Rapid Detection of Flusilazole in Pears with Au@Ag Nanoparticles for Surface-Enhanced Raman Scattering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Standard Solutions
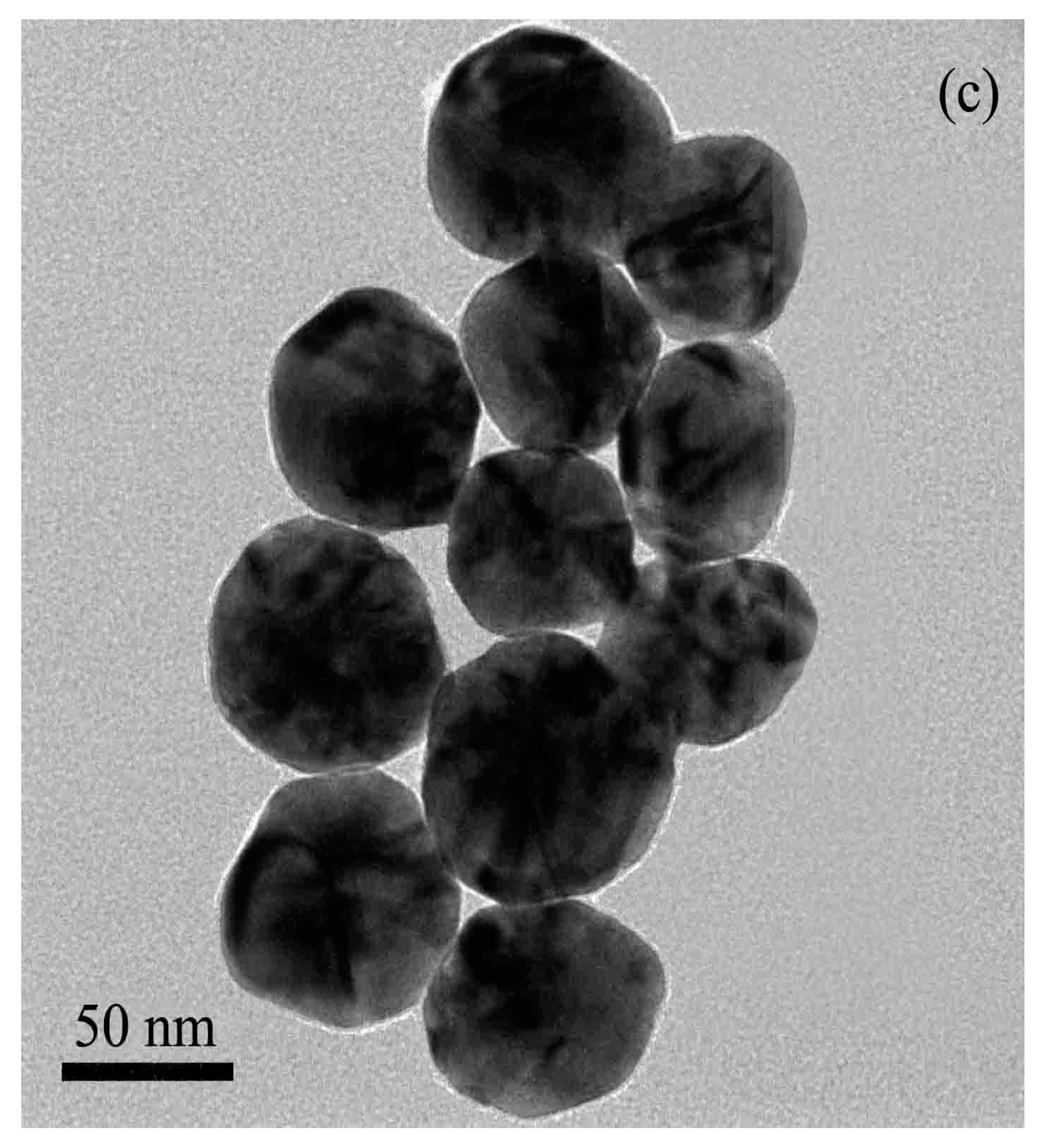
2.2. Synthesis of Au@Ag Nanoparticles
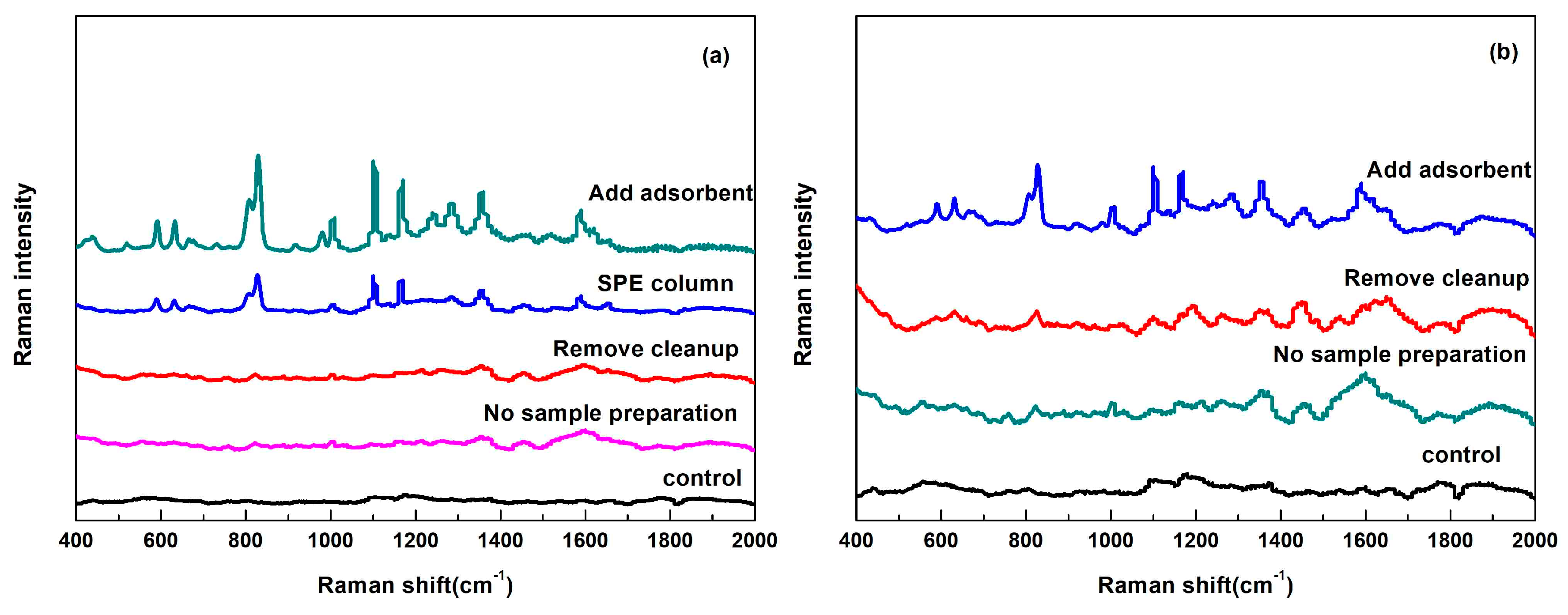
2.3. Sample Preparation
2.4. SERS Measurement
3. Results and Discussion
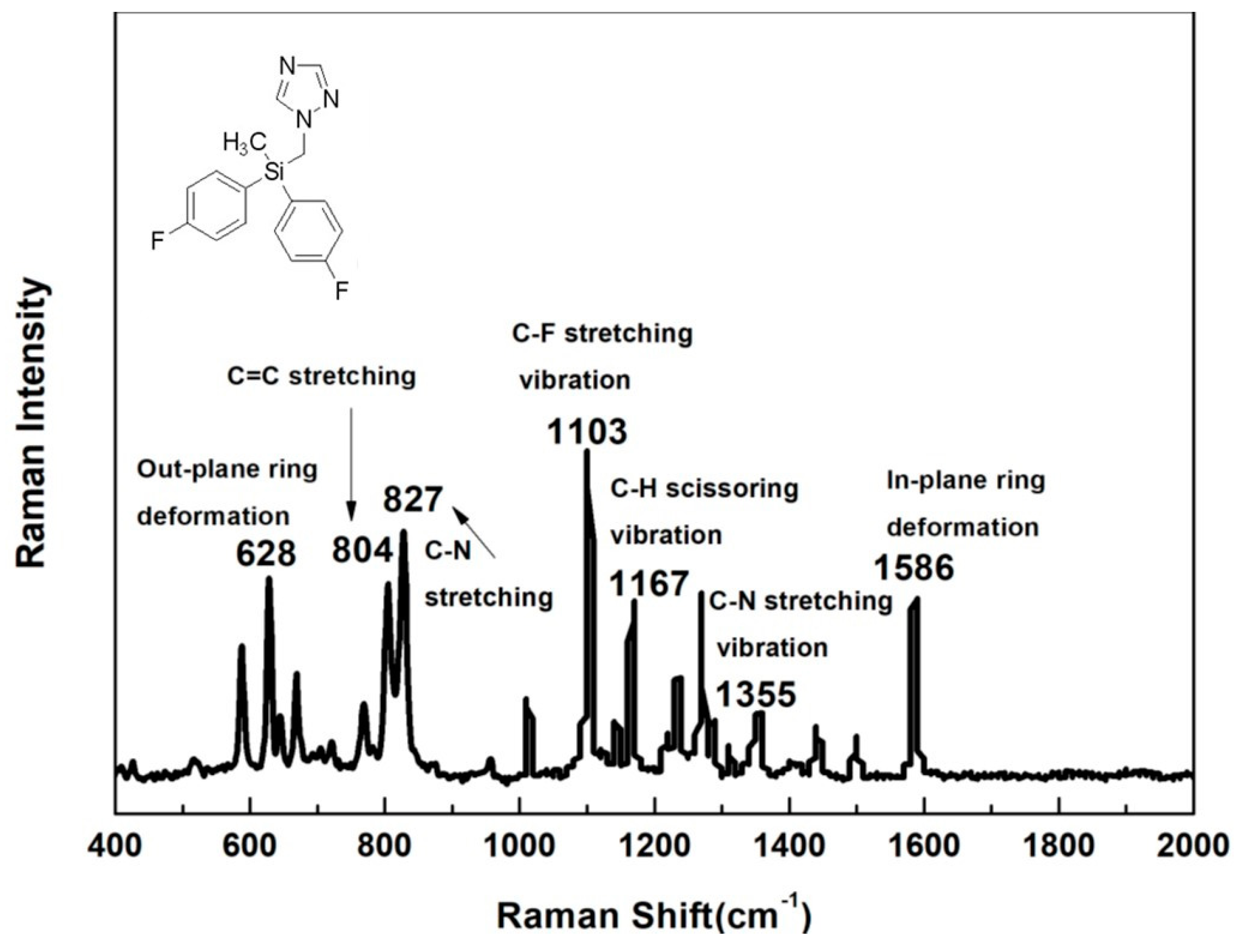
3.1. Spectral Features of Flusilazole
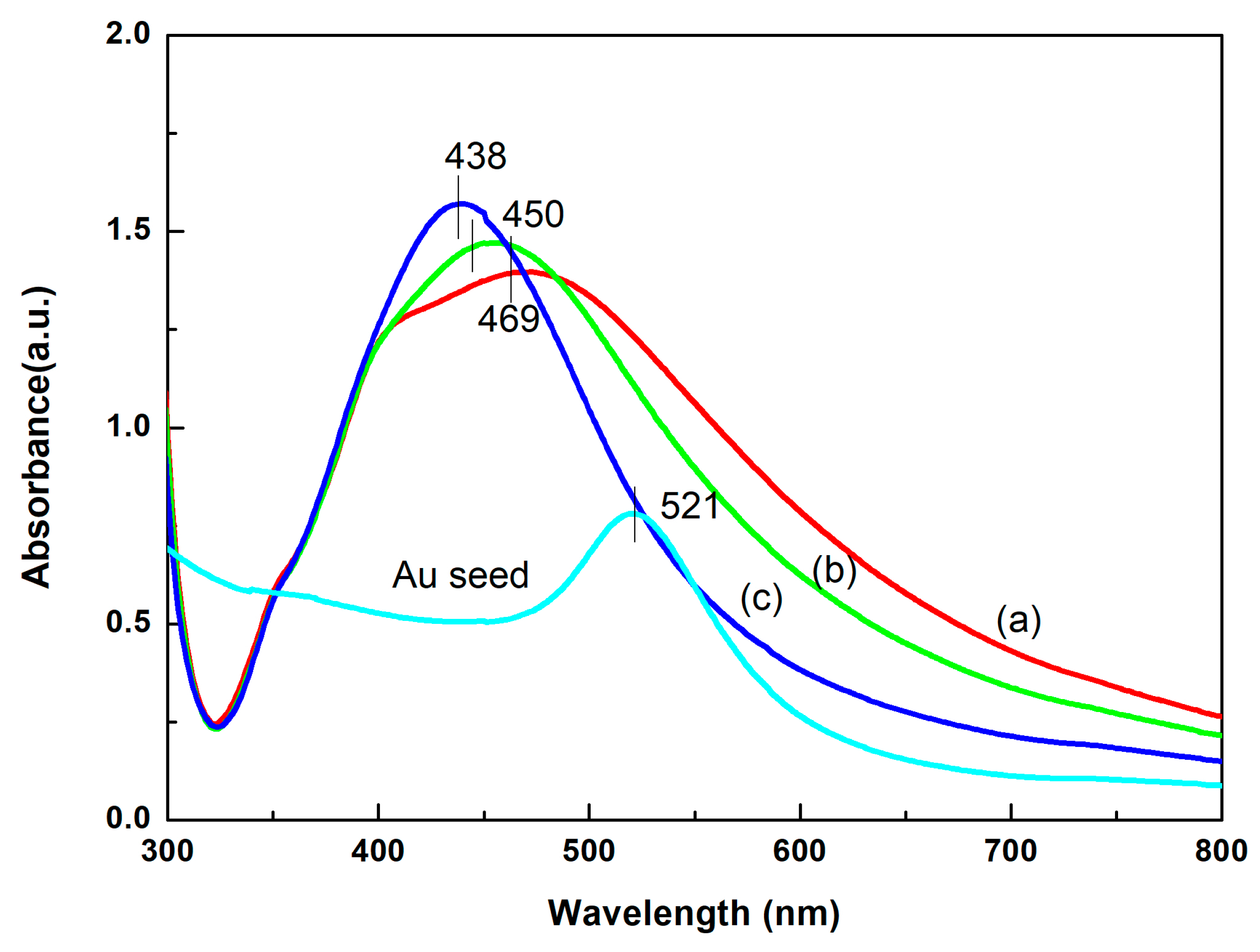
3.2. Selection of Different Size Au@Ag Nanoparticles for Fulsilazole
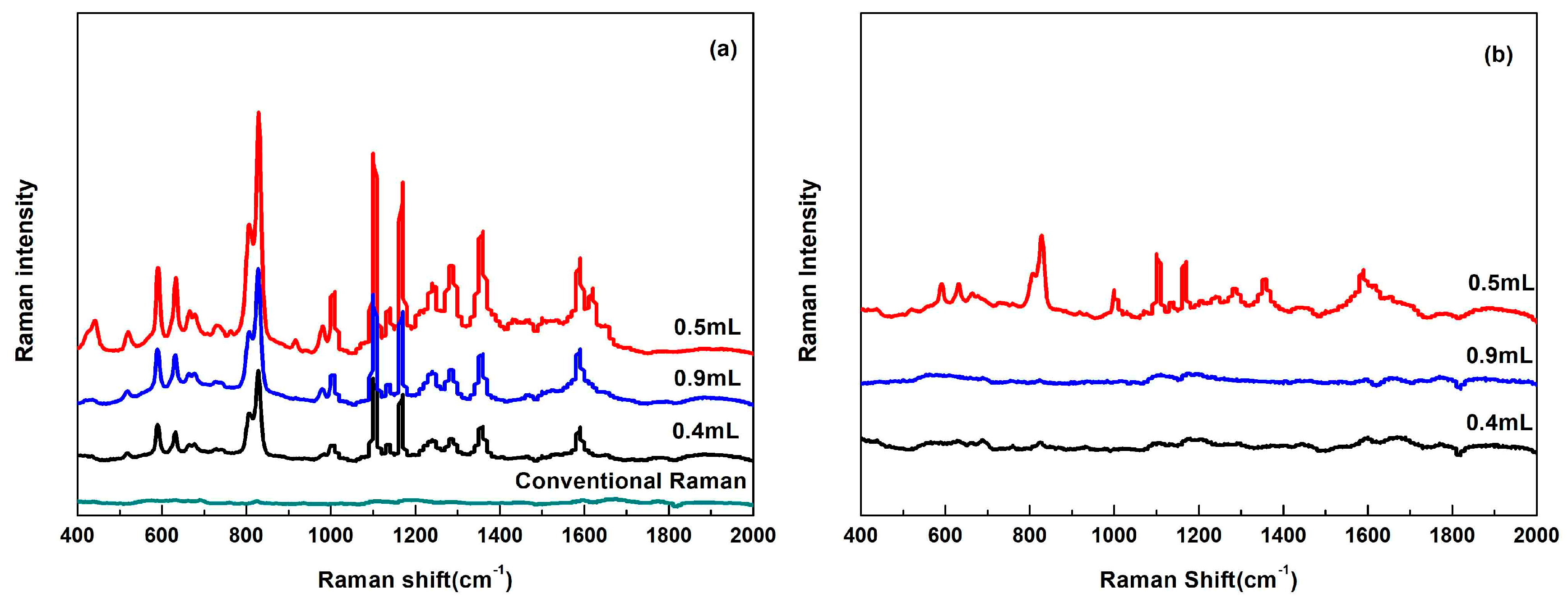
3.3. Analysis of Flusilazole Standard Solutions
3.4. SERS Analysis of Flusilazole in Pears
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozakca, D.U.; Silah, H. Genotoxicity effects of flusilazole on the somatic cells of Allium cepa. Pestic. Biochem. Phys. 2013, 107, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Standards for Pesticide Residue Limits in Foods. Available online: www.fda.gov.tw/tc/includes/GetFile.ashx?mID=172&id=54271 (accessed on 3 October 2017).
- Ma, J.; Yao, Z.; Hou, L.; Lu, W.; Yang, Q.; Li, J.; Chen, L. Metal organic frameworks (MOFs) for magnetic solid-phase extraction of pyrazole/pyrrole pesticides in environmental water samples followed by HPLC-DAD determination. Talanta 2016, 161, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, T.; Zhao, J.; Zhang, L.; Chen, R.; Wu, Y. Determination of eight pesticides in Lycium barbarum by LC-MS/MS and dietary risk assessment. Food Chem. 2017, 218, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.M.; Gadalla, S.A. Development of an efficient method for multi residue analysis of 160 pesticides in herbal plant by ethyl acetate hexane mixture with direct injection to GC-MS/MS. Talanta 2017, 174, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Zhang, Y.; Du, R.; Zhai, F.; Rasco, B.A.; Huang, Y. Determination of chloramphenicol and crystal violet with surface enhanced Raman spectroscopy. Sens. Instrum. Food Qual. 2011, 5, 19–24. [Google Scholar] [CrossRef]
- Lai, K.; Zhai, F.; Zhang, Y.; Wang, X.; Rasco, B.A.; Huang, Y. Application of surface enhanced Raman spectroscopy for analyses of restricted sulfa drugs. Sens. Instrum. Food Qual. 2011, 5, 91–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Pei, L.; Lai, K.; Rasco, B.A.; Huang, Y. Rapid analysis of malachite green and leucomalachite green in fish muscles with surface-enhanced resonance Raman scattering. Food Chem. 2015, 169, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Kim, N.J.; Lin, M. A nanoporous metallic mat showing excellent and stable surface enhanced Raman spectroscopy activities. J. Nanosci. Nanotechnol. 2010, 10, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, M.; Li, H.; Kim, N.J. Surface-enhanced Raman spectroscopy coupled with dendritic silver nanosubstrate for detection of restricted antibiotics. J. Raman Spectrosc. 2010, 41, 739–744. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, X.; Liou, P.; Lin, M. Development of nanofibrillated cellulose coated with gold nanoparticles for measurement of melamine by SERS. Cellulose 2017, 24, 2801–2811. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.J.; Li, J.J.; Li, X.; Zhao, J.W. Multi-branched gold nanostars with fractal structure for SERS detection of the pesticide Thiram. Spectrochim. Acta A 2017, 189, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, C.; Xu, S.; Zhang, C.; Li, Z.; Liu, X.; Jiang, S.; Huo, Y.; Liu, A.; Man, B. Ag2O@Ag core-shell structure on PMMA as low-cost and ultra-sensitive flexible surface-enhanced Raman scattering substrate. J. Alloys Compd. 2017, 695, 1677–1684. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, X.; Zhang, S.J.; Liu, L.; Zhao, Y.M.; Xu, H.J. Ultrasensitive and quantitative detection of paraquat on fruits skins via surface-enhanced Raman spectroscopy. Sens. Actuator B-Chem. 2015, 213, 452–456. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Surface-enhanced Raman spectroscopy coupled with gold nanoparticles for rapid detection of phosmet and thiabendazole residues in apples. Food Control 2016, 68, 229–235. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Analysis of trace methylene blue in fish muscle using ultra-sensitive surface-enhanced Raman spectroscopy. Food Control 2016, 65, 99–105. [Google Scholar] [CrossRef]
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and robust SERS active substrates for conformal rapid detection of pesticide residues from fruits. Sens. Actuator B-Chem. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Lu, L.; Wang, H.; Zhou, Y.; Xi, S.; Zhang, H.; Hu, J.; Zhao, B. Seed-mediated growth of large, monodisperse core-shell gold-silver nanoparticles with Ag-like optical properties. Chem. Commun. 2002, 2, 144–145. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.W.; Qin, D. Galvanic replacement-freedeposition of Au on Ag for core−shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef] [PubMed]
- Samal, A.K.; Polavarapu, L.; Rodalcedeira, S.; Lizmarzán, L.M.; Pérezjuste, J.; Pastorizasantos, I. Size tunable Au@Ag core-shell nanoparticles: Synthesis and surface-enhanced Raman scattering properties. Langmuir 2013, 29, 15076–15082. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Ou, Y.; Yu, W.; Fan, Y.; Huang, Y.; Lai, K. Au-Ag core-shell nanospheres for surface-enhanced Raman scattering detection of Sudan I and Sudan II in chili powder. J. Nanomater. 2015, 16, 215. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Lehotay, S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [PubMed]
- Tang, H.; Li, Q.; Ren, Y.; Geng, J.; Cao, P.; Sui, T.; Wang, X.; Du, Y. Surface enhanced Raman spectroscopy signals of mixed pesticides and their identification. Chin. Chem. Lett. 2011, 22, 1477–1480. [Google Scholar] [CrossRef]
- Pan, Y.C.; Wen, Y.; Zhang, R.; Wang, Y.Y.; Zhang, Z.R.; Yang, H.F. Electrochemical and SERS spectroscopic investigations of 4-methyl-4h-1,2,4-triazole-3-thiol monolayers self-assembled on copper surface. Appl. Surf. Sci. 2012, 258, 3956–3961. [Google Scholar] [CrossRef]
- Sun, Y.; Song, W.; Zhu, X.; Zhang, R.; Pang, Q.; Zhang, Z.; Yang, H. Electrochemical and in situ SERS spectroelectrochemical investigations of 4-methyl-4h-1,2,4-triazole-3-thiol monolayers at a silver electrode. J. Raman Spectrosc. 2010, 40, 1306–1311. [Google Scholar] [CrossRef]
- Boca, S.C.; Farcau, C.; Astilean, S. The study of Raman enhancement efficiency as function of nanoparticle size and shape. Nucl. Instrum. Methods B 2009, 267, 406–410. [Google Scholar] [CrossRef]
- Güzel, R.; Üstündağ, Z.; Ekşi, H.; Keskin, S.; Taner, B.; Durgun, Z.G.; Turan, A.A.; Solak, A.O. Effect of Au and Au@Ag core–shell nanoparticles on the SERS of bridging organic molecules. J. Colloid Interface Sci. 2010, 351, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Njoki, P.N.; Lim, I.S.; Mott, D.; Park, H.Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C. Size correlation of optical and spectroscopic properties for gold nanoparticles. J. Phys Chem. C. 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Smith, W.E. Practical understanding and use of surface enhanced Raman scattering/surface enhanced resonance Raman scattering in chemical and biological analysis. Chem. Soc. Rev. 2008, 37, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Z.; Cao, X.; Su, H.; Shao, H.; Jin, F.; Add EI-Aty, A.M.; Wang, J. Simultaneous determination of three pesticide adjuvant residues in plant-derived agro-products using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1528, 53–60. [Google Scholar] [CrossRef] [PubMed]


| - | Peaks/cm−1 | Regression Equation | R2 |
|---|---|---|---|
| Standard Solution | 632 | Y = 1300.06x + 513.57 | 0.924 |
| - | 807 | Y = 2186.26x + 498.59 | 0.956 |
| - | 829 | Y = 4242.81x + 1044.33 | 0.951 |
| - | 1103 | Y = 3644.11x + 783.79 | 0.952 |
| - | 1168 | Y = 2940.66x + 549.86 | 0.962 |
| - | 1358 | Y = 1677.96x + 368.57 | 0.946 |
| - | 1588 | Y = 1511.46x + 564.80 | 0.924 |
| Absorber Dosage | Flusilazole’s Concentration | ||
|---|---|---|---|
| MgSO4 | PSA | C18 | 0.1 μg/g |
| 0.45 g | 0.1 g | 0.1 g | - |
| 0.6 g | 0.1 g | 0.1 g | - |
| 0.6 g | 0.2 g | 0.1 g | - |
| 0.6 g | 0.2 g | 0.2 g | + |
| 0.45 g | 0.2 g | 0.2 g | - |
| - | Peaks/cm−1 | Regression Equation | R2 |
|---|---|---|---|
| Pear Extracts | 632 | Y = 232.05x + 219.72 | 0.922 |
| - | 808 | Y = 365.26x + 302.72 | 0.886 |
| - | 829 | Y = 749.85x + 533.64 | 0.914 |
| - | 1103 | Y = 655.32x + 557.58 | 0.921 |
| - | 1167 | Y = 442.63x + 525.25 | 0.879 |
| - | 1356 | Y = 301.85x + 450.82 | 0.741 |
| - | 1589 | Y = 216.17x + 350.04 | 0.708 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Huang, Y.; Fan, Y.; Lai, K. Rapid Detection of Flusilazole in Pears with Au@Ag Nanoparticles for Surface-Enhanced Raman Scattering. Nanomaterials 2018, 8, 94. https://doi.org/10.3390/nano8020094
Zhao Z, Huang Y, Fan Y, Lai K. Rapid Detection of Flusilazole in Pears with Au@Ag Nanoparticles for Surface-Enhanced Raman Scattering. Nanomaterials. 2018; 8(2):94. https://doi.org/10.3390/nano8020094
Chicago/Turabian StyleZhao, Zhihui, Yiqun Huang, Yuxia Fan, and Keqiang Lai. 2018. "Rapid Detection of Flusilazole in Pears with Au@Ag Nanoparticles for Surface-Enhanced Raman Scattering" Nanomaterials 8, no. 2: 94. https://doi.org/10.3390/nano8020094




