Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with β-Cyclodextrin
Abstract
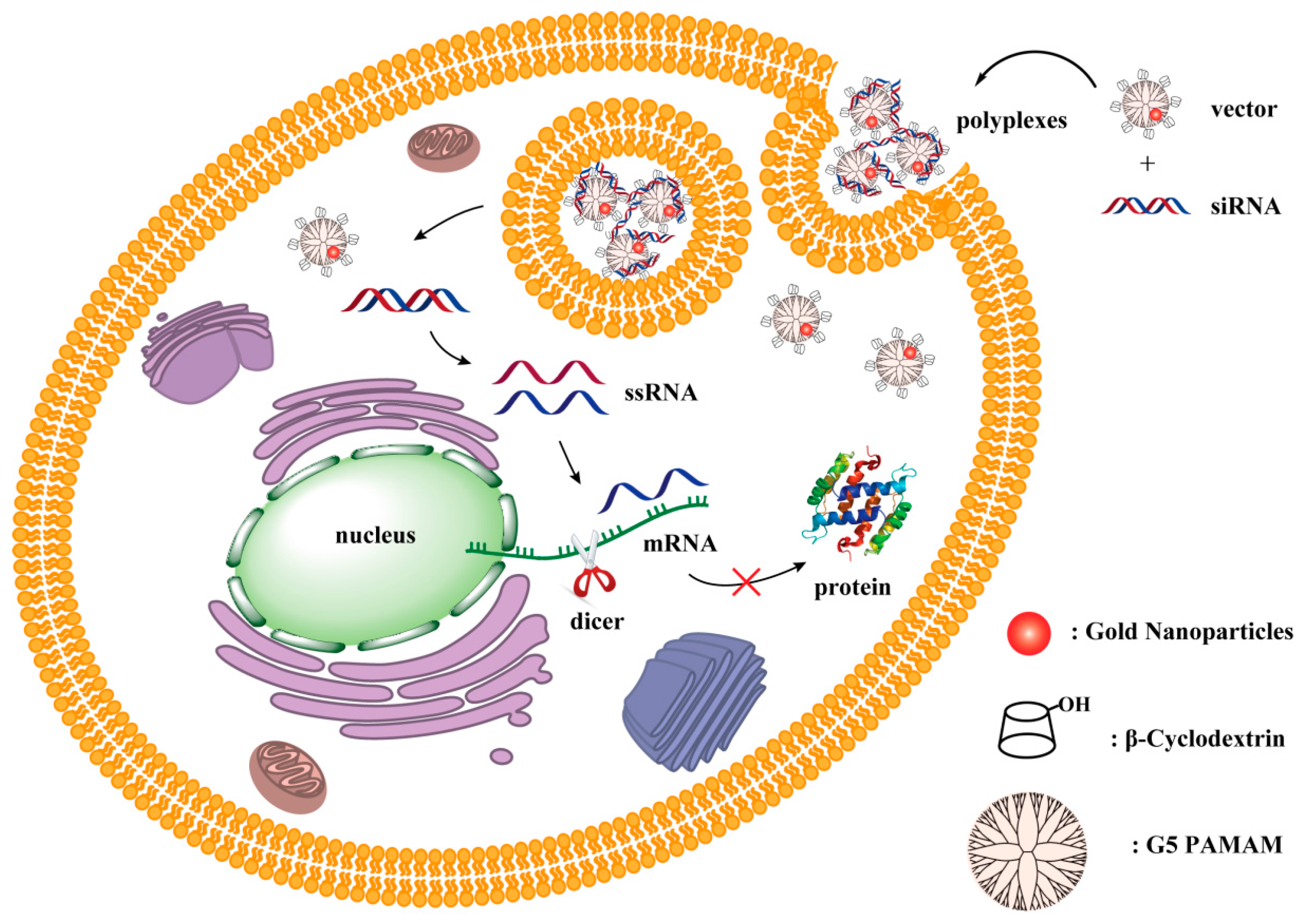
:1. Introduction
2. Results and Discussion
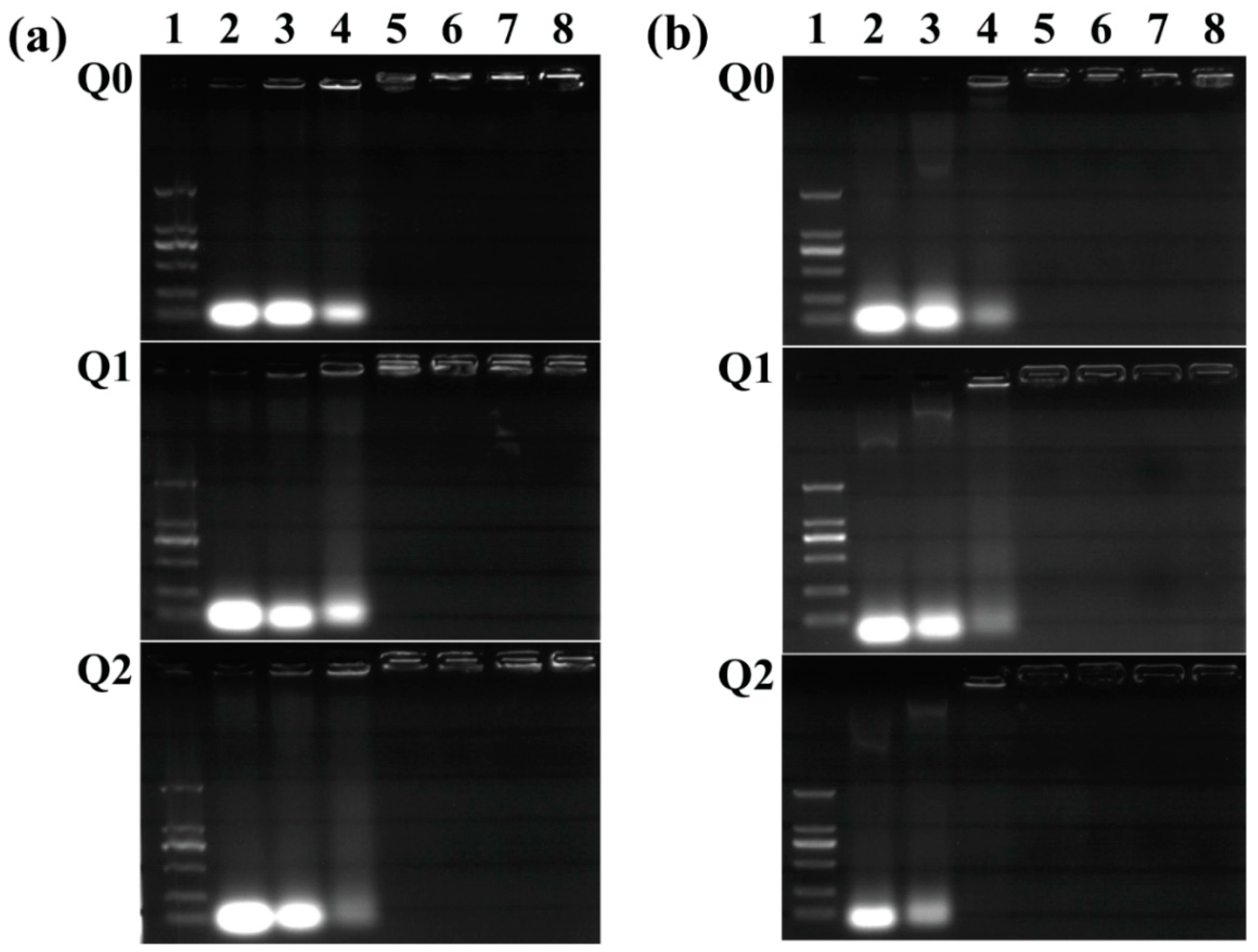
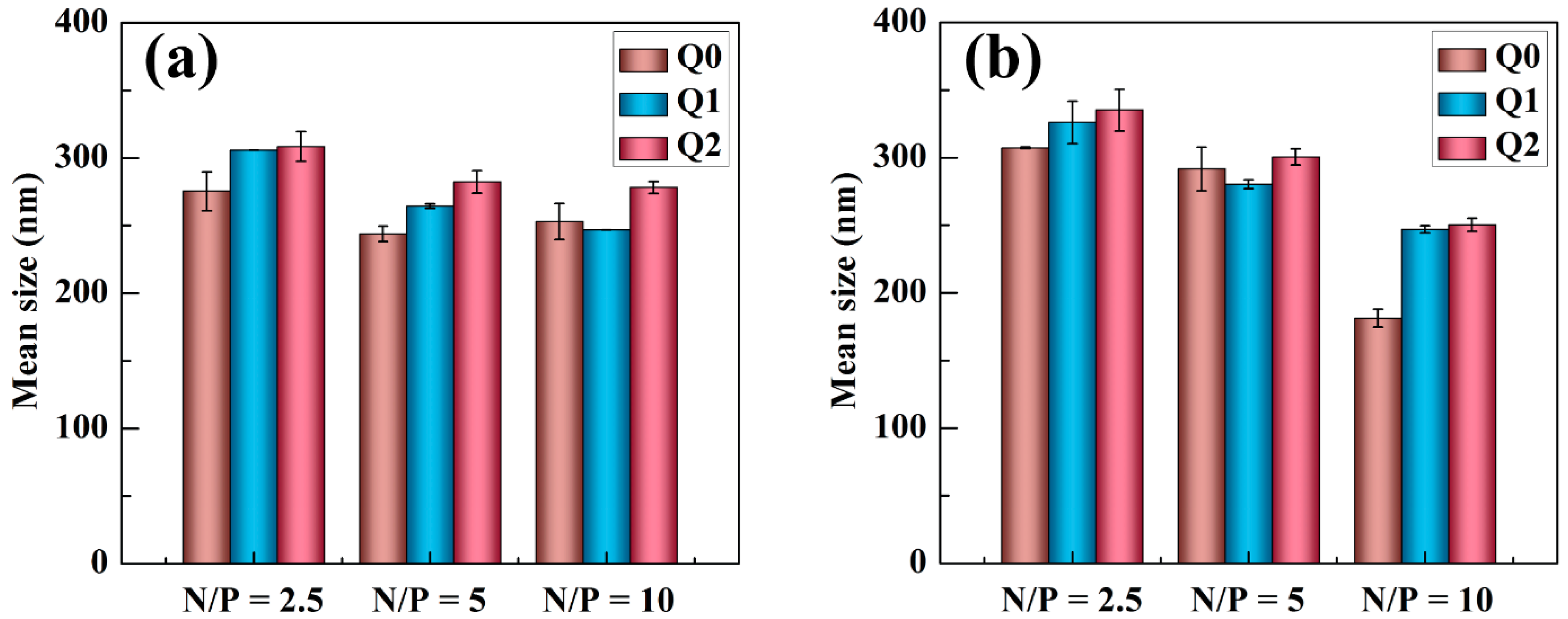
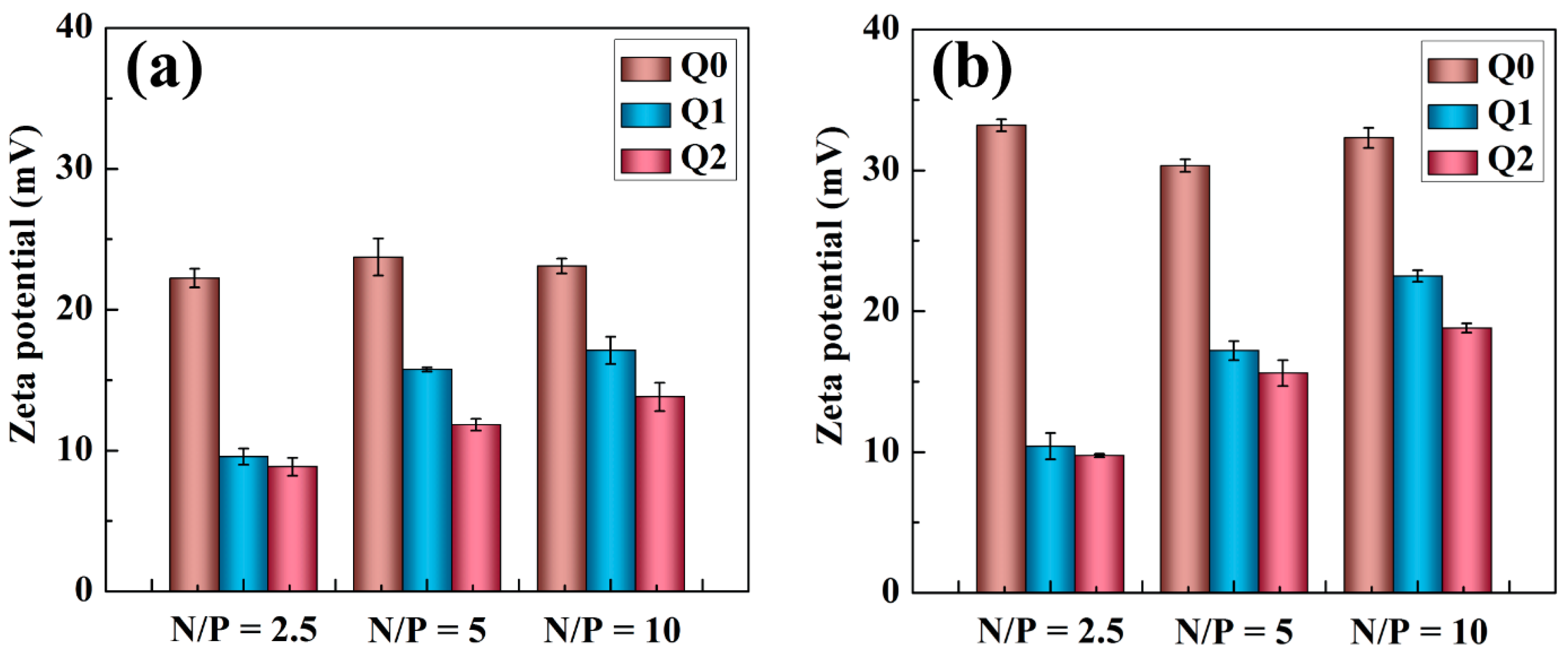
2.1. Characterization of Vector/siRNA Polyplexes
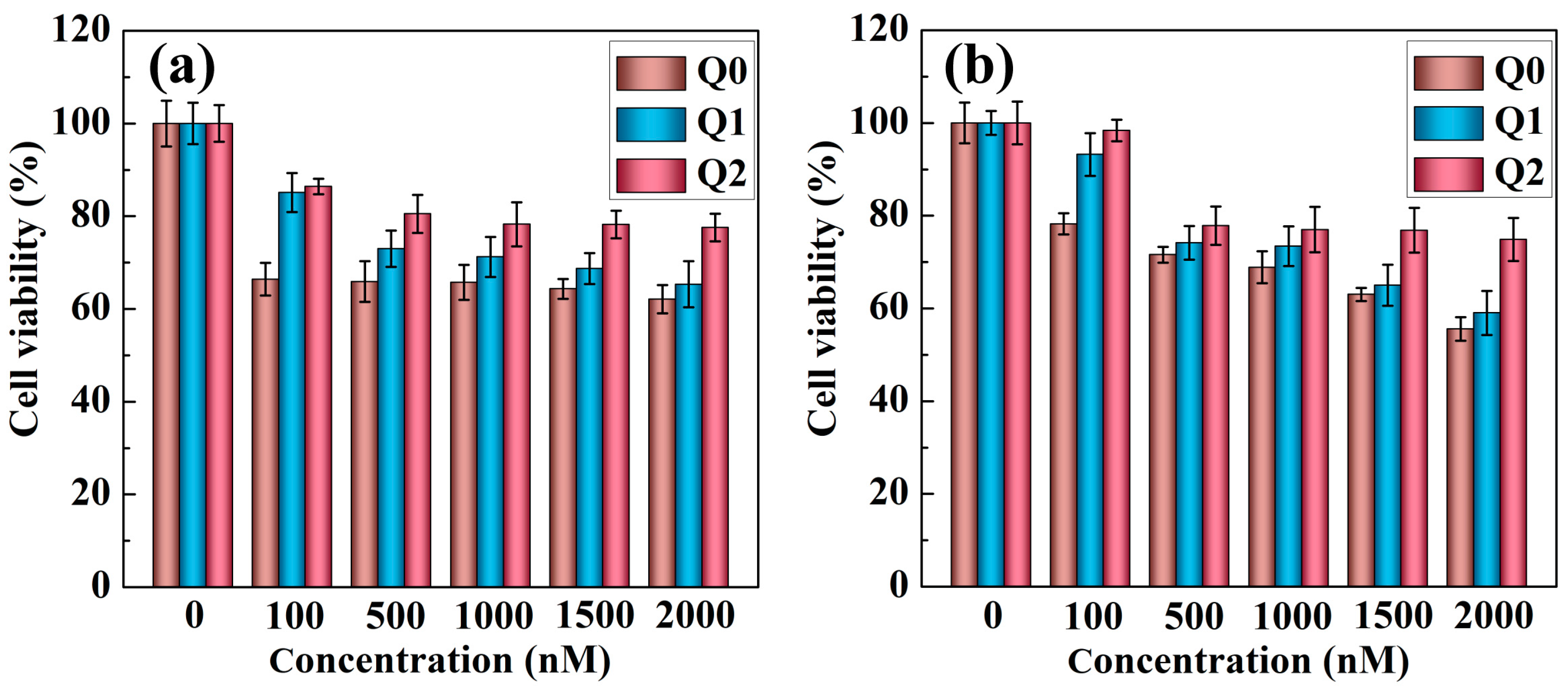
2.2. Cytotoxicity Assay
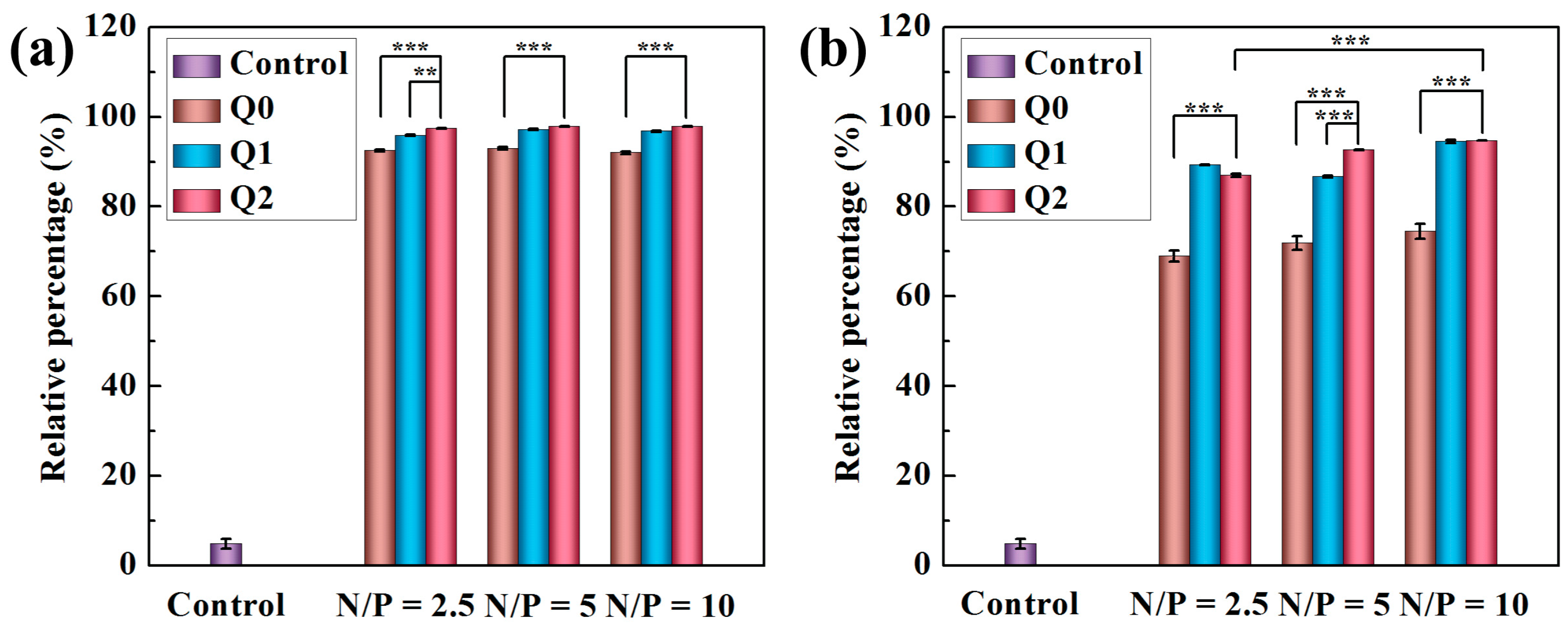
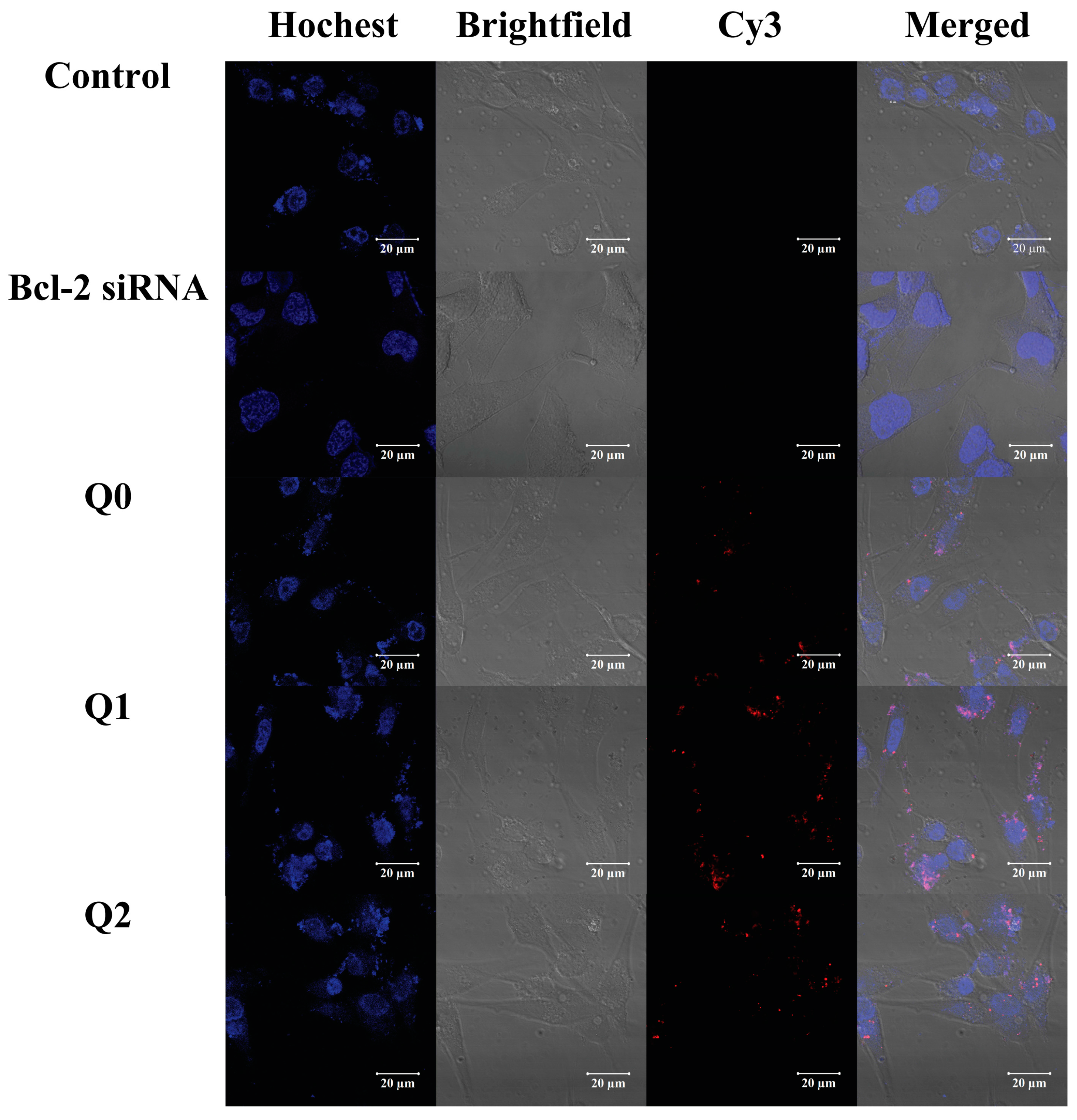
2.3. Uptake of Vector/siRNA Polyplexes by Cancer Cells
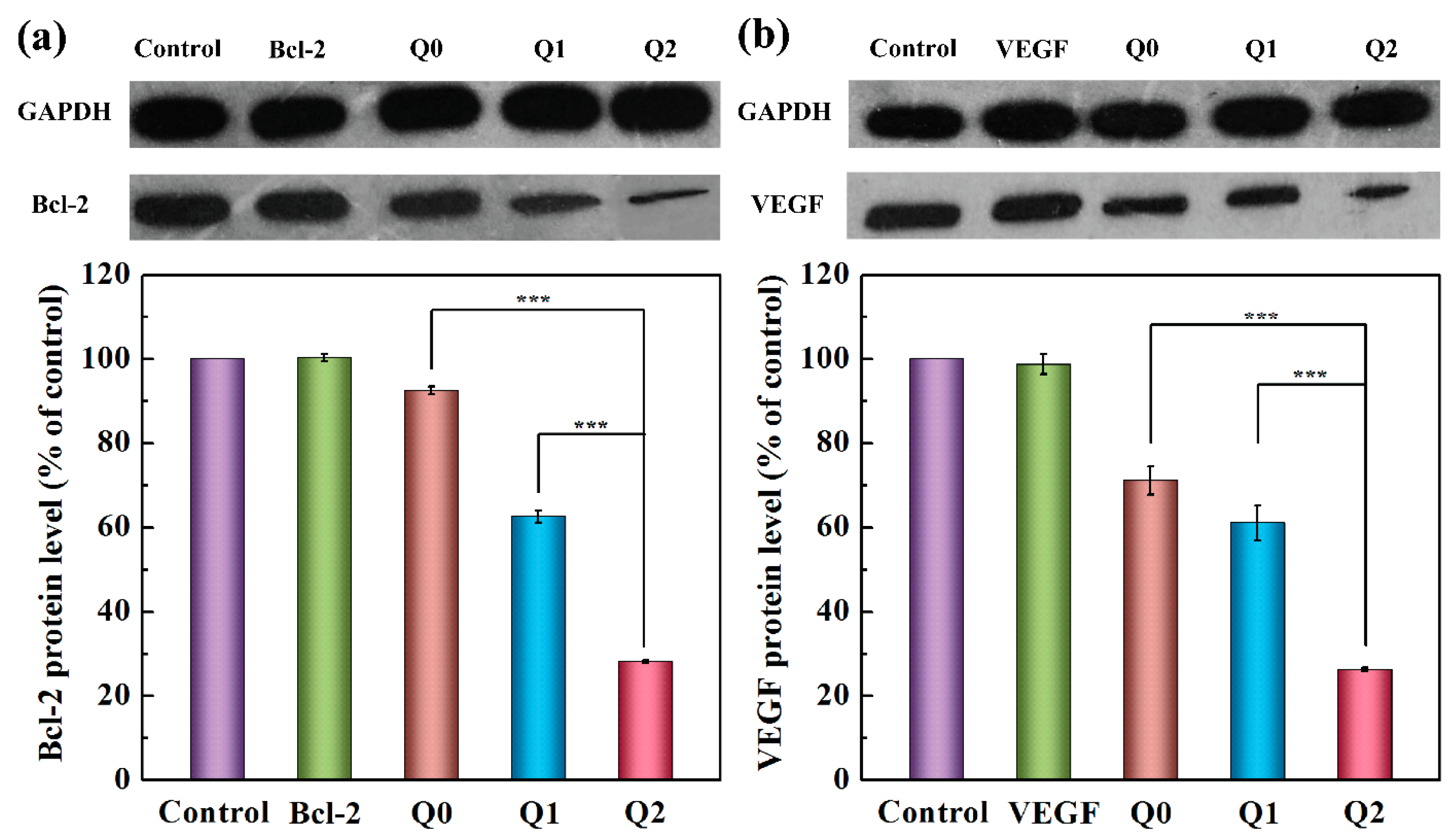
2.4. Gene Silencing with VEGF and Bcl-2 siRNAs
3. Experimental Section
3.1. Synthesis of Au DENPs-β-CD
3.2. Preparation of Vectors/siRNA Polyplexes
3.3. Gene Silencing with VEGF and Bcl-2 siRNAs
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sonawane, N.D.; Szoka, F.C.; Verkman, A.S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.P.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew. Chem. Int. Edit. 2004, 43, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Borgheti-Cardoso, L.N.; Depieri, L.V.; Diniz, H.; Calzzani, R.A.J.; Fantini, M.C.D.; Iyomasa, M.M.; Vicentini, F.; Bentley, M. Self-assembling gelling formulation based on a crystalline-phase liquid as a non-viral vector for siRNA delivery. Eur. J. Pharm. Sci. 2014, 58, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Li, Y.; Mozhi, A.; Zhang, L.; Liu, Y.L.; Xu, X.; Xing, J.M.; Liang, X.J.; Ma, G.H.; Yang, J.; et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials 2014, 35, 6519–6533. [Google Scholar] [CrossRef] [PubMed]
- Van Hauwermeiren, F.; Vandenbroucke, R.E.; Grine, L.; Puimege, L.; Van Wonterghem, E.; Zhang, H.; Libert, C. Antisense oligonucleotides against TNFR1 prevent toxicity of TNF/IFN gamma treatment in mouse tumor models. Int. J. Cancer 2014, 135, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nakaura, M.; Imanishi, T.; Obika, S. Target gene knockdown by 2′,4′-BNA/LNA antisense oligonucleotides in zebrafish. Nucleic Acid Ther. 2014, 24, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Putral, L.N.; Gu, W.Y.; McMillan, N.A.J. RNA interference for the treatment of cancer. Drug News Perspect. 2006, 19, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M. Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther. 2005, 12, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Castano, S.; Delord, B.; Fevrier, A.; Lehn, J.M.; Lehn, P.; Desbat, B. Brewster angle microscopy and PMIRRAS study of DNA interactions with BGTC, a cationic lipid used for gene transfer. Langmuir 2008, 24, 9598–9606. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Qiu, J.R.; Sun, W.J.; Yang, J.; Shen, M.W.; Wang, L.; Shi, X.Y. Multifunctional PEI-entrapped gold nanoparticles enable efficient delivery of therapeutic siRNA into glioblastoma cells. Biomater. Sci. 2017, 5, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Wu, Y.L.; Alves, C.S.; Shi, X.Y. Efficient delivery of therapeutic siRNA into glioblastoma cells using multifunctional dendrimer-entrapped gold nanoparticles. Nanomedicine 2016, 11, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.Y.; Hou, W.X.; Cao, X.Y.; Wen, S.H.; Shen, M.W.; Shi, X.Y. Dendrimer-entrapped gold nanoparticles modified with folic acid for targeted gene delivery applications. Biomater. Sci. 2013, 1, 1172–1180. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tziveleka, L.A.; Sideratou, Z.; Tsiourvas, D. Gene delivery using functional dendritic polymers. Expert Opin. Drug Deliv. 2009, 6, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yamanouchi, D.; Liu, B.; Chu, C.C. Biodegradable arginine-based poly(ether ester amide)s as a non-viral DNA delivery vector and their structure-function study. J. Mater. Chem. 2012, 22, 18983–18991. [Google Scholar] [CrossRef]
- Stamatatos, L.; Leventis, R.; Zuckermann, M.J.; Silvius, J.R. Interactions of cationic lipid vesicles with negatively charged phospholipid-vesicles and biological-membranes. Biochemistry 1988, 27, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P.; Demeneix, B.; Loeffler, J.P.; Mutul, J.P. Efficient gene-transfer into mammalian primary endocrine-cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. USA 1989, 86, 6982–6986. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualch, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in-vivo-polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Haralambidis, J.; Duncan, L.; Tregear, G.W. The solid-phase synthesis of oligonucleotides containing a 3′-peptide moiety. Tetrahedron Lett. 1987, 28, 5199–5202. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The 3rd helix of the antennapedia homeodomain translocates through biological-membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [PubMed]
- Kuriyama, S.; Mitoro, A.; Tsujinoue, H.; Nakatani, T.; Yoshiji, H.; Tsujimoto, T.; Yamazaki, M.; Fukui, T. Particle-mediated gene transfer into murine livers using a newly developed gene gun. Gene Ther. 2000, 7, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, C.; Lee, J.; Papadimitrakopoulos, F.; Silbart, L.K.; Zhao, M.H.; Burgess, D.J. Labeling and intracellular tracking of functionally active plasmid DNA with semiconductor quantum dots. Mol. Ther. 2006, 14, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Nieckowski, M.; Eisler, S.; Diederich, F. Dendritic vectors for gene transfection. New J. Chem. 2007, 31, 1111–1127. [Google Scholar] [CrossRef]
- Li, S.; Huang, L. Nonviral gene therapy: Promises and challenges. Gene Ther. 2000, 7, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Itaka, K.; Kanayama, N.; Nishiyama, N.; Jang, W.D.; Yamasaki, Y.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. Supramolecular nanocarrier of siRNA from peg-based block catiomer carrying diamine side chain with distinctive pk(a) directed to enhance intracellular gene silencing. J. Am. Chem. Soc. 2004, 126, 13612–13613. [Google Scholar] [CrossRef] [PubMed]
- Minakuchi, Y.; Takeshita, F.; Kosaka, N.; Sasaki, H.; Yamamoto, Y.; Kouno, M.; Honma, K.; Nagahara, S.; Hanai, K.; Sano, A.; et al. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004, 32, e109. [Google Scholar] [CrossRef] [PubMed]
- Spagnou, S.; Miller, A.D.; Keller, M. Lipidic carriers of siRNA: Differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry 2004, 43, 13348–13356. [Google Scholar] [CrossRef] [PubMed]
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced transfection with lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Unnamalai, N.; Kang, B.G.; Lee, W.S. Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Lett. 2004, 566, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers-starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules-synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Chen, C.L.; Johnson, J.; Baker, J.R. DNA complexing with polyamidoamine dendrimers: Implications for transfection. Bioconjug. Chem. 1999, 10, 843–850. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.R.; Du, J.; Ying, X.; Lu, W.L.; Lou, J.N.; Wei, Y. PEGylated poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Shakhbazau, A.; Isayenka, I.; Kartel, N.; Goncharova, N.; Seviaryn, I.; Kosmacheva, S.; Potapnev, M.; Shcharbin, D.; Bryszewska, M. Transfection efficiencies of PAMAM dendrimers correlate inversely with their hydrophobicity. Int. J. Pharm. 2010, 383, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.W.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: From molecular recognition to self-assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Wu, J.Y.; Hafdi, N.; Behr, J.P.; Erbacher, P.; Peng, L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006, 22, 2362–2364. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, K.; Hahn, H.J.; Kim, B.H.; Lim, H.J.; Kim, H.J.; Choi, J.S.; Park, J.S. Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials 2009, 30, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; de Ilarduya, C.T. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.X.; Wei, P.; Kong, L.D.; Guo, R.; Wang, S.G.; Shi, X.Y. Partially PEGylated dendrimer-entrapped gold nanoparticles: A promising nanoplatform for highly efficient DNA and siRNA delivery. J. Mater. Chem. B 2016, 4, 2933–2943. [Google Scholar] [CrossRef]
- Shan, Y.B.; Luo, T.; Peng, C.; Sheng, R.L.; Cao, A.M.; Cao, X.Y.; Shen, M.W.; Guo, R.; Tomas, H.; Shi, X.Y. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Gao, Y.; Tang, Y.; He, R.R.; Liu, T.L.; He, Y.; Sun, S.; Li, B.Y.; Li, Y.B.; Liu, G. PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J. 2009, 11, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.Y.; Cao, X.Y.; Hou, W.X.; Peng, C.; Qiu, J.R.; Shi, X.Y. Poly(amidoamine) dendrimers modified with 1,2-epoxyhexane or 1,2-epoxydodecane for enhanced gene delivery applications. J. Nanosci. Nanotechnol. 2015, 15, 10134–10140. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.X.; Wen, S.H.; Guo, R.; Wang, S.G.; Shi, X.Y. Partially acetylated dendrimer-entrapped gold nanoparticles with reduced cytotoxicity for gene delivery applications. J. Nanosci. Nanotechnol. 2015, 15, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.R.; Kong, L.D.; Cao, X.Y.; Li, A.J.; Tan, H.R.; Shi, X.Y. Dendrimer-entrapped gold nanoparticles modified with β-cyclodextrin for enhanced gene delivery applications. RSC Adv. 2016, 6, 25633–25640. [Google Scholar] [CrossRef]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. In vitro and in vivo gene transfer by an optimized alpha-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjug. Chem. 2003, 14, 342–350. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Kong, L.; Cao, X.; Li, A.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with β-Cyclodextrin. Nanomaterials 2018, 8, 131. https://doi.org/10.3390/nano8030131
Qiu J, Kong L, Cao X, Li A, Wei P, Wang L, Mignani S, Caminade A-M, Majoral J-P, Shi X. Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with β-Cyclodextrin. Nanomaterials. 2018; 8(3):131. https://doi.org/10.3390/nano8030131
Chicago/Turabian StyleQiu, Jieru, Lingdan Kong, Xueyan Cao, Aijun Li, Ping Wei, Lu Wang, Serge Mignani, Anne-Marie Caminade, Jean-Pierre Majoral, and Xiangyang Shi. 2018. "Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with β-Cyclodextrin" Nanomaterials 8, no. 3: 131. https://doi.org/10.3390/nano8030131





