A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of PMB-Protein Conjugates
2.3. Production and Purification of mAbs
2.4. IcELISA Procedure
2.5. Specificity of mAbs
2.6. Preparation of AuNPs-Labeled mAb
2.7. Construction and Principle of the Immunochromatographic Test Strip
2.8. Sample Analysis
3. Results and Discussion
3.1. Identification of Conjugates
3.2. Characterization of mAbs
3.3. Specificity of mAbs
3.4. Preparation of AuNPs
3.5. Optimization of the ICT Strip
3.6. Sensitivity of the ICT Strip
3.7. Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaheen, M.; Li, J.; Ross, A.C.; Vederas, J.C.; Jensen, S.E. Paenibacillus polymyxa PKB1 produces variants of polymyxin B-type antibiotics. Chem. Biol. 2011, 18, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Rabanal, F.; Cajal, Y. Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep. 2017, 34, 886–908. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Novel derivatives of polymyxins. J. Antimicrob. Chemother. 2013, 68, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed. Res. Int. 2015, 2015, 679109. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.P.; Wu, X.; McKelvey, G.R.; McGuire, J.; Schilke, K.F. Binding interactions of bacterial lipopolysaccharide and the cationic amphiphilic peptides polymyxin B and WLBU2. Colloids Surf. B Biointerfaces 2014, 120, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Velkov, T.; Zhu, Y.; Roberts, K.D.; Le Brun, A.P.; Chow, S.H.; Gutu, A.D.; Moskowitz, S.M.; Shen, H.H.; Li, J. Polymyxin-Induced Lipid A Deacylation in Pseudomonas aeruginosa Perturbs Polymyxin Penetration and Confers High-Level Resistance. ACS Chem. Biol. 2018, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Justo, J.A.; Bosso, J.A. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 2015, 35, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kadar, B.; Kocsis, B.; Nagy, K.; Szabo, D. The Renaissance of Polymyxins. Curr. Med. Chem. 2013, 20, 3759–3773. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Landersdorfer, C.B.; Lee, H.J.; Li, J.; Nation, R.L. ‘Old’ antibiotics for emerging multidrug-resistant bacteria. Curr. Opin. Infect. Dis. 2012, 25, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Singh, O.; Juneja, D.; Tyagi, N.; Khurana, A.S.; Qamra, A.; Motlekar, S.; Barkate, H. Resurgence of Polymyxin B for MDR/XDR Gram-Negative Infections: An Overview of Current Evidence. Crit. Care Res. Pract. 2017, 2017, 3635609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China a microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeannot, K.; Bolard, A.; Plesiat, P. Resistance to polymyxins in Gram-negative organisms. Int. J. Antimicrob. Agents 2017, 49, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Sitzlar, B.; Vajravelu, R.K.; Jury, L.; Donskey, C.J.; Jump, R.L. Environmental decontamination with ultraviolet radiation to prevent recurrent Clostridium difficile infection in 2 roommates in a long-term care Facility. Infect. Control Hosp. Epidemiol. 2012, 33, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Cao, H.; Ledesma, K.R.; Hu, M. In vitro potency of various polymyxin B components. Antimicrob. Agents Chemother. 2011, 55, 4490–4491. [Google Scholar] [CrossRef] [PubMed]
- Covelli, J.; Ruszaj, D.; Straubinger, R.; Li, J.; Rao, G.G. The development and validation of a simple liquid chromatography-tandem mass spectrometry method for polymyxin B1 and B2 quantification in different matrices. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1065–1066, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, L.; Liu, S.; Jaber, O.M.; Gao, L.; Chevrette, L.; Reuschel, S. Simultaneous quantitation of polymyxin B1, polymyxin B2 and polymyxin B1-1 in human plasma and treated human urine using solid phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1012–1013, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Hee, K.H.; Leaw, Y.K.J.; Ong, J.L.; Lee, L.S. Development and validation of liquid chromatography tandem mass spectrometry method quantitative determination of polymyxin B1, polymyxin B2, polymyxin B3 and isoleucine-polymyxin B1 in human plasma and its application in clinical studies. J. Pharm. Biomed. Anal. 2017, 140, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cheah, S.E.; Bulitta, J.B.; Li, J.; Nation, R.L. Development and validation of a liquid chromatography-mass spectrometry assay for polymyxin B in bacterial growth media. J. Pharm. Biomed. Anal. 2014, 92, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Saita, T.; Yoshida, M.; Nakashima, M.; Matsunaga, H.; Fujito, H.; Mori, M. A high sensitive ELISA for the quantification of polymyxin B sulfate in Human serum. Biol. Pharm. Bull. 1999, 22, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, G.; Hayat, A.; Andreescu, S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors 2015, 15, 30736–30758. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, L.L.; Xu, L.G.; Song, S.S.; Kuang, H.; Cui, G.; Xu, C.L. Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro) quinolones by one monoclonal antibody. Nano Res. 2016, 10, 108–120. [Google Scholar] [CrossRef]
- Omidfar, K.; Khorsand, F.; Azizi, M.D. New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens. Bioelectron. 2013, 43, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Dharmarajan, R.; Megharaj, M.; Naidu, R. Gold nanoparticle-based optical sensors for selected anionic contaminants. TrAC Trends Anal. Chem. 2017, 86, 143–154. [Google Scholar] [CrossRef]
- Yoo, J.H.; Woo, D.H.; Chang, M.S.; Chun, M.S. Microfluidic based biosensing for Escherichia coli detection by embedding antimicrobial peptide-labeled beads. Sens. Actuators B Chem. 2014, 191, 211–218. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Establishment of a monoclonal antibody-based indirect enzyme-linked immunosorbent assay for the detection of trimethoprim residues in milk, honey and fish samples. Food Agric. Immunol. 2016, 27, 830–840. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, W.; Zhou, J.; Wen, K.; Wang, Z.; Jiang, H.; Ding, S. Production of Monoclonal Antibody and Development of a New Immunoassay for Apramycin in Food. J. Agric. Food Chem. 2014, 62, 3108–3113. [Google Scholar] [CrossRef] [PubMed]
- Uchigashima, M.; Watanabe, E.; Ito, S.; Iwasa, S.; Miyake, S. Development of immunoassay based on monoclonal antibody reacted with the neonicotinoid insecticides clothianidin and dinotefuran. Sensors 2012, 12, 15858–15872. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Xing, C.; Hao, C.; Liu, L.; Wang, L.; Xu, C. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors 2013, 13, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Song, S.; Peng, J.; Liu, L.; Kuang, H.; Xu, C. Sensitive, fast and specific immunoassays for methyltestosterone detection. Sensors 2015, 15, 10059–10073. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Liu, L.; Ma, W.; Xu, C.; Wang, L.; Kuang, H. Rapid on-site determination of melamine in raw milk by an immunochromatographic strip. Int. J. Food Sci. Technol. 2012, 47, 1505–1510. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Huang, X.; Guo, X.; Cai, Q.; Zhou, S. Rapid Detection of Chloramphenicol Residues in Aquatic Products Using Colloidal Gold Immunochromatographic Assay. Sensors 2014, 14, 21872–21888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, H.; An, Z.; Xu, Z.; Yang, X.; Huang, H.; Wang, Z. Development of an Heterologous Immunoassay for Ciprofloxacin Residue in Milk. Phys. Procedia 2012, 25, 1829–1836. [Google Scholar] [CrossRef]
- Kong, D.; Liu, L.; Song, S.; Suryoprabowo, S.; Li, A.; Kuang, H.; Wang, L.; Xu, C. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Liu, L.; Song, S.; Zheng, Q.; Wu, X.; Kuang, H. Rapid detection of tenuazonic acid in cereal and fruit juice using a lateral-flow immunochromatographic assay strip. Food Agric. Immunol. 2017, 28, 1293–1303. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a Rainbow Lateral Flow Immunoassay for the Simultaneous Detection of Four Mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
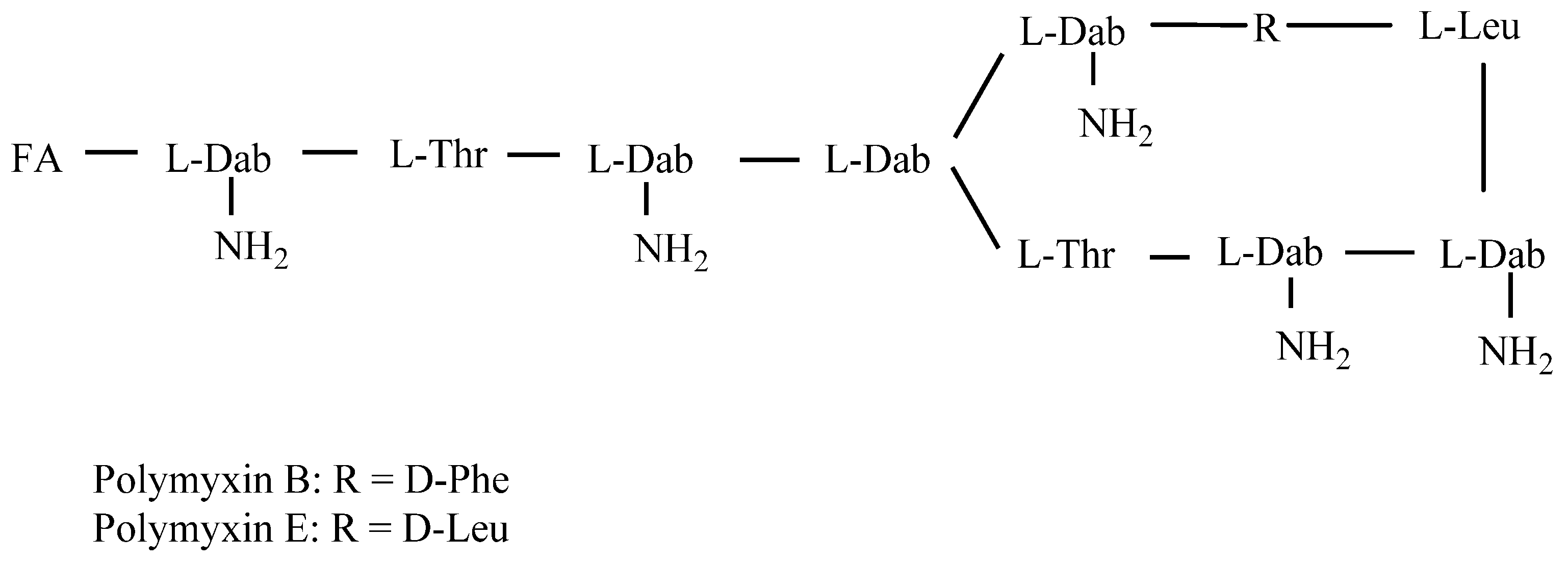
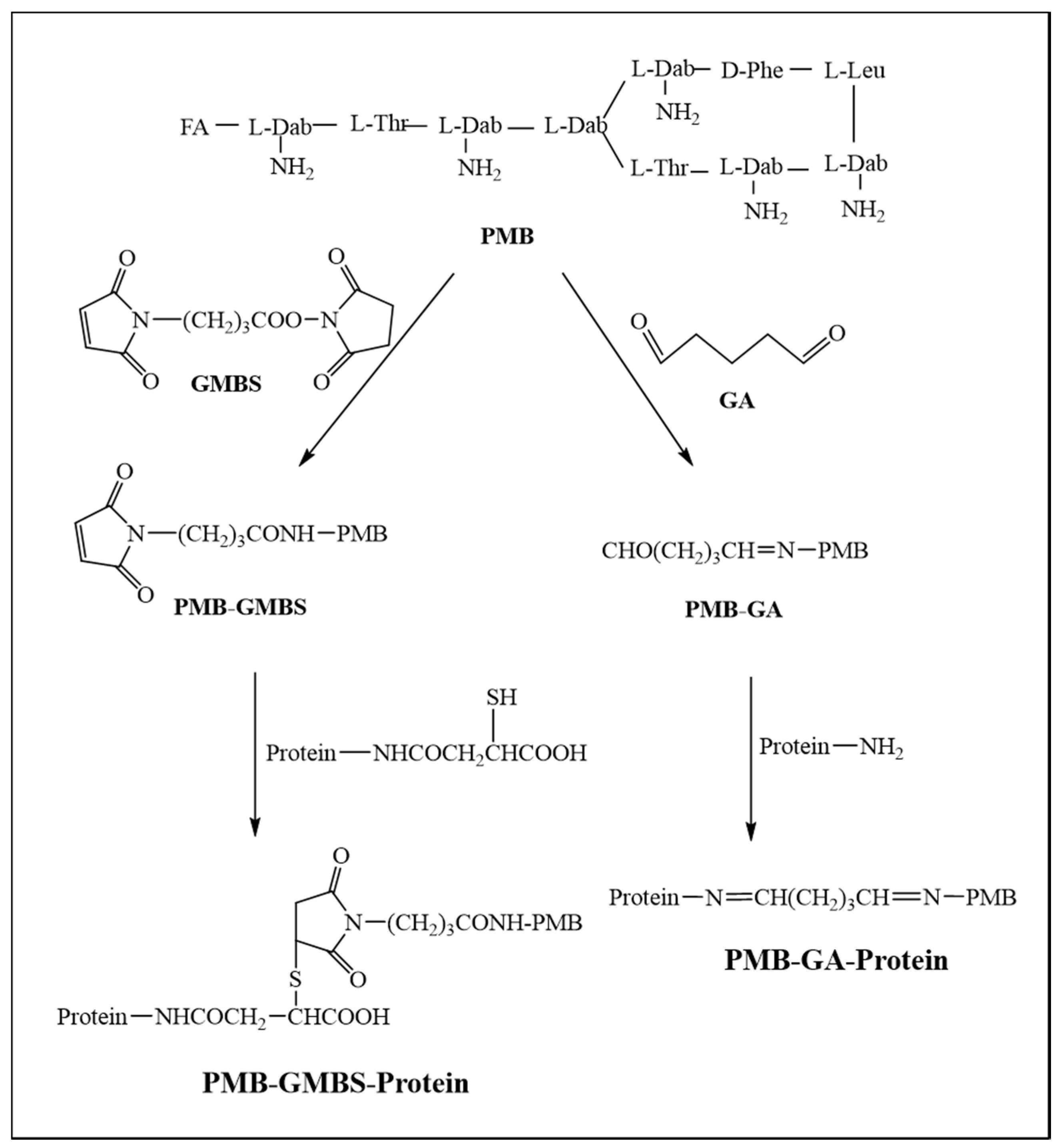
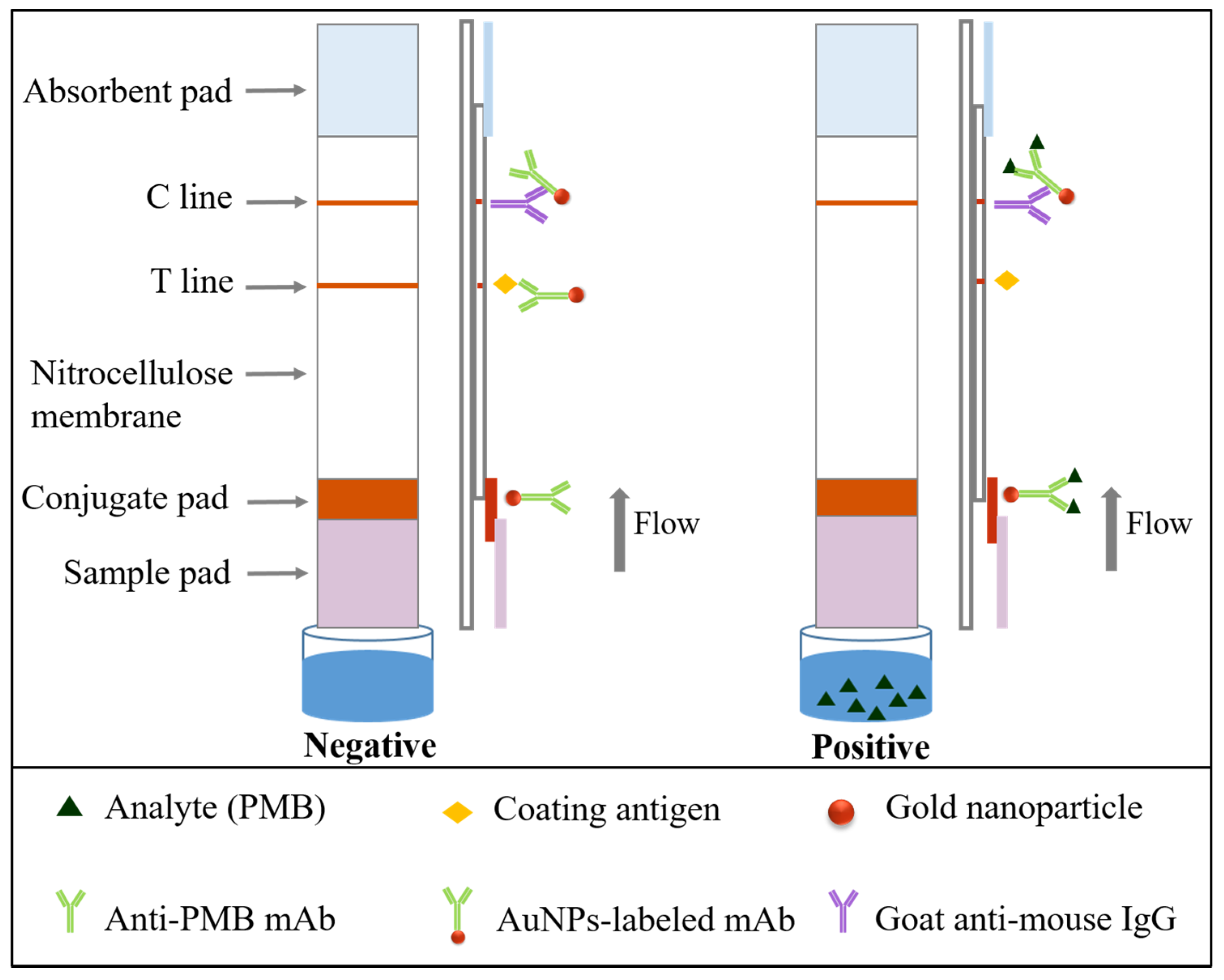
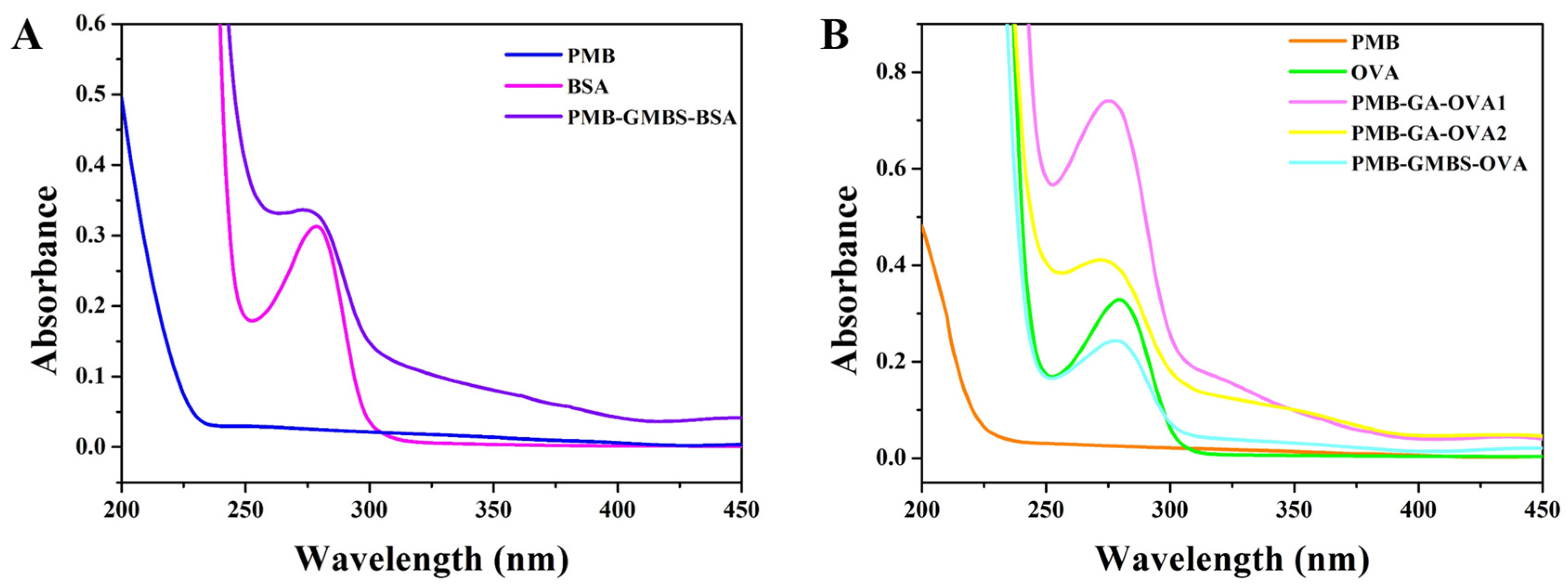
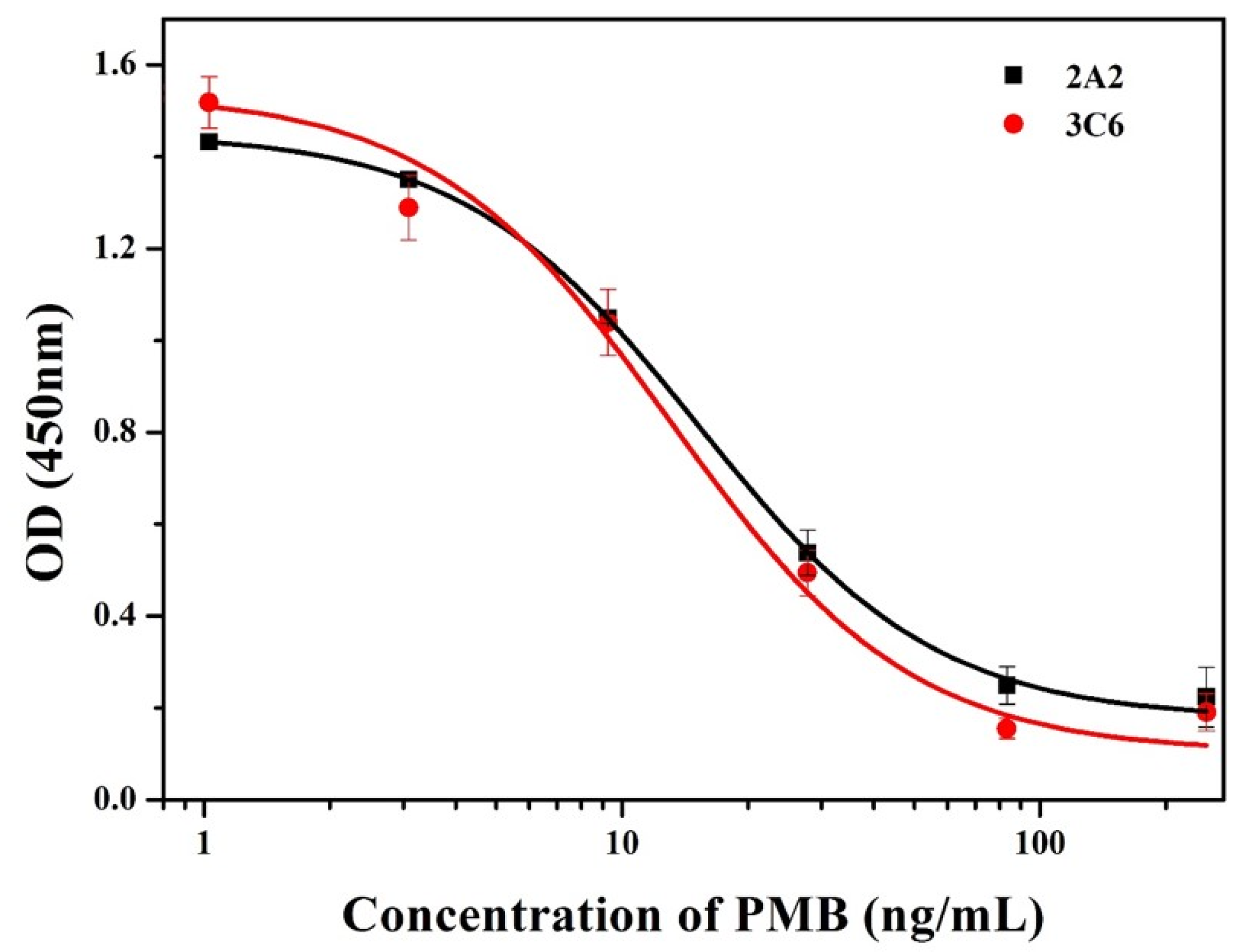
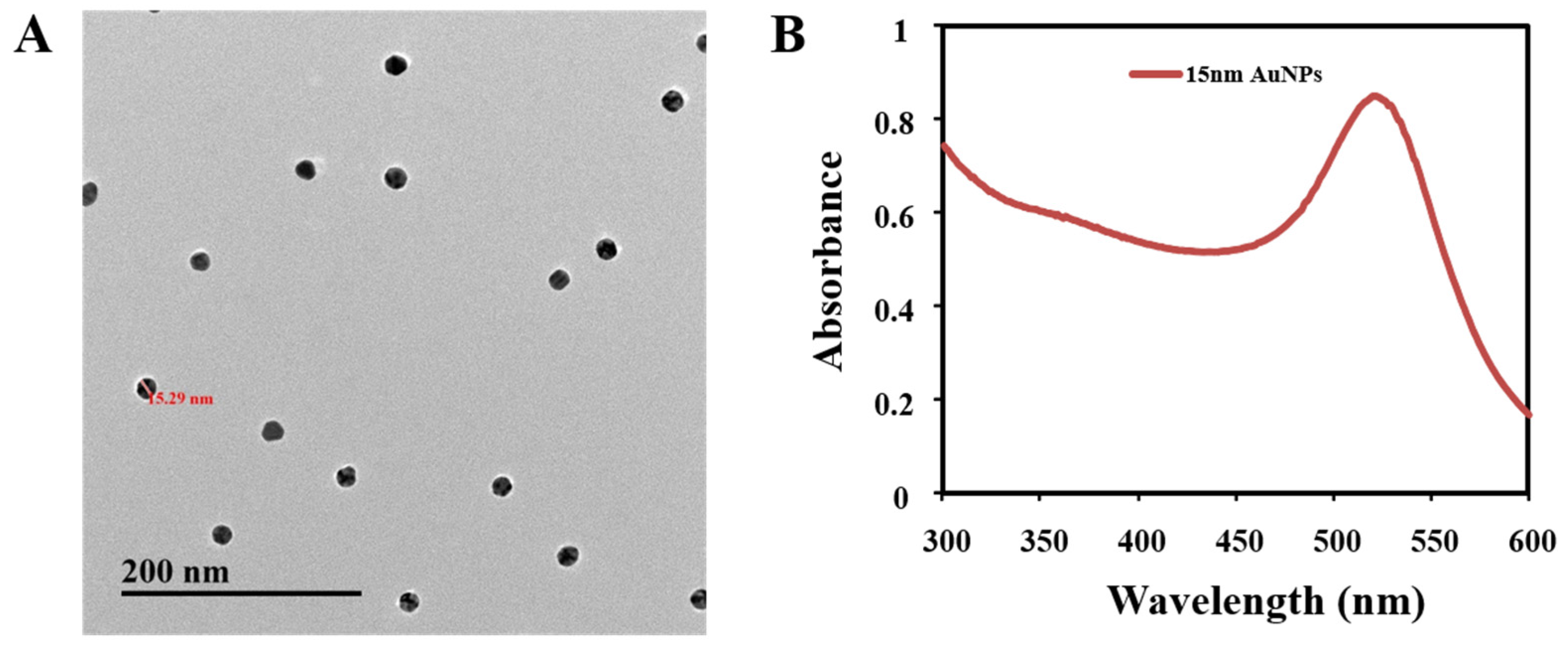


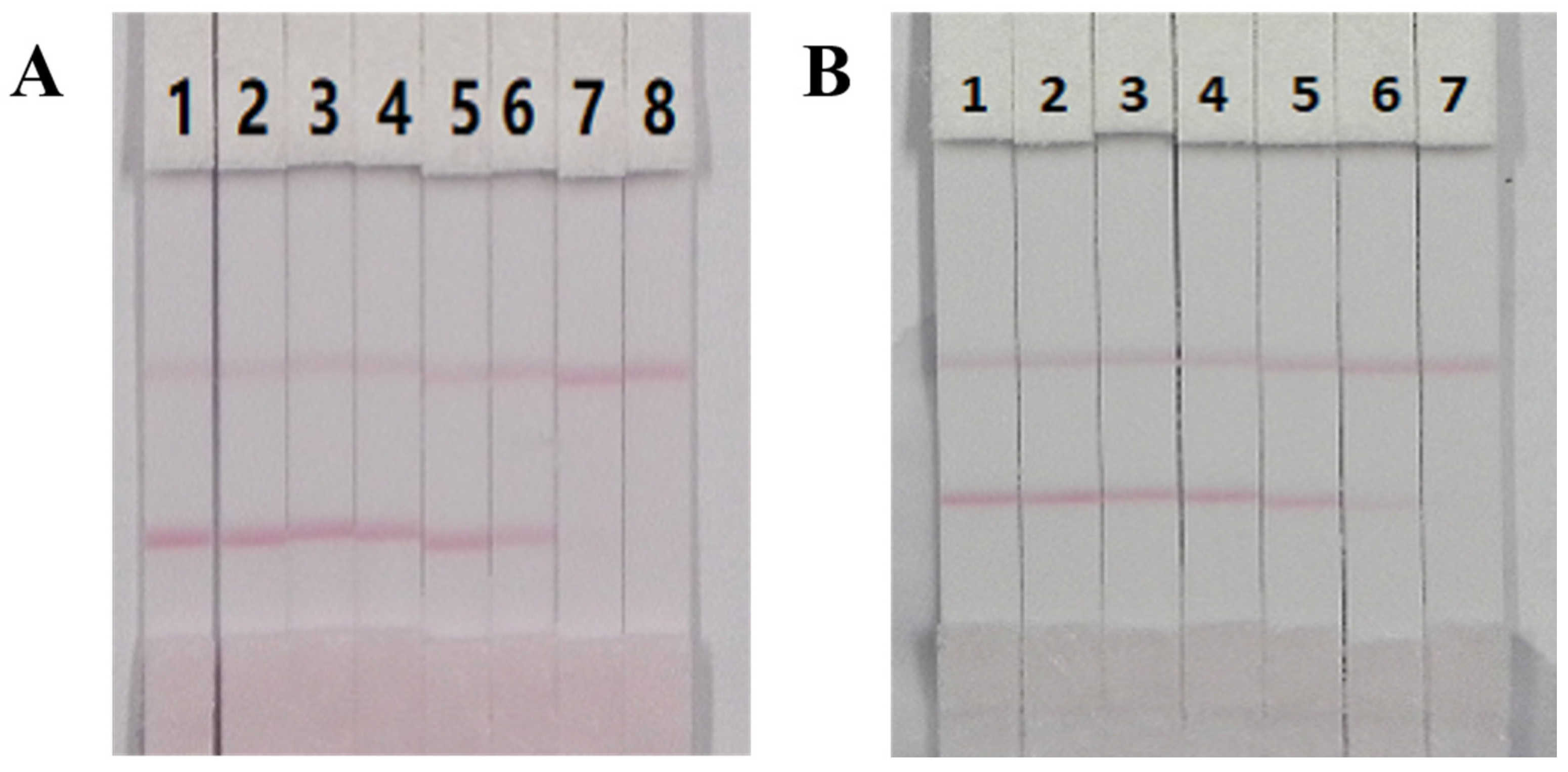
| mAb | Analytes | IC50 (ng/mL) | CR (%) |
|---|---|---|---|
| 2A2 | PMB | 15.26 | 100% |
| PME | >1000 | - | |
| 3C6 | PMB | 13.13 | 100% |
| PME | 259 | 5.07% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, L.; Song, S.; Kuang, H.; Xu, C. A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues. Nanomaterials 2018, 8, 144. https://doi.org/10.3390/nano8030144
Li Y, Liu L, Song S, Kuang H, Xu C. A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues. Nanomaterials. 2018; 8(3):144. https://doi.org/10.3390/nano8030144
Chicago/Turabian StyleLi, Yue, Liqiang Liu, Shanshan Song, Hua Kuang, and Chuanlai Xu. 2018. "A Rapid and Semi-Quantitative Gold Nanoparticles Based Strip Sensor for Polymyxin B Sulfate Residues" Nanomaterials 8, no. 3: 144. https://doi.org/10.3390/nano8030144




