Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles
Abstract
:1. Introduction
2. Results
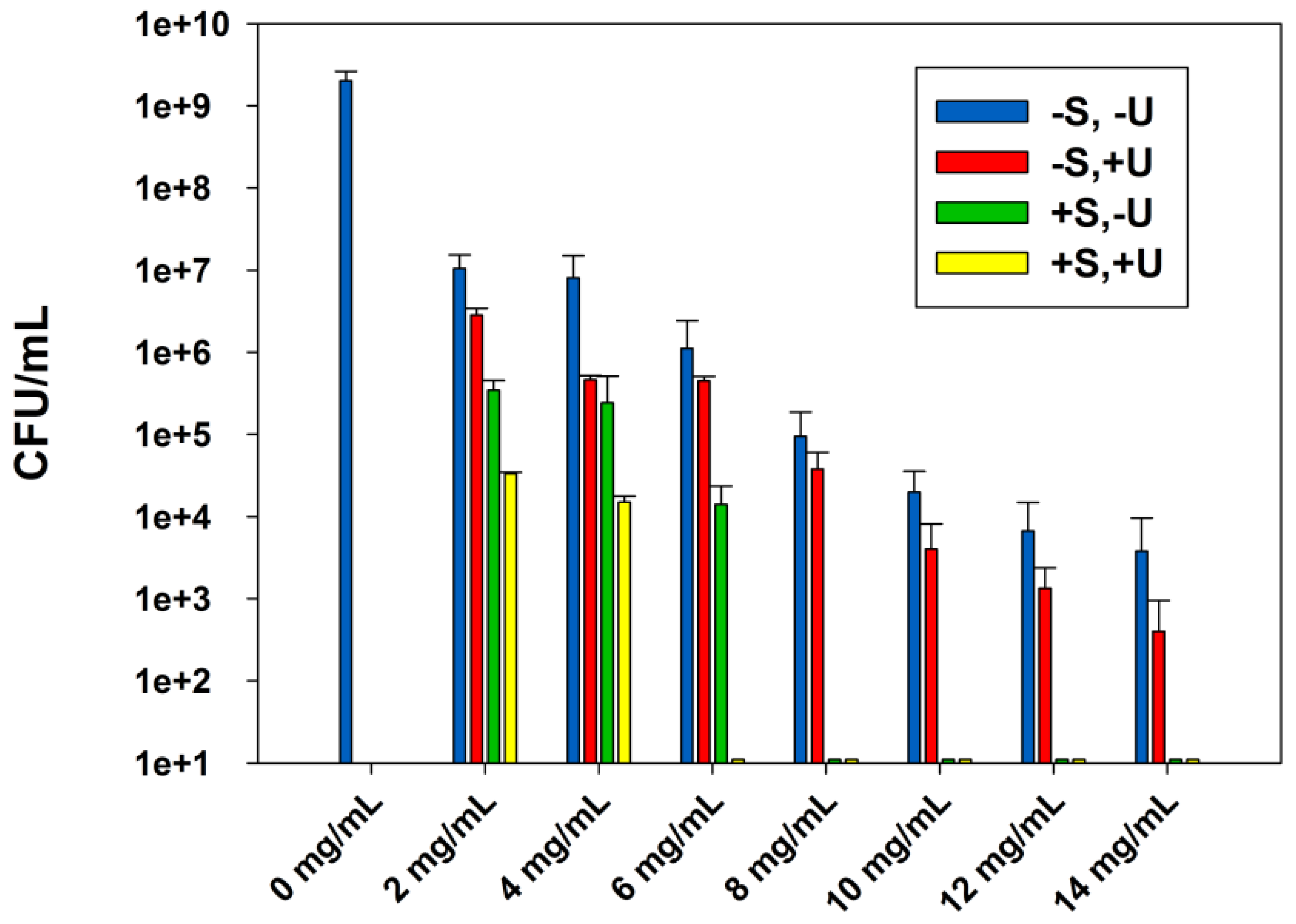
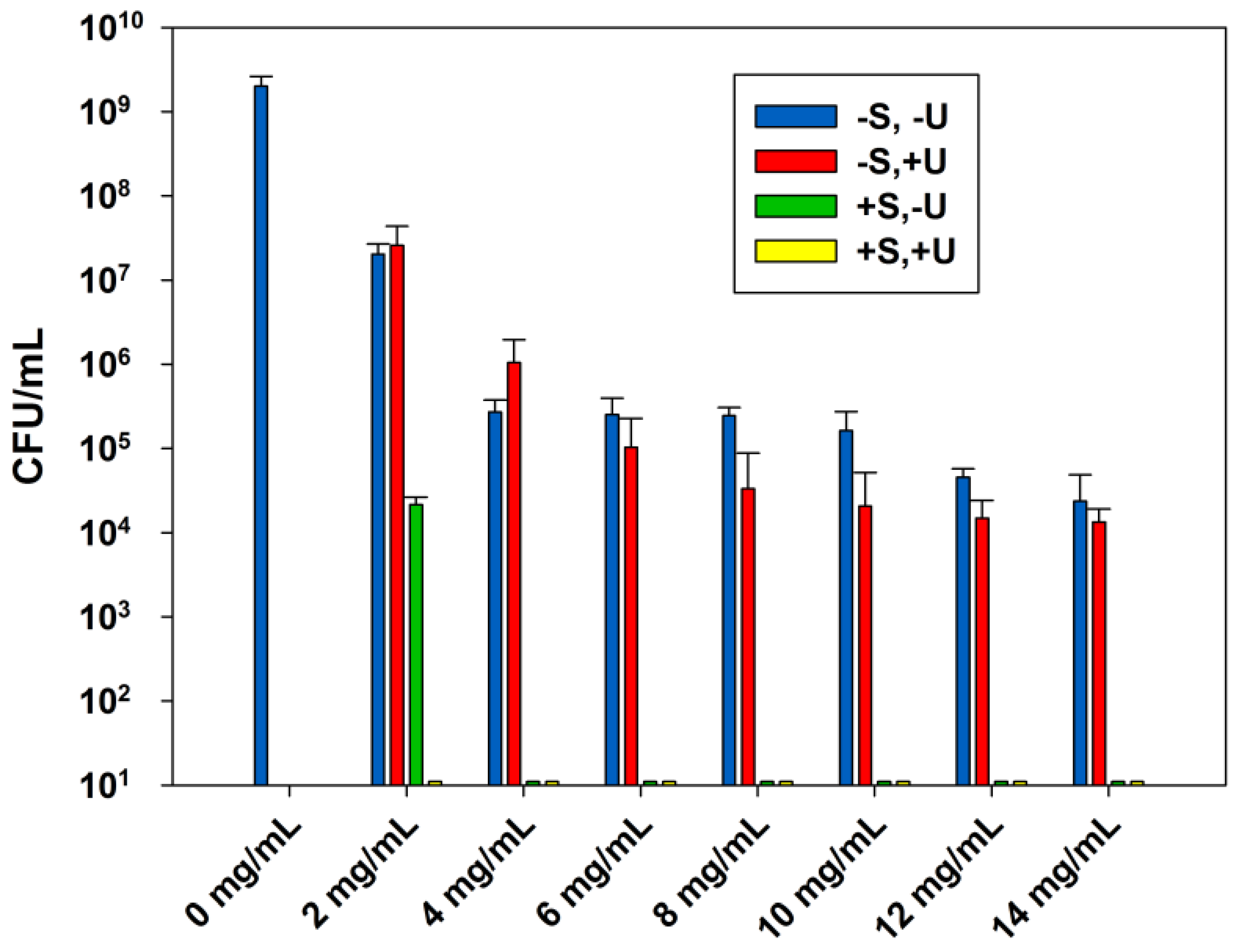
2.1. Effect of the Combined Effect of Sterilization and Ultrasound Stimulations on the ABA of Zinc Oxide Suspensions
2.2 MICs and MBCs of the Zinc Oxide Suspensions
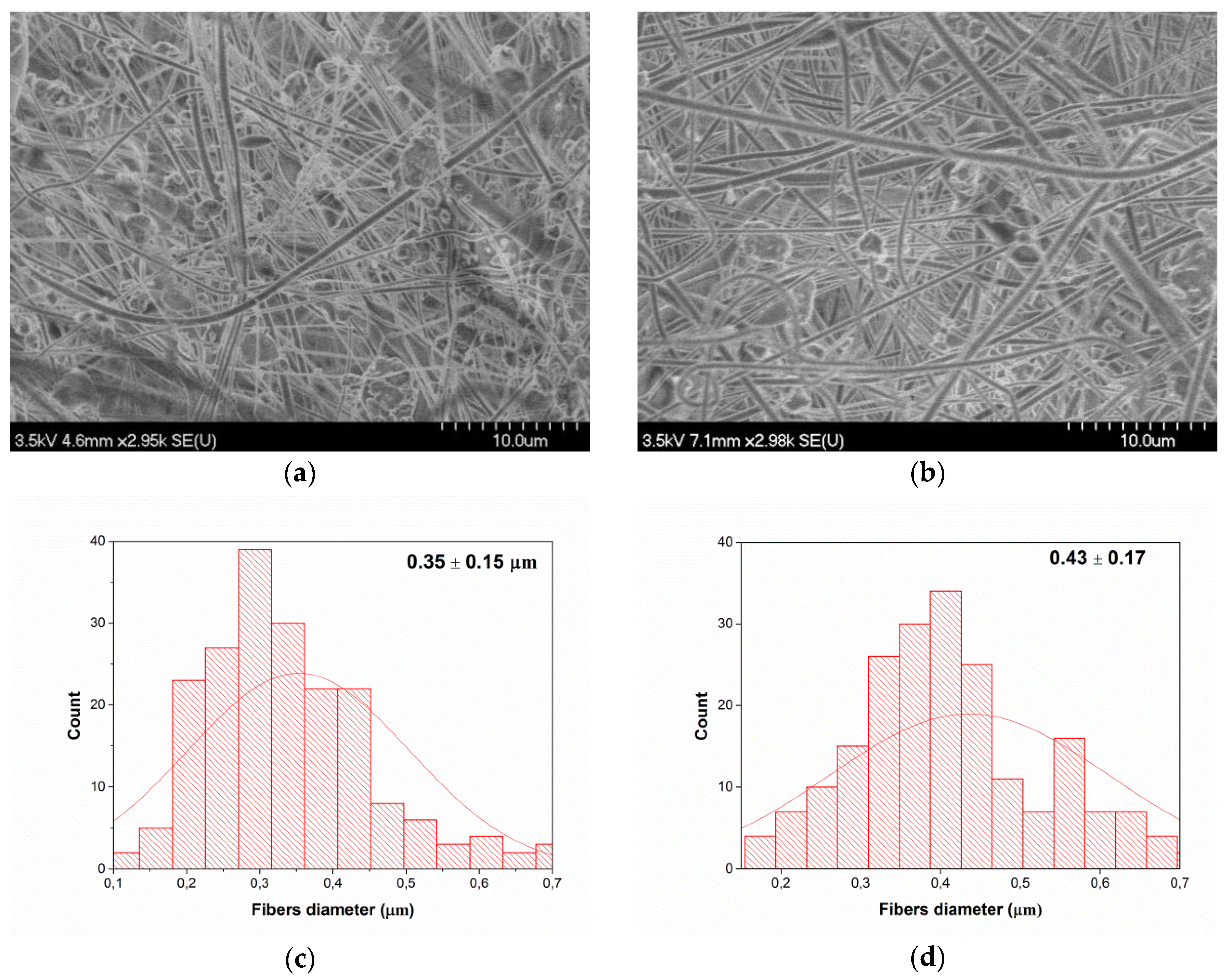
2.3. Fiber Porosity Measurement
2.4. Thermogravimetric Analysis (TGA)
2.5. Scanning Electron Microscopy (SEM)
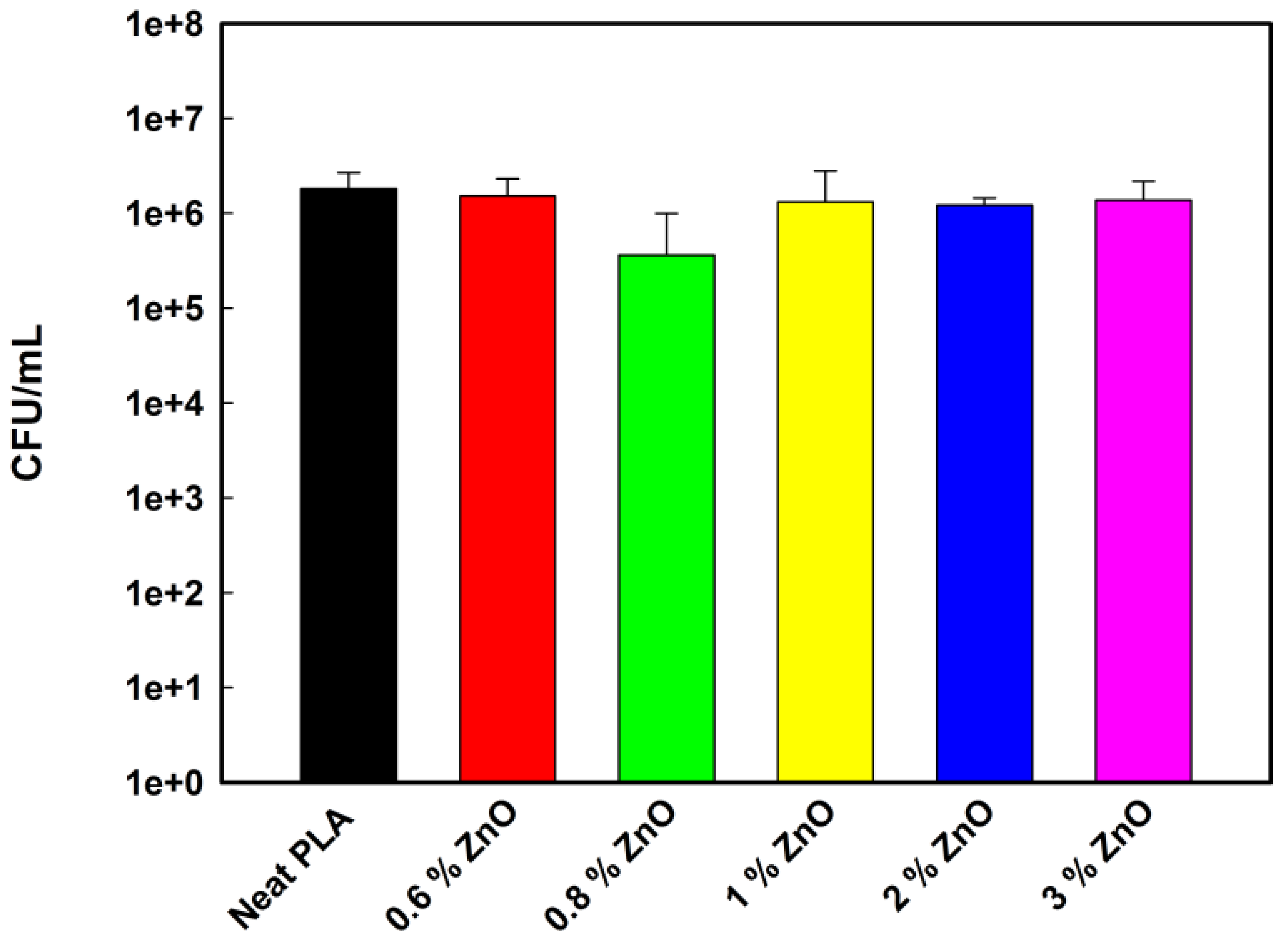
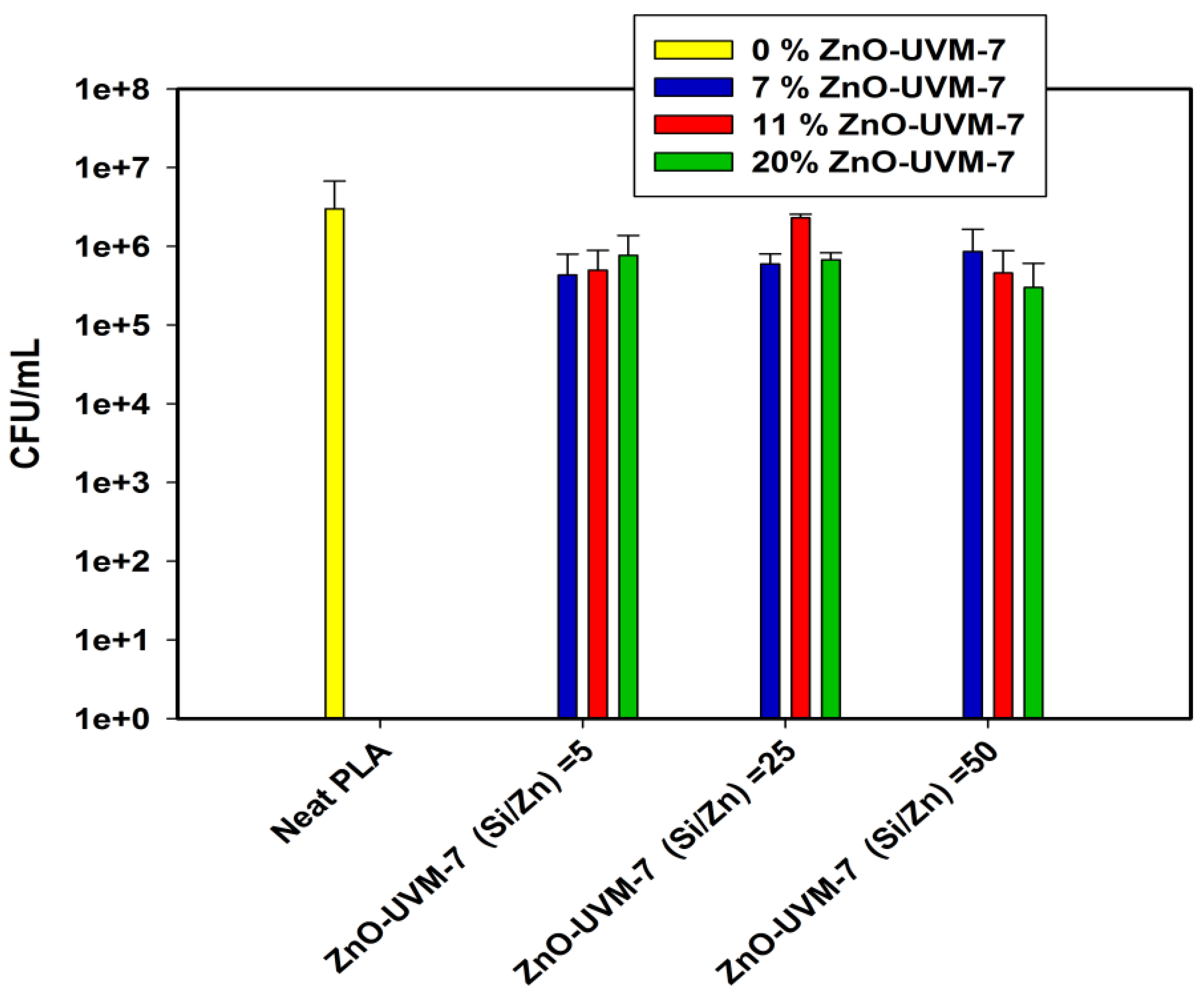
2.6. ABA of PLA-Based Nanofibers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Solutions Preparation
4.3. Electrospinning Process
4.4. Scanning Electron Microscopy (SEM)
4.5. Thermogravimetric Analysis (TGA)
4.6. Fiber's Porosity Measurement
4.7. Antibacterial Test
4.7.1. Effect of the Combined Effect of Sterilization and Ultrasound Stimulations on the ABA of the Zinc Oxide Suspensions
4.7.2. In Vitro Antibacterial Efficiency of Nanocomposite PLA Nanofibers
5. Conclusions
Acknowledgment
Author Contributions
Conflict of Interest
References
- Tawakkal, I.S.; Cran, M.J.; Miltz, J.; Bigger, S.W. A review of poly (lactic acid)-based materials for antimicrobial packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kim, S.H.; Lee, C.H. Electrospinning of polylactide fibers containing silver nanoparticles. Macromol. Res. 2010, 18, 215–221. [Google Scholar] [CrossRef]
- Conn, R.; Kolstad, J.; Borzelleca, J.; Dixler, D.; Filer, L.; LaDu, B.; Pariza, M. Safety assessment of polylactide (pla) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Li, D.; Frey, M.W.; Baeumner, A.J. Electrospun polylactic acid nanofiber membranes as substrates for biosensor assemblies. J. Membrane Sci. 2006, 279, 354–363. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-food packaging: An overview of market, migration research, and safety regulations. J. Food Sci. 2015, 80. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of zno nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of zno nanoparticles: A review. Food Addit. Contam. A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of zno nanoparticles (zno nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (zno, mgo and cao) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing ag and zno on inactivation of lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing ag and zno on shelf life of fresh orange juice. Innov. Food Sci. Emerg. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Electrospinning and electrospraying techniques for designing antimicrobial polymeric biocomposite mats. Nanofiber Res. 2016, 91. [Google Scholar]
- Shalumon, K.; Anulekha, K.; Nair, S.V.; Nair, S.; Chennazhi, K.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano zno composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone membranes incorporated with zno nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv. 2014, 4, 24777–24785. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Effect of zinc oxide nanoparticles on the in vitro degradation of electrospun polycaprolactone membranes in simulated body fluid. Int. J. Polym. Mater. PO 2016, 65, 28–37. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. Electrospinning: A comparative study on the design of poly (l-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Improvement of mechanical properties and antibacterial activity of electrospun poly(d,l-lactide)-based mats by incorporation of zno-graft-poly(d,l-lactide) nanoparticles. Mater. Chem. Phys. 2016, 182, 324–331. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Grande, D. Novel antibacterial electrospun mats based on poly(d,l-lactide) nanofibers and zinc oxide nanoparticles. J. Mater. Sci. 2014, 49, 8373–8385. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of zno nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mat. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of zno nanoparticles against e. Coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. Zno nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Wahab, R.; Mishra, A.; Yun, S.-I.; Hwang, I.; Mussarat, J.; Al-Khedhairy, A.A.; Kim, Y.-S.; Shin, H.-S. Fabrication, growth mechanism and antibacterial activity of zno micro-spheres prepared via solution process. Biomass Bioenerg. 2012, 39, 227–236. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against escherichia coli o157: H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Saliani, M.; Jalal, R.; Goharshadi, E.K. Effects of ph and temperature on antibacterial activity of zinc oxide nanofluid against escherichia coli o157: H7 and staphylococcus aureus. Jundishapur J. Microb. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of zno nanoparticles—the role of ros mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on escherichia coli bacteria in ultrafine zno nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale tio 2, sio 2, and zno water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. Zno nanofluids–a potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against campylobacter jejuni. Appl. Environ. Microb. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Seil, J.T.; Webster, T.J. Antibacterial effect of zinc oxide nanoparticles combined with ultrasound. Nanotechnology 2012, 23, 495101. [Google Scholar] [CrossRef] [PubMed]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of zno nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011, 5, 1368–1373. [Google Scholar]
- Tayel, A.A.; EL-TRAS, W.F.; Moussa, S.; EL-BAZ, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Ohira, T.; Yamamoto, O. Correlation between antibacterial activity and crystallite size on ceramics. Chem. Eng. Sci. 2012, 68, 355–361. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, R.; Huang, T.; Worley, S. Polymerization of a hydantoinylsiloxane on particles of silicon dioxide to produce a biocidal sand. J. Appl. Polym. Sci. 2005, 97, 1161–1166. [Google Scholar] [CrossRef]
- Spoiala, A.; Nedelcu, I.; Ficai, D.; Ficai, A.; Andronescu, E. Zinc based antibacterial formulations for cosmetic applications. Dig. J. Nanomater. Bios. 2013, 8, 1235. [Google Scholar]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Applerot, G.; Perkas, N.; Amirian, G.; Girshevitz, O.; Gedanken, A. Coating of glass with zno via ultrasonic irradiation and a study of its antibacterial properties. Appl. Surf. Sci. 2009, 256, S3–S8. [Google Scholar] [CrossRef]
- Binan, L.; Tendey, C.; De Crescenzo, G.; El Ayoubi, R.; Ajji, A.; Jolicoeur, M. Differentiation of neuronal stem cells into motor neurons using electrospun poly-l-lactic acid/gelatin scaffold. Biomaterials 2014, 35, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Hadjizadeh, A.; Savoji, H.; Ajji, A. A facile approach for the mass production of submicro/micro poly (lactic acid) fibrous mats and their cytotoxicity test towards neural stem cells. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- El Haskouri, J.; Dallali, L.; Fernández, L.; Garro, N.; Jaziri, S.; Latorre, J.; Guillem, C.; Beltrán, A.; Beltrán, D.; Amorós, P. Zno nanoparticles embedded in uvm-7-like mesoporous silica materials: Synthesis and characterization. Phys. E 2009, 42, 25–31. [Google Scholar] [CrossRef]
- El Haskouri, J.; de Zárate, D.O.; Guillem, C.; Latorre, J.; Caldés, M.; Beltrán, A.; Beltrán, D.; Descalzo, A.B.; Rodríguez-López, G.; Martínez-Máñez, R. Silica-based powders and monoliths with bimodal pore systems. Chem. Commun. 2002, 330–331. [Google Scholar] [CrossRef]
- Cabrera, S.; El Haskouri, J.; Guillem, C.; Latorre, J.; Beltrán-Porter, A.; Beltrán-Porter, D.; Marcos, M.D.; Amoros, P. Generalised syntheses of ordered mesoporous oxides: The atrane route. Solid State Sci. 2000, 2, 405–420. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.C.; Giliberto, J.P.; Mrowczynski, W.; Gurny, R.; Walpoth, B.H.; Möller, M. Advantages of bilayered vascular grafts for surgical applicability and tissue regeneration. Acta Biomater. 2012, 8, 3914–3920. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Lerouge, S.; Ajji, A.; Wertheimer, M.R. Plasma-etching for controlled modification of structural and mechanical properties of electrospun pet scaffolds. Plasma Process Polym. 2015, 12, 314–327. [Google Scholar] [CrossRef]
| Treatment Antibacterial NPs | −S,−U | −S,+U | +S,−U | +S,+U | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| ZnO | 2 | >14 | 2 | >14 | 2 | 8 | 2 | 6 |
| ZnO-UVM-7 | 2 | >14 | 2 | >14 | 2 | 4 | <2 | 2 |
| Samples | Porosity ɛ (%) |
|---|---|
| Neat PLA nanofibers | 90.08 |
| PLA/0.8 wt % ZnO nanofibers | 87.48 |
| PLA/3 wt % ZnO nanofibers | 87.07 |
| PLA/0.6 wt % ZnO-UVM-7 (Si/Zn = 5) nanofibers | 86.95 |
| PLA/0.8 wt % ZnO-UVM-7 (Si/Zn = 5) nanofibers | 84.59 |
| ZnO Content | ZnO Weight in Solutions (mg) | ZnO Weight in PLA Nanocomposites Mats (mg) | Percentage of ZnO in the PLA Nanocomposite Mats (%) (Compared to 100% if No Loss) |
|---|---|---|---|
| PLA/0.6 wt % ZnO | 12 | 9.49 | 79.08 |
| PLA/0.8 wt % ZnO | 16 | 10.13 | 63.31 |
| PLA/1 wt % ZnO | 20 | 4.31 | 21.55 |
| PLA/2 wt % ZnO | 24 | 5.48 | 22.83 |
| PLA/3 wt % ZnO | 28 | 8.03 | 28.67 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokbani, H.; Daigle, F.; Ajji, A. Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles. Nanomaterials 2018, 8, 129. https://doi.org/10.3390/nano8030129
Rokbani H, Daigle F, Ajji A. Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles. Nanomaterials. 2018; 8(3):129. https://doi.org/10.3390/nano8030129
Chicago/Turabian StyleRokbani, Hajer, France Daigle, and Abdellah Ajji. 2018. "Combined Effect of Ultrasound Stimulations and Autoclaving on the Enhancement of Antibacterial Activity of ZnO and SiO2/ZnO Nanoparticles" Nanomaterials 8, no. 3: 129. https://doi.org/10.3390/nano8030129





