of Cancer. Biomaterials 2016, 76, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.; et al. Controllable Synthesis of dual-MOFs Nanostructures for pH-responsive Artemisinin Delivery, Magnetic Resonance and Optical Dual-Model Imaging-Guided Chemo/Photothermal Combinational Cancer Therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhang, H.; Zhang, X.; Liu, D.; Chen, D.; Zhang, W.; Zhang, L.; Santos, H.A.; Hai, M. Biodegradable Photothermal and pH Responsive Calcium Carbonate@Phospholipid@Acetalated Dextran Hybrid Platform for Advancing Biomedical Applications. Adv. Funct. Mater. 2016, 26, 6158–6169. [Google Scholar] [CrossRef]
- Meng, Z.; Wei, F.; Wang, R.; Xia, M.; Chen, Z.; Wang, H.; Zhu, M. NIR-Laser-Switched in vivo Smart Nanocapsules for Synergic Photothermal and Chemotherapy of Tumors. Adv. Mater. 2016, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, X.; Wang, Y.; Deng, G.; Zhou, F.; Wang, Q.; Zhang, L.; Lu, J. Facile One-Pot Synthesis of Fe3O4@Chitosan Nano-Spheres for MRI and Fluorescence Imaging Guided Chemo-Photothermal Combinational Cancer Therapy. Dalton Trans. 2016, 45, 19519–19528. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, H.; Bie, N.; Xu, Q.; Yong, T.; Wang, Q.; Gan, L.; Yang, X. Zwitterionic Temperature/Redox-Sensitive Nanogels for Near-Infrared Light-Triggered Synergistic Thermo-Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 23564–23573. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Luo, L.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Yang, J.; Zhu, C.; Du, Y.; et al. External Magnetic Field-Enhanced Chemo-Photothermal Combination Tumor Therapy via Iron Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 16581–16593. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, X.; Deng, G.; Zhou, F.; Zhang, L.; Wang, Q.; Lu, J. Fe3O4@mSiO2-FA-CuS-PEG Nanocomposites for Magnetic Resonance Imaging and Targeted Chemo-Photothermal Synergistic Therapy of Cancer Cells. Dalton Trans. 2016, 45, 13456–13465. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, G.; Li, J.; Sun, L.; Wang, J.; Li, J.; Li, P.; Chen, W.; Wang, Q.; Tong, T. Preparation of Hyaluronic Acid-Modified Mesoporous Silica-Coated Gold Nanorods and their Application in Chemo-Photothermal Therapy of Cancer. Chem. J. Chin. Univ. 2016, 37, 224–231. (In Chinese) [Google Scholar]
- Li, J.; Zhang, F.; Hu, Z.; Song, W.; Li, G.; Liang, G.; Zhou, J.; Li, K.; Cao, Y.; Luo, Z.; et al. Drug “Pent-Up” in Hollow Magnetic Prussian Blue Nanoparticles for NIR-Induced Chemo-Photothermal Tumor Therapy with Trimodal Imaging. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Lu, M.; Li, L.; Zhang, Y.; Zheng, X.; Shao, D.; Li, J.; Dong, W. Janus Au-mesoporous Silica Nanocarriers for Chemo-Photothermal Treatment of Liver Cancer Cells. RSC Adv. 2016, 6, 44498–44505. [Google Scholar] [CrossRef]
- Frangioni, J.V. in vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Cheng, R.; Zhang, X.; Jiang, J. NIR- and UV-dual Responsive Amphiphilic Copolymer Micelles with Light-Dissociable PAG-side Groups. Colloid Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Tang, Y.; McGoron, A.J. Combined Effects of laser-ICG Photothermotherapy and Doxorubicin Chemotherapy On Ovarian Cancer Cells. J. Photochem. Photobiol. B Biol. 2009, 97, 138–144. [Google Scholar] [CrossRef] [PubMed]
- De Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Deng, Z. A Review of Hyperthermia Combined with Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Xi, W.; Wang, H.; Liu, J.; Mayr, T.; Shi, L.; Sun, L. In Situ Crystal Growth of Gold Nanocrystals On Upconversion Nanoparticles for Synergistic Chemo-Photothermal Therapy. Nanoscale 2017, 9, 12885–12896. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Salipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic Studies of Anticancer Aptamer AS1411 Reveal a Novel Role for Nucleolin in Regulating Rac1 Activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Souery, W.N.; Bishop, C.J. Clinically Advancing and Promising Polymer-Based Therapeutics. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, G.; Marsaud, V.; Plassat, V.; Renoir, J. 10 Nanocarriers Targeting Breast Cancers to Deliver Modulators of Oestrogen Receptor. In Lipid Nanocarriers in Cancer Diagnosis and Therapy; Souto, E., Ed.; Smithers Information Limited: Shrewsbury, UK, 2011; pp. 279–308. ISBN 978-1-84735-479-2. [Google Scholar]
- Beyer, S.; Xie, L.; Grafe, S.; Vogel, V.; Dietrich, K.; Wiehe, A.; Albrecht, V.; Mantele, W.; Wacker, M.G. Bridging Laboratory and Large Scale Production: Preparation and in Vitro-Evaluation of Photosensitizer-Loaded Nanocarrier Devices for Targeted Drug Delivery. Pharm. Res 2015, 32, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Singh, K.K.; Müller, R.H. Production & Stability of Stavudine Solid Lipid Nanoparticles—From Lab to Industrial Scale. Int. J. Pharm. 2011, 416, 461–470. [Google Scholar] [CrossRef] [PubMed]

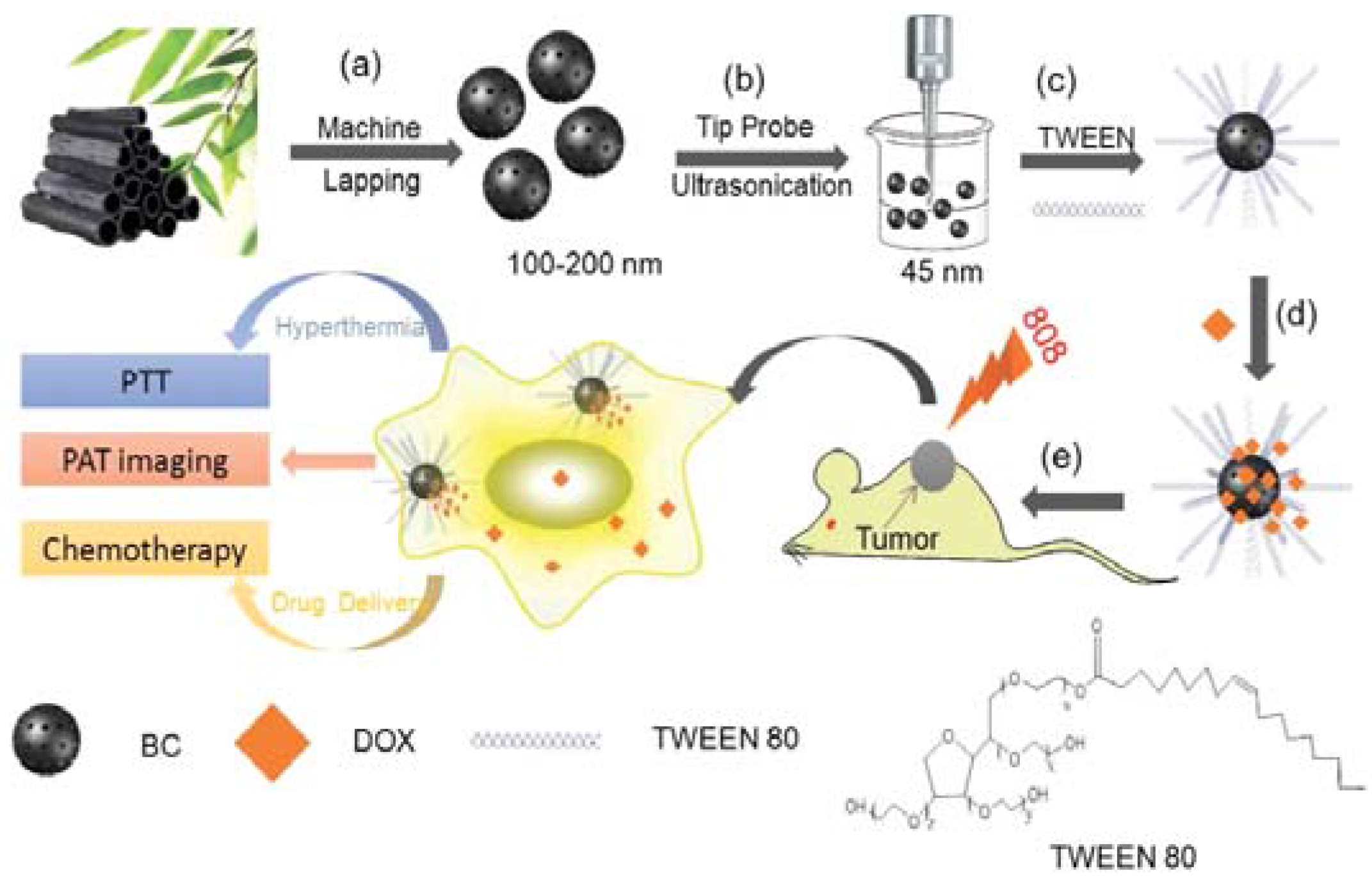
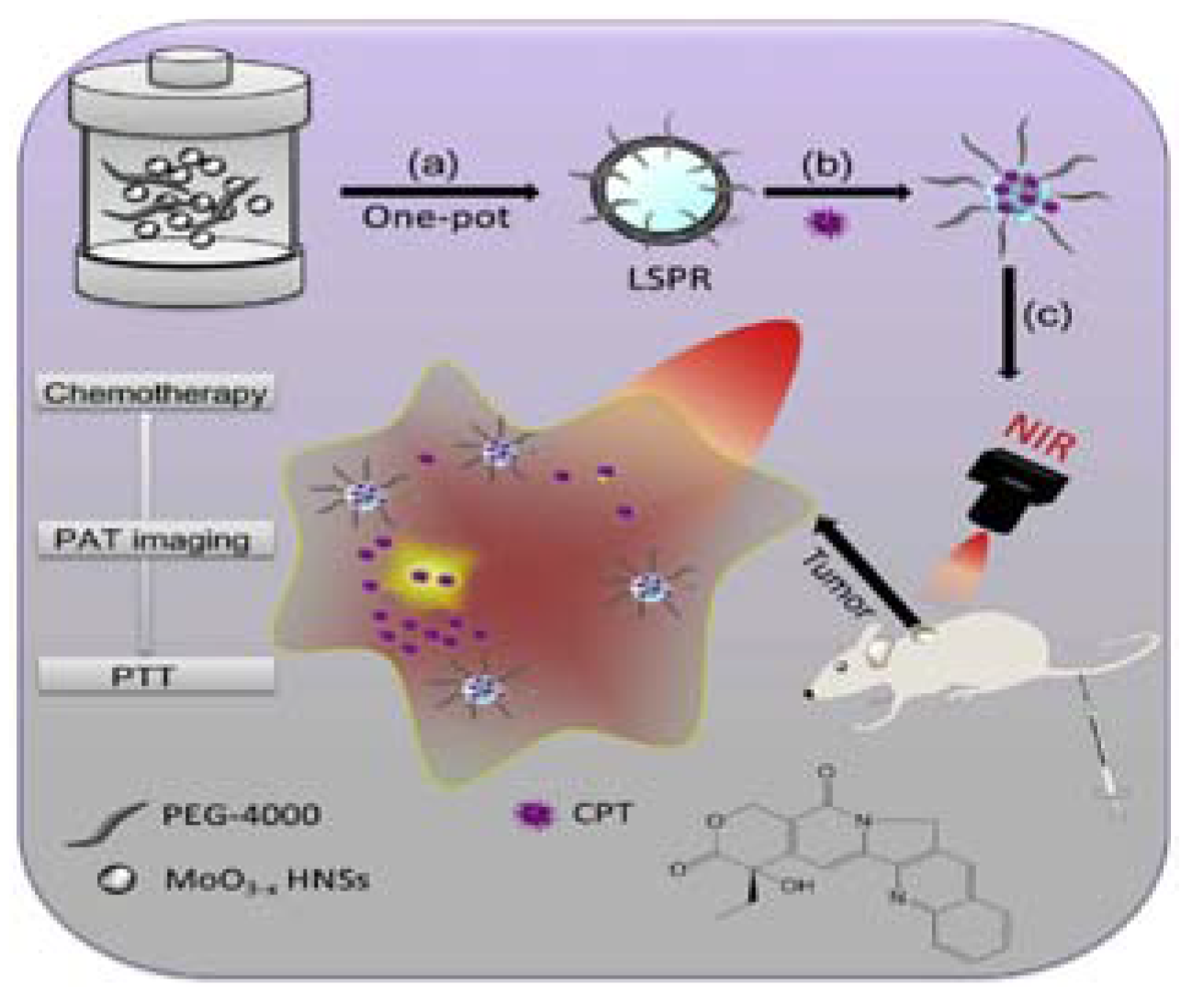
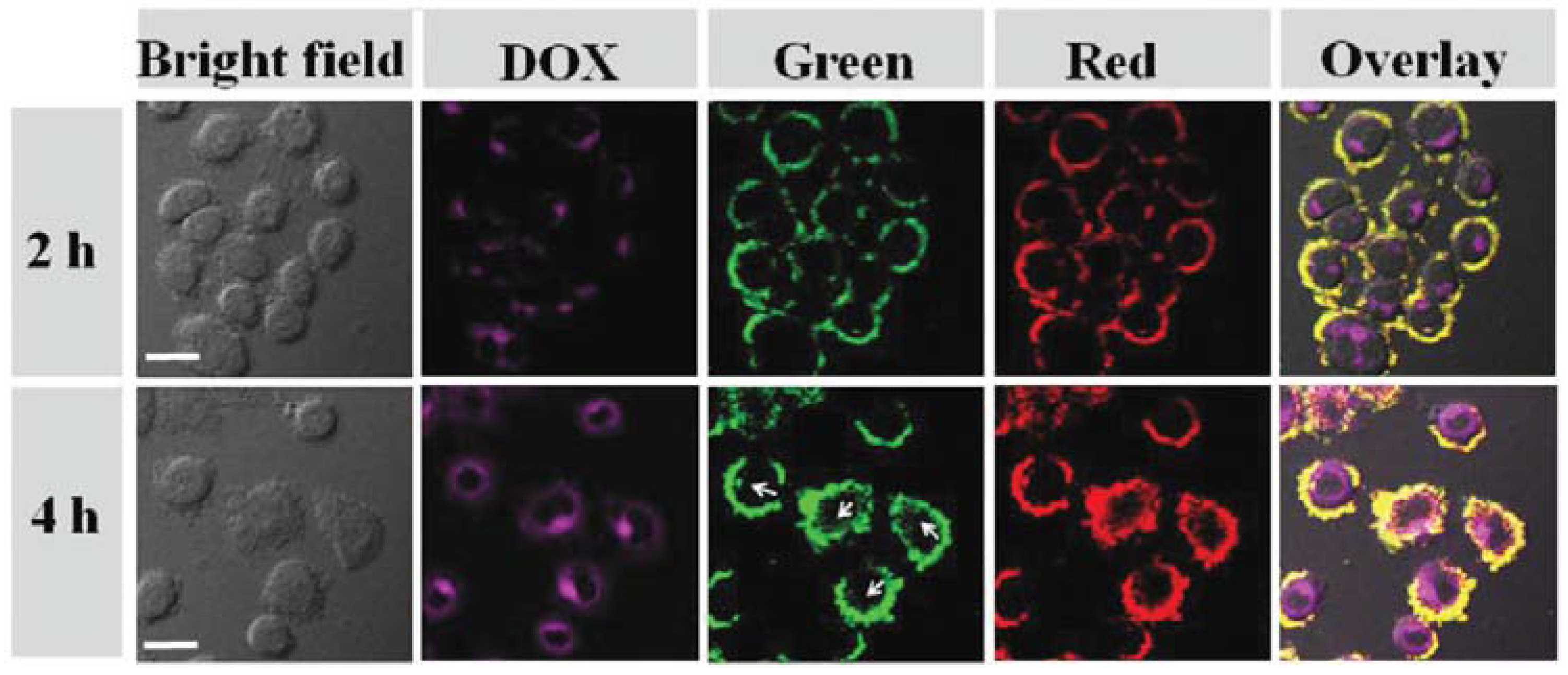
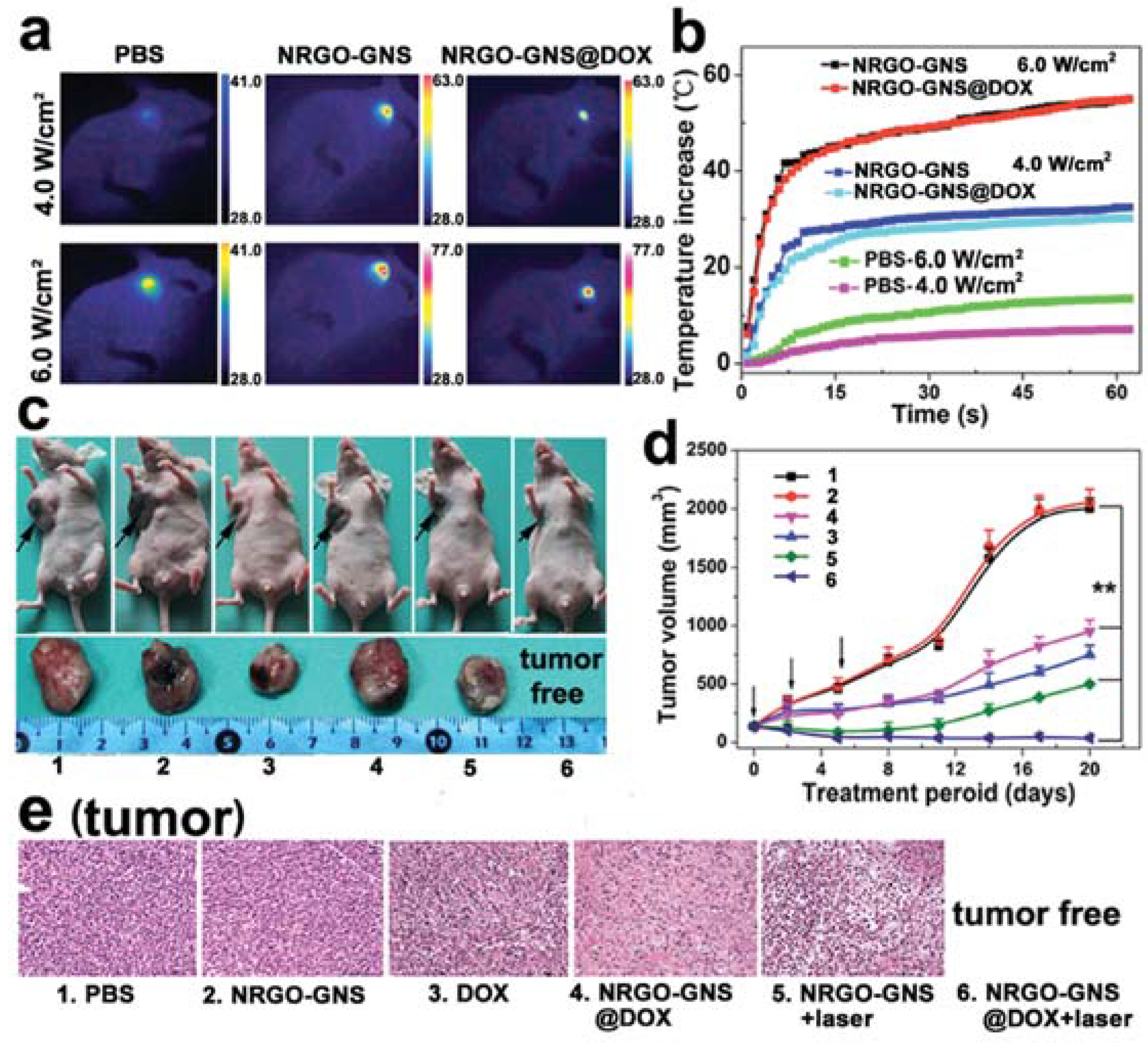
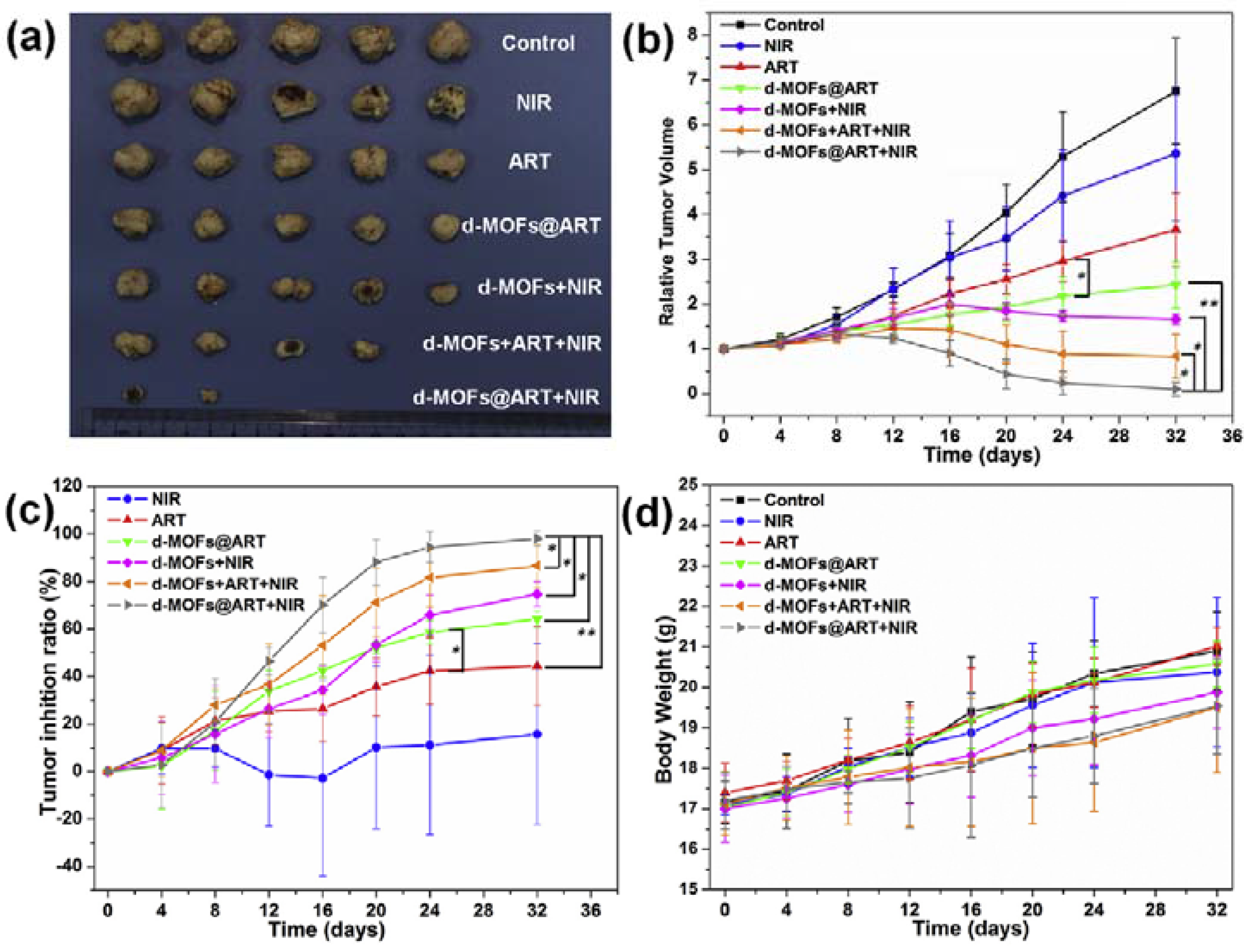
| Photothermal Material | Drug(s) | Polymer | Targeting Ligand | Reference |
|---|---|---|---|---|
| IR780 | AMD3100 | PC, MCT and trilaurin | AMD3100 | [3] |
| ICG | DOX | Carboxyl-terminated pluronic F68 and polyethyleneimine-coated perfluorocarbon | HER2 antibody | [9] |
| Fe3O4 | DOX | PMMA and PMMA-co-DMAEMA-co-DVB-co-PEG360-co-AA-co-DS | None | [14] |
| IR780 | SN38 | IR780-mPEG and IR780-mPEG-cRGD | cRGD | [45] |
| RGO | Resveratrol | Folate acid-terminated PEG-phospholipid | Folate acid | [21] |
| Au-S nanorod | DOX, NaHCO3 and Salicylic acid | PVA, PLGA and TPGS | None | [23] |
| AuNS | Resveratrol | Resveratrol-binding lipid and CTS | None | [24] |
| CuS and MoS2 | DOX | Aptamer, NH2-(SH)-PEG | None | [60] |
| PDA | DOX | PDA and PLGA | None | [26] |
| OA-PB NP | DOX | DSPE-PEG 2000 | None | [27] |
| BCNP | Curcumin | TPGS | None | [48] |
| PPy NP | Rapamycin | DPPC, cholesterol, CHEMS and DSPE-PEG2000-Maleimide | Trastuzumab | [49] |
| ICG | Wedelolactone | Soybean phosphatidylcholine, Cholesterol and PEG-2000 | None | [50] |
| NGO | DOX and Rapamycin | PEGylated lipid | None | [51] |
| ICG | DOX | PEG-PLGA | HER2 antibody | [61] |
| RGO | DOX | PVA | Lactoferrin | [64] |
| HMPB | DOX | Red blood cell membrane | None | [76] |
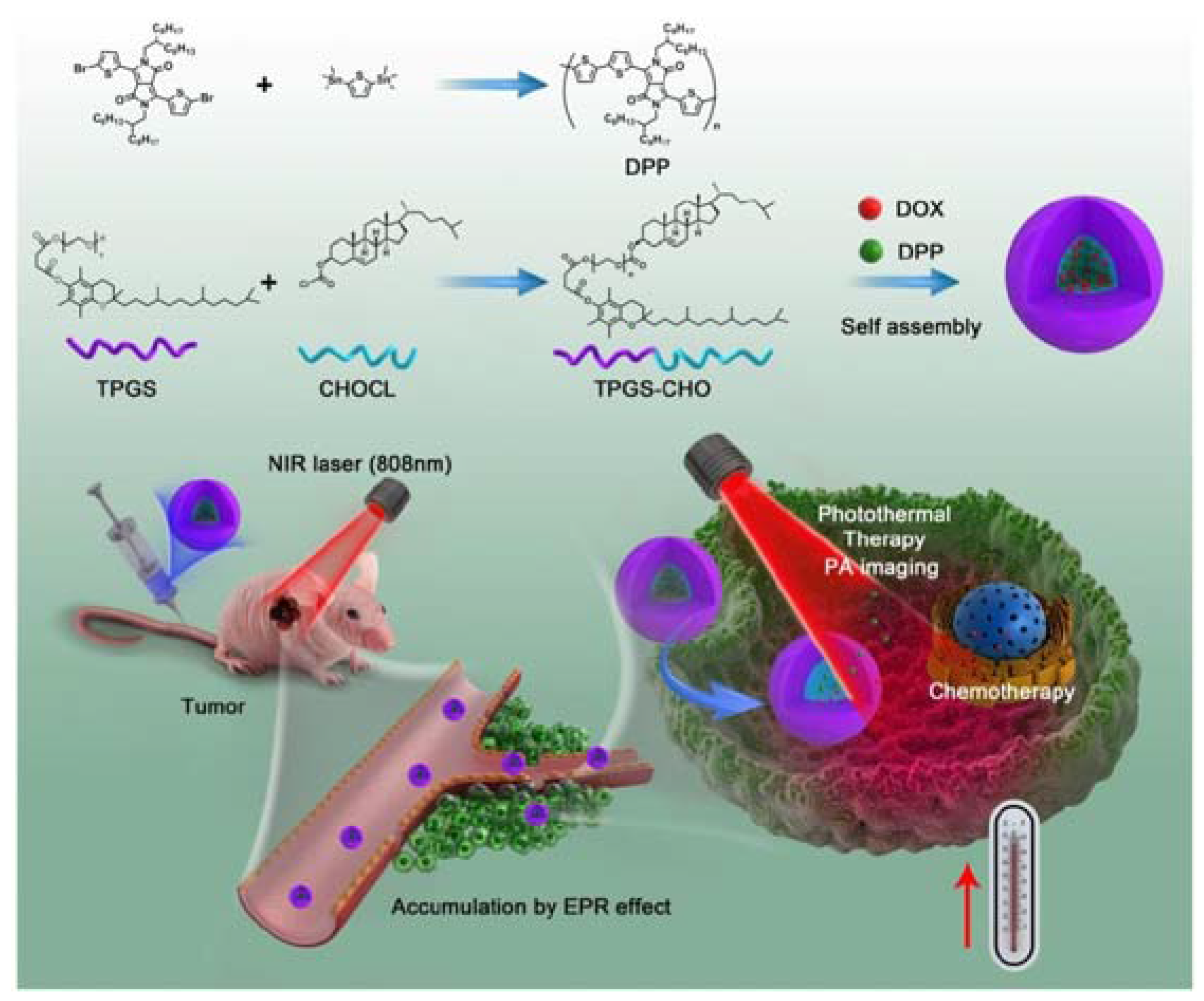
| Diketopyrrolopyrrole-based polymers | DOX | TPGS-CHO copolymers | None | [80] |
| CuS | DTX | Povidone and chitosan | Folic acid | [81] |
| OA-CuS-NP | DOX | Chitosan | None | [82] |
| ICG | DOX | PLGA and NAcHis-TPGS | None | [83] |
| Graphene nanosponge | DTX and Perfluorohexane | DOPE and DSPE-PEG2000-NH2 | Lactoferrin | [84] |
| BCNP | DOX | Tween 80 | None | [85] |
| Nanogold and Fe3O4 | DOX | PSMA | None | [86] |
| Fe3O4 | DOX | poly(N-isopropylacrylamide)-co-1-Vinyl-2-pyrrolidone | None | [87] |
| ICG | DOX | PPE-Chol1-HA | Hyaluronic acid | [88] |
| Porphyrin-phospholipid (PoP) | DOX | DOTAP and PoP | None | [89] |
| PDA NP | Bortezomib and DOX | pNIPAAm-co-pAAm | None | [90] |
| SPION (Fe2O3) | DOX | -PLGA-PEG-PLGA-urethane-SS- | Folic acid | [91] |
| Fe3O4 | DOX | mPEG-g-PDAEAIM | None | [92] |
| Frame | Photothermal Material | Drug(s) | Polymer | Targeting Ligand | Reference |
|---|---|---|---|---|---|
| Monoolein | GO | DTX | CTS-PEG | GO | [11] |
| Si/C NP | C in Si/C NPs | DOX | PEG | None | [39] |
| MWNT | MWNT | DOX | CTS | Transactivator of transcription | [41] |
| PdNP | Gold | DOX | ZIF-8 | None | [22] |
| GO@Gd | GO | DOX | PEG-2000 | Folic acid | [79] |
| PDA | PDA | DOX and SN-38 | PEG | None | [28] |
| SWNT | SWNT | DOX | PEG | None | [29] |
| SiNP | Polyaniline | DOX | Polyaniline | None | [30,35] |
| Silica | Palladium phthalocyanine | PTX | Pluroic F108 | None | [31] |
| ZnO nanosheet | ZnO NS | DOX | PEG | Folic acid | [35] |
| NGO | NGO and ICG | PTX | PEG | None | [36] |
| MCN | MCN | DOX | PEG-PCDA | None | [37] |
| PDA NP | PDA NP | CP | PEG | None | [47] |
| Fluorinated GO | Fe3O4 | DOX | FGO | None | [59] |
| GO | GO | Mitoxantrone | Hyaluronic acid and Pluronic | Hyaluronic acid | [63] |
| MWNT@PPy@Au shell | MWNT@PPy@Au shell | DOX | FA-PEG-SH | Folic acid | [94] |
| PPy@Mil-100(Fe) | PPy | DOX | PPy | None | [73] |
| PPy | PPy | DOX | Hyaluronic acid | Hyaluronic acid | [72] |
| PNA | PPy | DOX | Chitosan (CTS) | None | [70] |
| PPy@DSN | PPy | DOX | PEG | None | [71] |
| ZrO2 | (PPy) | DOX | PPy | None | [69] |
| NGO | NGO and GNS | DOX | PDA-PEG | None | [66] |
| MPDA | MPDA | DOX | TPGS | None | [67] |
| RGO | PDA | Ara or HCPT | PDA | None | [68] |
| PEI and DNA | AuNR | DOX and PND | PEI and DNA | Biotin | [74] |
| GNP | GNP | DOX | Hairpin-like aptamer AS1411 | AS1411 | [75] |
| AuNC with tetradecanol | AuNC | DOX | Biotin-PEG-PCDA | Biotin | [77] |
| AuNC with tetradecanol | AuNC and ICG | DOX | PEG | Biotin | [78] |
| HPMO | GNR | PTX | Mesenchymal stem cells (MSCs) | MSCs per se | [93] |
| NGO | NGO | DOX | PEG | Octreotide | [95] |
| SWCNT | CNT | DOX | CS-OA and PNIPAAm | None | [96] |
| RGO | rGO and Bi2S3 | DOX | PVP | None | [97] |
| Nano graphene | Nano graphene | Curcumin | Hyperbranched polyglycerol | None | [98] |
| SiO2@Au | Gold and GO | DTX | HS-PEG-NH2 and GO | None | [99] |
| Gold nanorod (AuNR) | AuNR | DOX and TMPyP4 | DNA and/or AS1411 | None | [100] |
| AuNC | AuNC | DOX | Hyaluronic acid and PEG | A54 peptide | [101] |
| HAuNs | HAuNs | DOX and Ce6 | p(OEGMA-co-MEMA) | None | [102] |
| AuNS | AuNS | DOX | Hyaluronic acid (HA) | HA, Cationic peptide R8 and mitochondria-targeting peptide TPP-KLA | [103] |
| PPy@Mil-100(Fe) | PPy | DOX | PPy | None | [104] |
| OA-Cu2Te NC | Cu2Te | DOX | DSPE-PEG2000 | None | [105] |
| MoO3−x HNS | MoO3−x HNS | Camptothecin | PEG | None | [106] |
| PB-NP | PB-NP | Artemisinin | Mil-100(Fe) | No | [107] |
| CaCO3 | AuNR | DOX, 17-AAG Afatinib HER-2 antibody Amylase | Phospholipid, POPC Acetalated dextran | None | [108] |
| G (a nano gel) | CuS | DOX | G | None | [109] |
| CTS | Fe3O4 and ICG | DOX | CTS | None | [110] |
| PNIPAM, SBMA, MAA and BAC | ICG | DOX | The same as the frame | None | [111] |
| Fe3O4 | Fe3O4 | DOX | CMCTS | None | [112] |
| Fe3O4@mSiO2 | Fe3O4 and CuS | DOX | PEG | Folic acid | [113] |
| MSN | AuNR | DOX | Hyaluronic acid | None | [114] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Li, A.; Zhang, A.; Sun, Y.; Liu, J. Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers. Nanomaterials 2018, 8, 85. https://doi.org/10.3390/nano8020085
Yan G, Li A, Zhang A, Sun Y, Liu J. Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers. Nanomaterials. 2018; 8(2):85. https://doi.org/10.3390/nano8020085
Chicago/Turabian StyleYan, Guowen, Aihua Li, Aitang Zhang, Yong Sun, and Jingquan Liu. 2018. "Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers" Nanomaterials 8, no. 2: 85. https://doi.org/10.3390/nano8020085




