Understanding the Effects of NaCl, NaBr and Their Mixtures on Silver Nanowire Nucleation and Growth in Terms of the Distribution of Electron Traps in Silver Halide Crystals
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Silver Nanowires
2.2. Fabrication of Ag Nanowire Electrode
2.3. Measurements
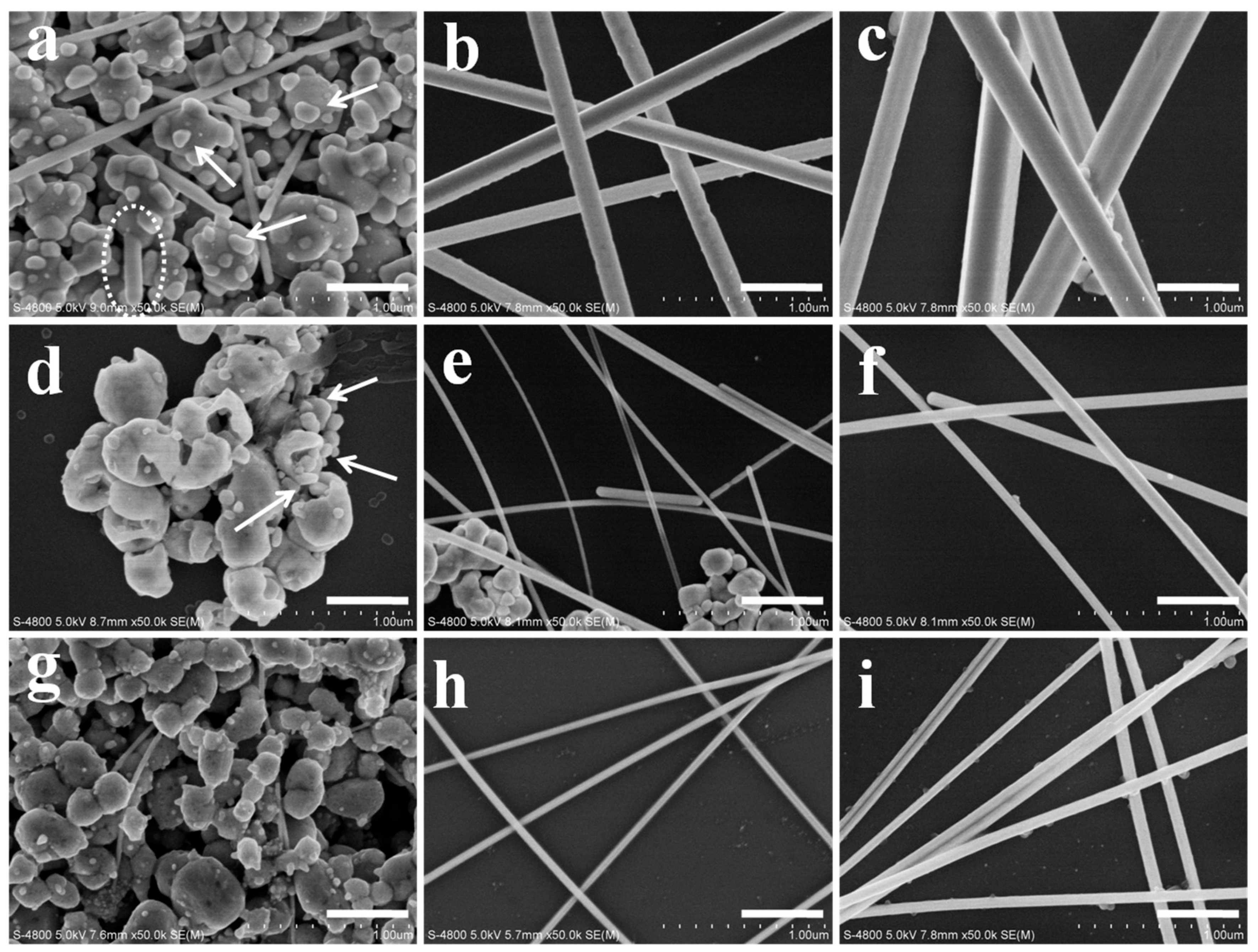
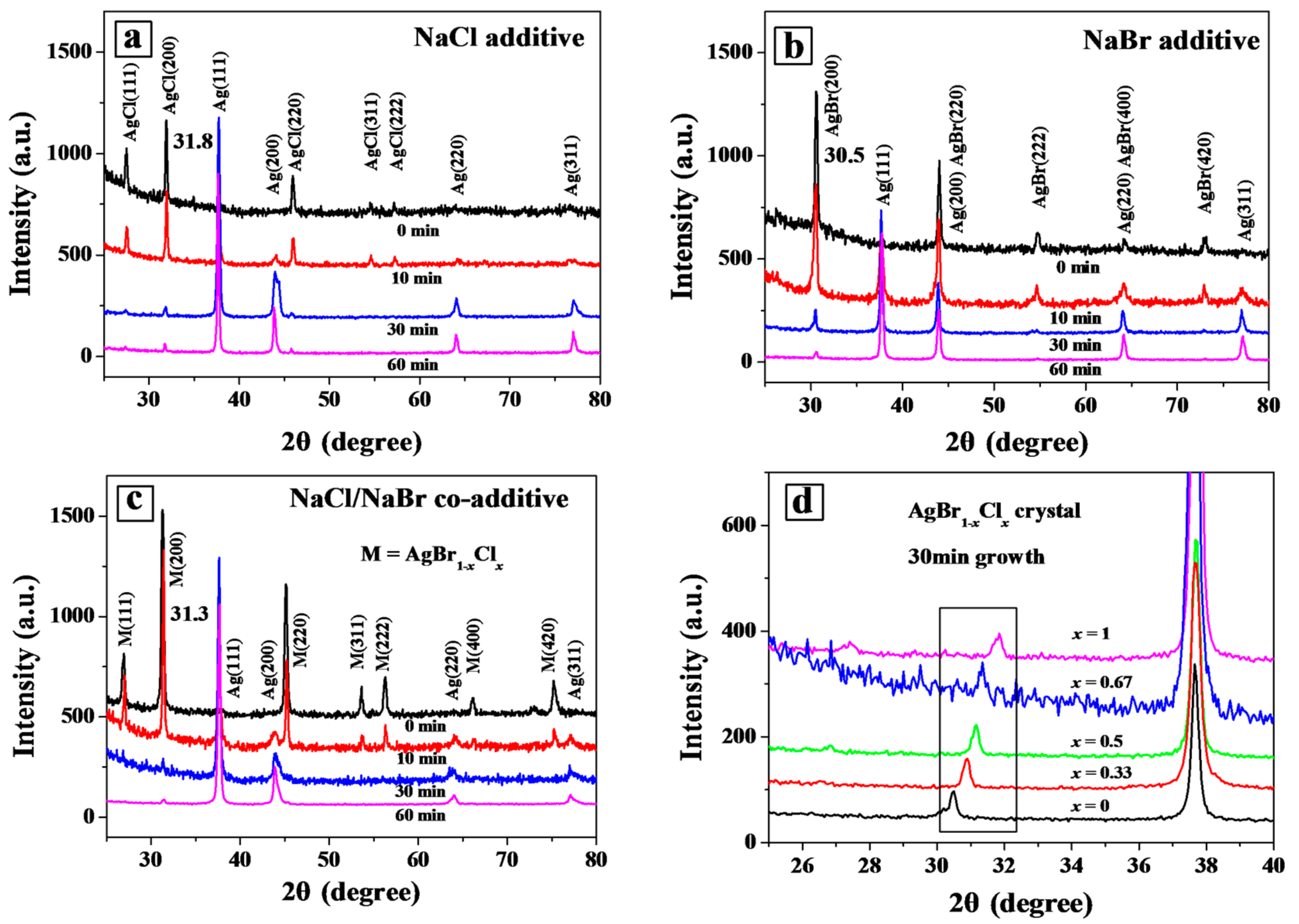
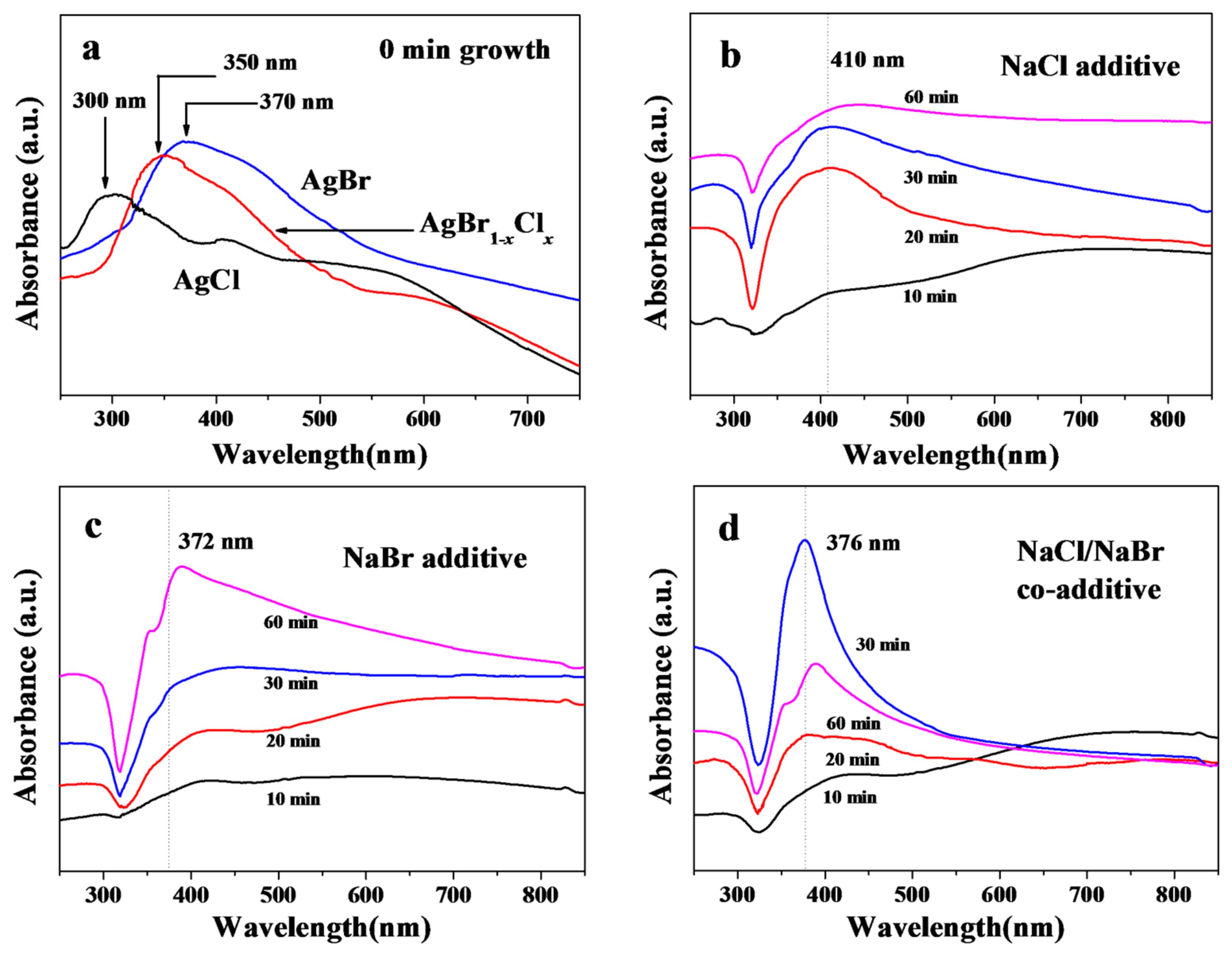
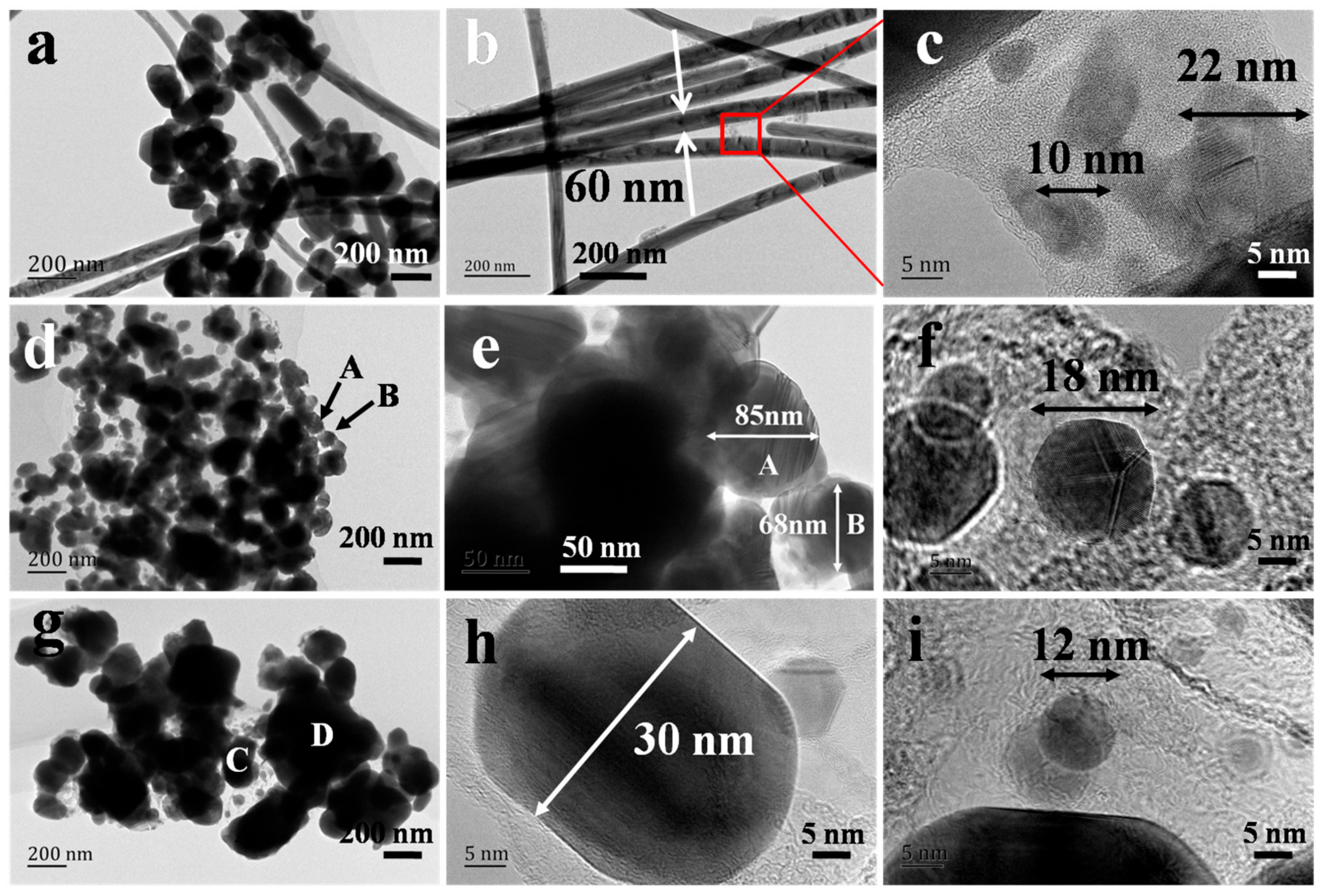
3. Results and Discussion
3.1. Characterization of AgNWs by SEM, XRD and Absorption Spectra
3.2. Analysis of AgNW Growth Mechanism
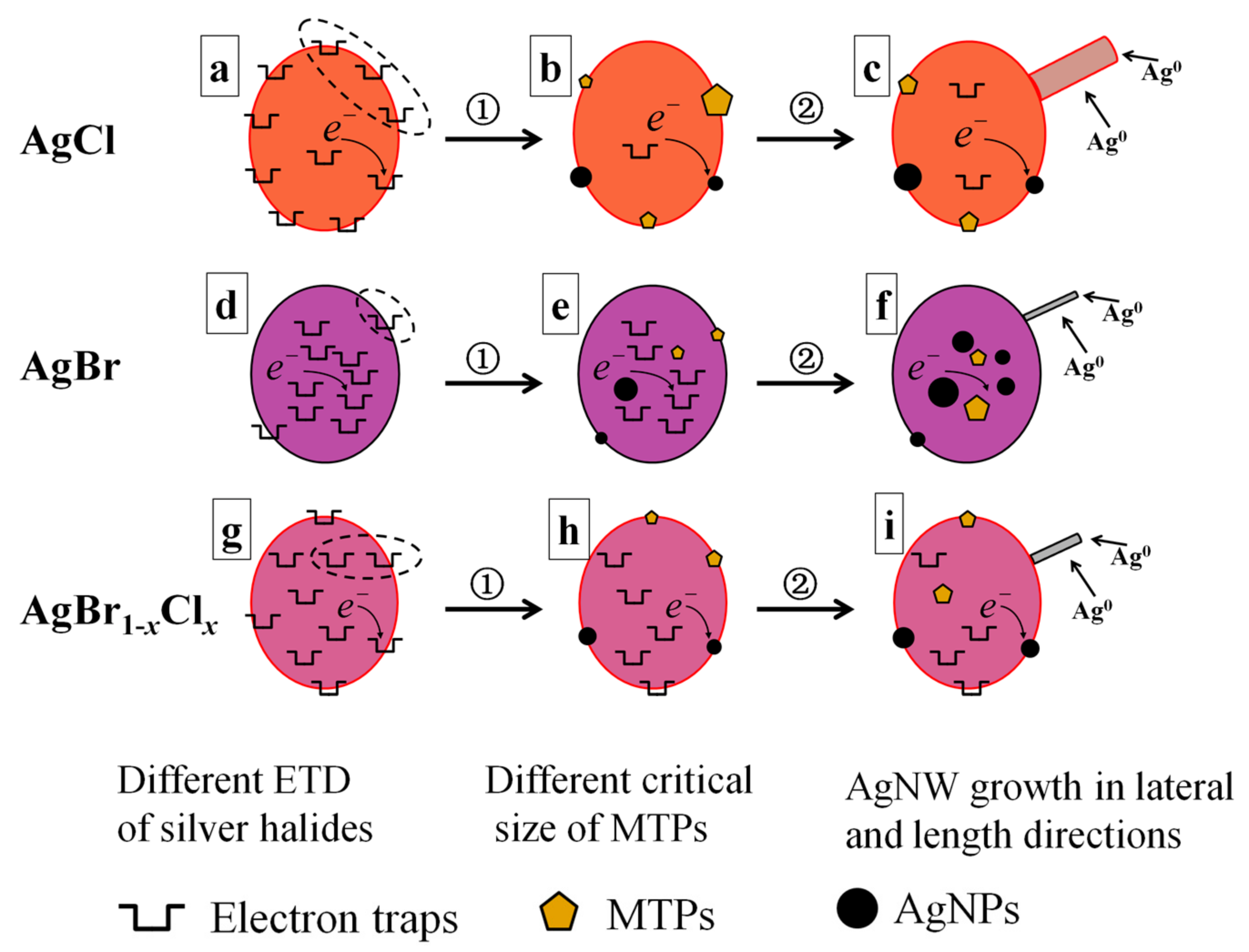
3.3. Our Proposed ETD Mechanism for the Growth of AgNWs
3.4. AgNW Diameter Controlled by NaCl/NaBr Concentration
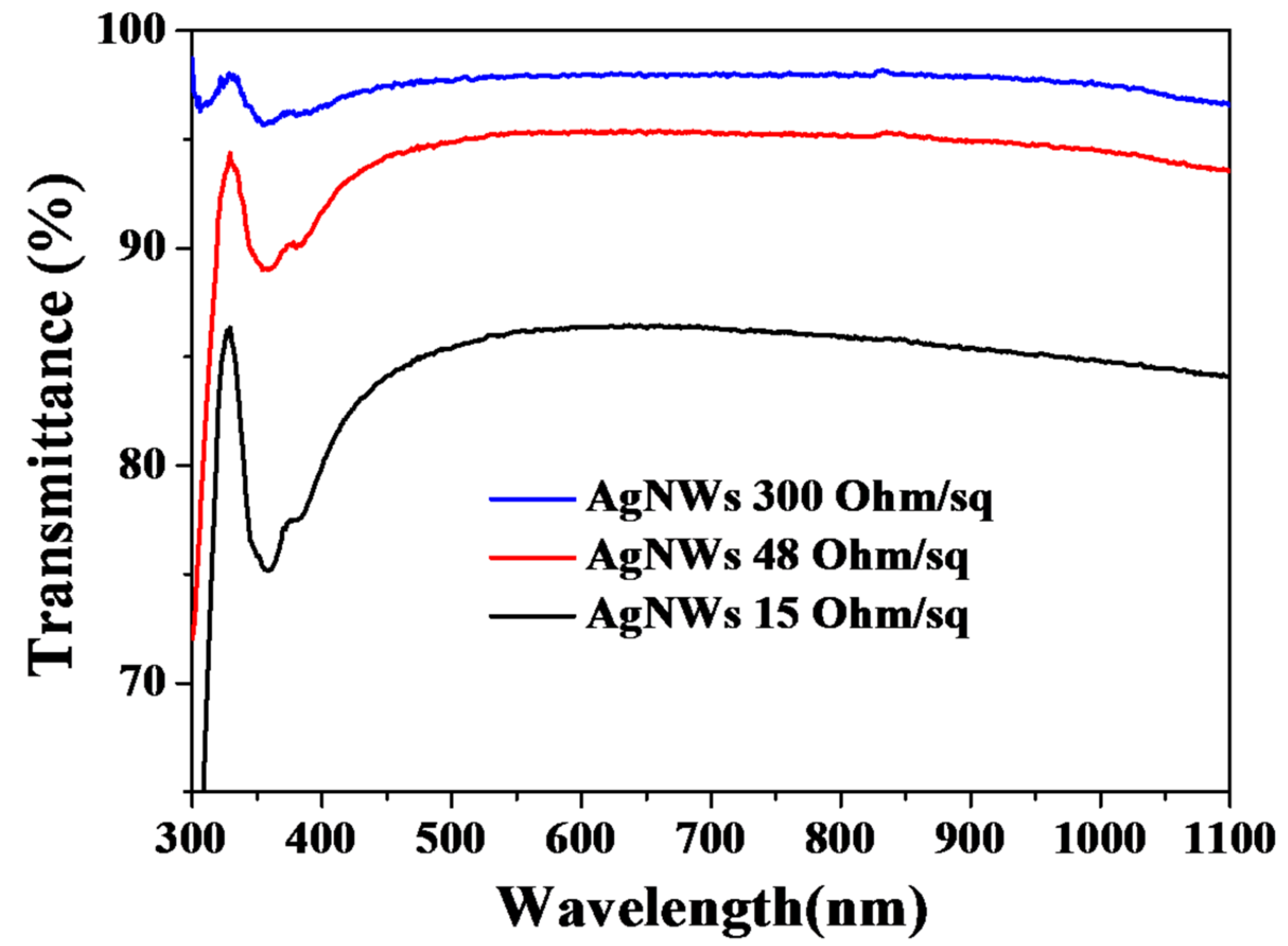
3.5. Properties of AgNW Transparent Electrodes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNWs | Silver nanowires |
| MTPs | Multiply twinned particles |
| AgNPs | Silver nanoparticles |
| Agt+ | Electron traps in the silver halides |
| ETD | Electron trap distribution |
| LSPR | Localized surface plasmon resonance |
| ITO | Indium tin oxide |
| FWHM | Full width at half maximum |
| PVP | Poly(vinyl pyrrolidone) |
| EG | Ethylene glycol |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray spectroscopy |
| UV-vis | Ultraviolet-visible spectra |
References
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef] [PubMed]
- Spechler, J.; Nagamatsu, K.; Sturm, J.; Arnold, C. Improved efficiency of hybrid organic photovoltaics by pulsed laser sintering of silver nanowire network transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 10556–10562. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, Y.; Fan, Y.; Zhao, Z.; Yu, W.; Rasco, B.A.; Lai, K. Detection of Prohibited Fish Drugs Using Silver Nanowires as Substrate for Surface-Enhanced Raman Scattering. Nanomaterials 2016, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kim, H.; Lee, J.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, S.; Stewart, I.; Alvarez, S.; Wiley, B. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef] [PubMed]
- Bergin, S.M.; Chen, Y.H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; He, W.; Wang, K.; Ji, S.; Ye, C. A one-step route to Ag nanowires with a diameter below 40 nm and an aspect ratio above 1000. Chem. Commun. 2014, 50, 14877–14880. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, P.; Liu, D.F.; Yuan, H.J.; Yan, X.Q.; Zhou, Z.P.; Wang, J.X.; Song, L.; Liu, L.F.; Zhou, W.Y.; et al. Evidence for the monolayer assembly of poly(vinylpyrrolidone) on the surfaces of silver nanowires. J. Phys. Chem. B 2004, 108, 12877–12881. [Google Scholar] [CrossRef]
- Korte, K.; Skrabalak, S.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
- Jiu, J.; Murai, K.; Kim, D.; Kim, K.; Suganuma, K. Preparation of Ag nanorods with high yield by polyol process. Mater. Chem. Phys. 2009, 114, 333–338. [Google Scholar] [CrossRef]
- Zhang, W.C.; Wu, X.L.; Chen, H.T.; Gao, Y.J.; Zhu, J.; Huang, G.S.; Chu, P.K. Self-organized formation of silver nanowires, nanocubes and bipyramids via a solvothermal method. Acta Mater. 2008, 56, 2508–2513. [Google Scholar] [CrossRef]
- Ma, J.; Zhan, M. Rapid production of silver nanowires based on high concentration of AgNO3 precursor and use of FeCl3 as reaction promoter. RSC Adv. 2014, 4, 21060–21071. [Google Scholar] [CrossRef]
- Li, M.; Jing, M.; Wang, Z.; Li, B.; Shen, X. Controllable growth of superfine silver nanowires by self-seeding polyol process. J. Nanosci. Nanotechnol. 2015, 15, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.; Yang, M.; Choi, S.I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.Y.; Camargo, P.H.; Ribeiro, S.J.L.; Xia, Y. Facile synthesis of sub-20 nm silver nanowires through a bromide-mediated polyol method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Du, Y.; Chen, S. Sub 30 nm silver nanowire synthesized using KBr as co-nucleant through one-pot polyol method for optoelectronic applications. Org. Electron. 2015, 26, 380–385. [Google Scholar] [CrossRef]
- Jia, D.; Zhao, Y.; Wei, W.; Chen, C.; Lei, G.; Wan, M.; Tao, J.; Li, S.; Ji, S.; Ye, C. Synthesis of very thin Ag nanowires with fewer particles by suppressing secondary seeding. CrystEngComm 2017, 19, 148–153. [Google Scholar] [CrossRef]
- Lee, E.; Kim, Y.; Hwang, D.; Choi, W.; Kim, J. Synthesis and optoelectronic characteristics of 20 nm diameter silver nanowires for highly transparent electrode films. RSC Adv. 2016, 6, 11702–11710. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H. Polyol synthesis of silver nanowires: An extensive parametric study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Jiang, W.; Zhang, M.; Ming, X.; Wang, S.; Zhang, Q. Strongly visible-light responsive plasmonic shaped AgX:Ag (X = Cl, Br) nanoparticles for reduction of CO2 to methanol. Nanoscale 2012, 4, 5646–5650. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dong, Q.; Shao, Y.; Yuan, Y.; Huang, J. Highly narrowband perovskite single-crystal photodetectors enabled by surface-charge recombination. Nat. Photon. 2015, 9, 679–687. [Google Scholar] [CrossRef]
- Cheng, Z.; Chu, X.; Sheng, Z.; Xu, J.; Zhong, H.; Zhang, L. Synthesis of quasi-spherical AgBr microcrystal via a simple ion-exchange route. Mater. Lett. 2016, 168, 99–102. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Zhao, Z.; Xiao, X.; Li, Z.; Zhang, J. Site-selected synthesis of novel Ag@AgCl nanoframes with efficient visible light induced photocatalytic activity. J. Mater. Chem. A 2014, 2, 12594–12600. [Google Scholar] [CrossRef]
- Zhu, J.; Kan, C.; Wu, Y.; Wan, J.; Han, M.; Wang, G. A novel discovery of growth process for Ag nanowires and plausible mechanism. J. Nanomater. 2016, 2016, 5812739. [Google Scholar] [CrossRef]
- Schuette, W.; Buhro, W. Silver chloride as a heterogeneous nucleant for the growth of silver nanowires. ASC Nano 2013, 7, 3844–3853. [Google Scholar] [CrossRef] [PubMed]
- Schuette, W.; Buhro, W. Polyol synthesis of silver nanowires by heterogeneous nucleation; mechanistic aspects influencing nanowire diameter and length. Chem. Mater. 2014, 26, 6410–6417. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Yang, G.; Zhang, S.; Yu, L.; Zhang, P. Large scale preparation of silver nanowires with different diameters by a one-pot method and their application in transparent conducting films. RSC Adv. 2016, 6, 8096–8102. [Google Scholar] [CrossRef]
- An, C.; Wang, J.; Wang, S.; Zhang, Q.; Yang, M.; Zhan, J. Converting AgCl nanocubes to silver nanowires through a glycerol-mediated solution route. CrystEngComm 2012, 14, 5886–5891. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, B.; Zhang, H.; Wan, P.; Luo, L.; Kuang, Y.; Sun, X. Probing the seeded protocol for high-concentration preparation of silver nanowires. Nano Res. 2016, 9, 1532–1542. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth echanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Matsunaga, K.; Tanaka, I.; Adachi, H. Electronic mechanism of Ag-cluster formation in AgBr and AgI. J. Phys. Soc. Jpn. 1998, 67, 2027–2036. [Google Scholar] [CrossRef]
- Hamilton, J.F. The silver halide photographic process. Adv. Phys. 1988, 37, 359–441. [Google Scholar] [CrossRef]
- Oikawa, T.; Saeki, N.; Kaneda, T.; Hirano, A.; Tani, T. Electronic properties and photographic behavior of AgCl emulsion grains. J. Imaging Sci. Technol. 1995, 39, 233–238. [Google Scholar]
- Tani, T. Nanoparticles and nanotechnology in silver halide imaging. J. Dispers. Sci. Technol. 2004, 25, 375–388. [Google Scholar] [CrossRef]
- Jiu, J.; Tokuno, T.; Nogi, M.; Suganuma, K. Synthesis and application of Ag nanowires via a trace salt assisted hydrothermal process. J. Nanopart. Res. 2012, 14, 975. [Google Scholar] [CrossRef]
- Wiley, B.J.; Xiong, Y.; Li, Z.Y.; Yin, Y.; Xia, Y. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Tang, X.; Tsuji, M.; Jiang, P.; Nishio, M.; Jang, S.M.; Yoon, S.H. Rapid and high-yield synthesis of silver nanowires using air-assisted polyol method with chloride ions. Colloids Surf. A Physicochem. Eng. Aspects 2009, 338, 33–39. [Google Scholar] [CrossRef]
- Sandifer, J.R. Effects of bromide on silver chloride electrodes. Anal. Chem. 1981, 53, 312–316. [Google Scholar] [CrossRef]
- Kelly, T.M.; Mason, M.G. Halide composition profiles in silver microcrystals. J. Appl. Phys. 1976, 47, 4721–4725. [Google Scholar] [CrossRef]
- Kawasaki, M.; Hada, H.; Uchida, H. Transfer of photoelectrons and photoholes through a AgBr/AgCl interface, and relative locations of the energy bands. J. Appl. Phys. 1986, 60, 3945–3953. [Google Scholar] [CrossRef]
- Haugh, E.F.; Kitts, E.L.; Michewich, D.J. Silver Halide Crystal with Two Surface Types. U.S. Patent 4496652, 29 January 1985. [Google Scholar]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of Silver Nanowire Transparent Electrodes at Room Temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, Y.H.; Kim, J.K.; Baik, H.; Park, J.H.; Lee, J.; Nam, J.; Park, J.H.; Lee, T.W.; Yi, G.R.; et al. A roll-to-roll welding process for planarized silver nanowire electrodes. Nanoscale 2014, 6, 11828–11834. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Kim, D.Y.; Lee, J.H.; Ray, S.S.; Yarin, A.L.; Swihart, M.T.; Kim, D.H.; Yoon, S.S. Production of Flexible Transparent Conducting Films of Self-Fused Nanowires via One-Step Supersonic Spraying. Adv. Funct. Mater. 2017, 27, 1602548. [Google Scholar] [CrossRef]

| Sample No. | Molar Ratio of AgNO3/NaCl/NaBr | Diameter (nm) | Aspect Ratio | Ag(111)/(200) Ratio | Yield of NWs (%) |
|---|---|---|---|---|---|
| A2 | 80/2/0 | 162.5 ± 21.8 | 360 | 4.2 | 94 |
| B2 | 80/0/2 | 27.5 ± 7.6 | 230 | 3.1 | 30 |
| C1 | 800/2/1 | 94.4 ± 11.5 | 380 | 4.6 | 97 |
| C2 | 80/2/1 | 40.8 ± 6.9 | 2100 | 8.8 | 90 |
| C3 | 16/2/1 | 29.0 ± 3.8 | 1400 | 7.0 | 85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rui, Y.; Zhao, W.; Zhu, D.; Wang, H.; Song, G.; Swihart, M.T.; Wan, N.; Gu, D.; Tang, X.; Yang, Y.; et al. Understanding the Effects of NaCl, NaBr and Their Mixtures on Silver Nanowire Nucleation and Growth in Terms of the Distribution of Electron Traps in Silver Halide Crystals. Nanomaterials 2018, 8, 161. https://doi.org/10.3390/nano8030161
Rui Y, Zhao W, Zhu D, Wang H, Song G, Swihart MT, Wan N, Gu D, Tang X, Yang Y, et al. Understanding the Effects of NaCl, NaBr and Their Mixtures on Silver Nanowire Nucleation and Growth in Terms of the Distribution of Electron Traps in Silver Halide Crystals. Nanomaterials. 2018; 8(3):161. https://doi.org/10.3390/nano8030161
Chicago/Turabian StyleRui, Yunjun, Weiliang Zhao, Dewei Zhu, Hengyu Wang, Guangliang Song, Mark T. Swihart, Neng Wan, Dawei Gu, Xiaobing Tang, Ying Yang, and et al. 2018. "Understanding the Effects of NaCl, NaBr and Their Mixtures on Silver Nanowire Nucleation and Growth in Terms of the Distribution of Electron Traps in Silver Halide Crystals" Nanomaterials 8, no. 3: 161. https://doi.org/10.3390/nano8030161




