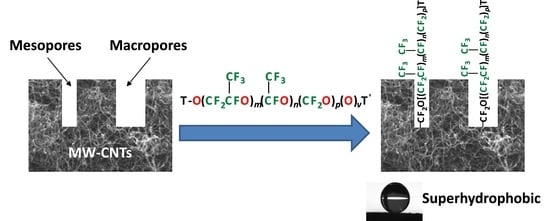
Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Results
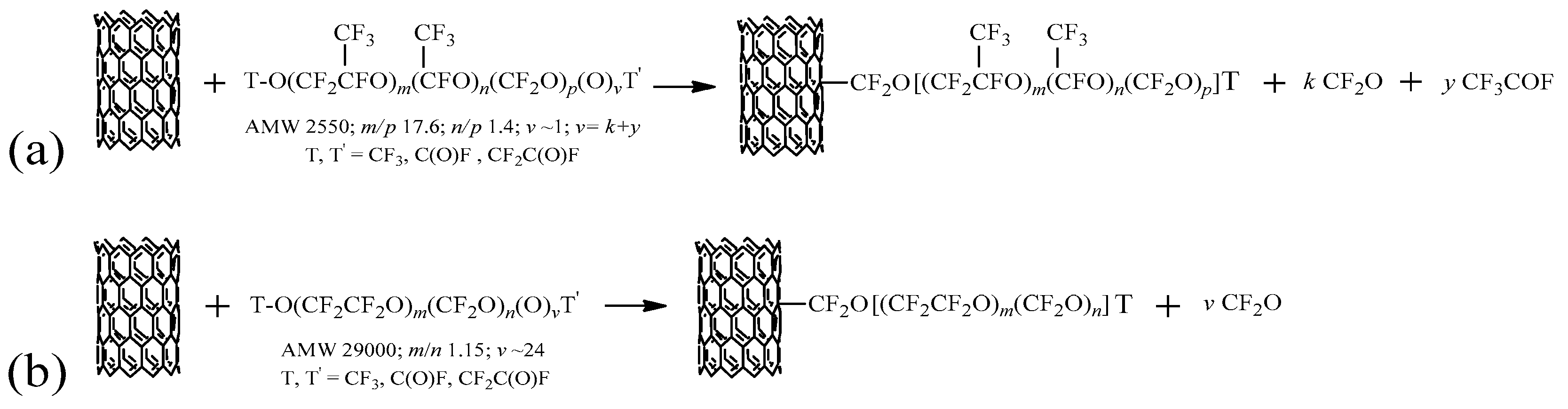
2.1. Functionalization Mechanism
2.2. Effect on Wettability
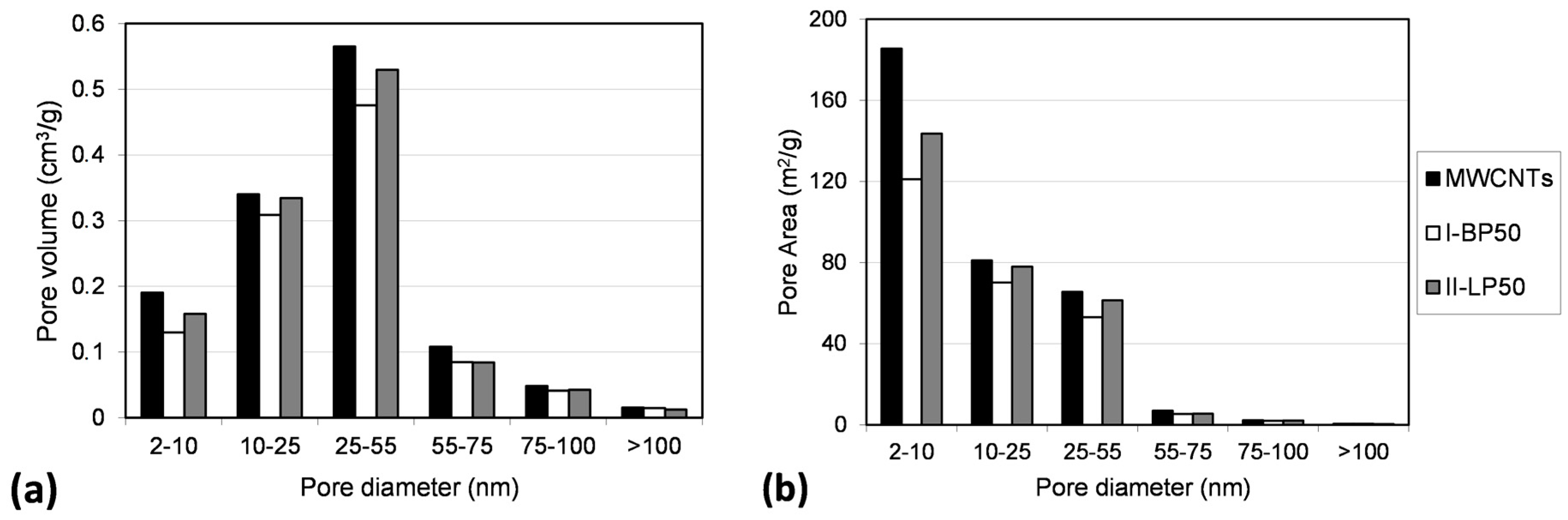
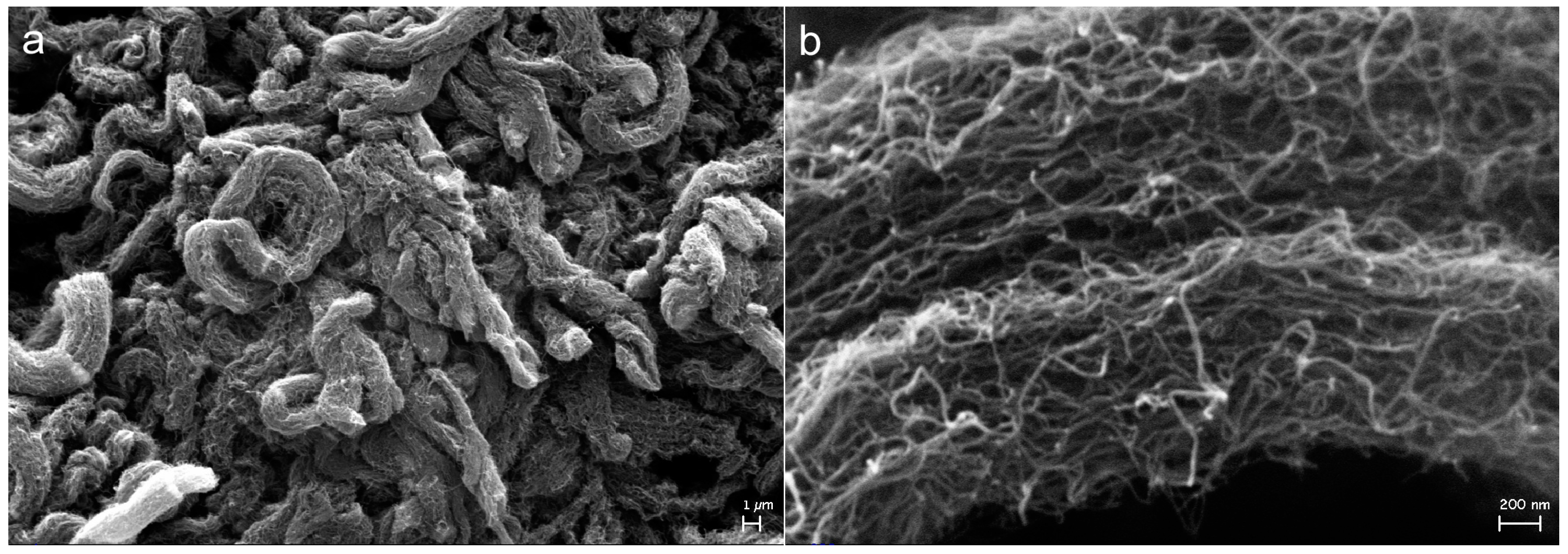
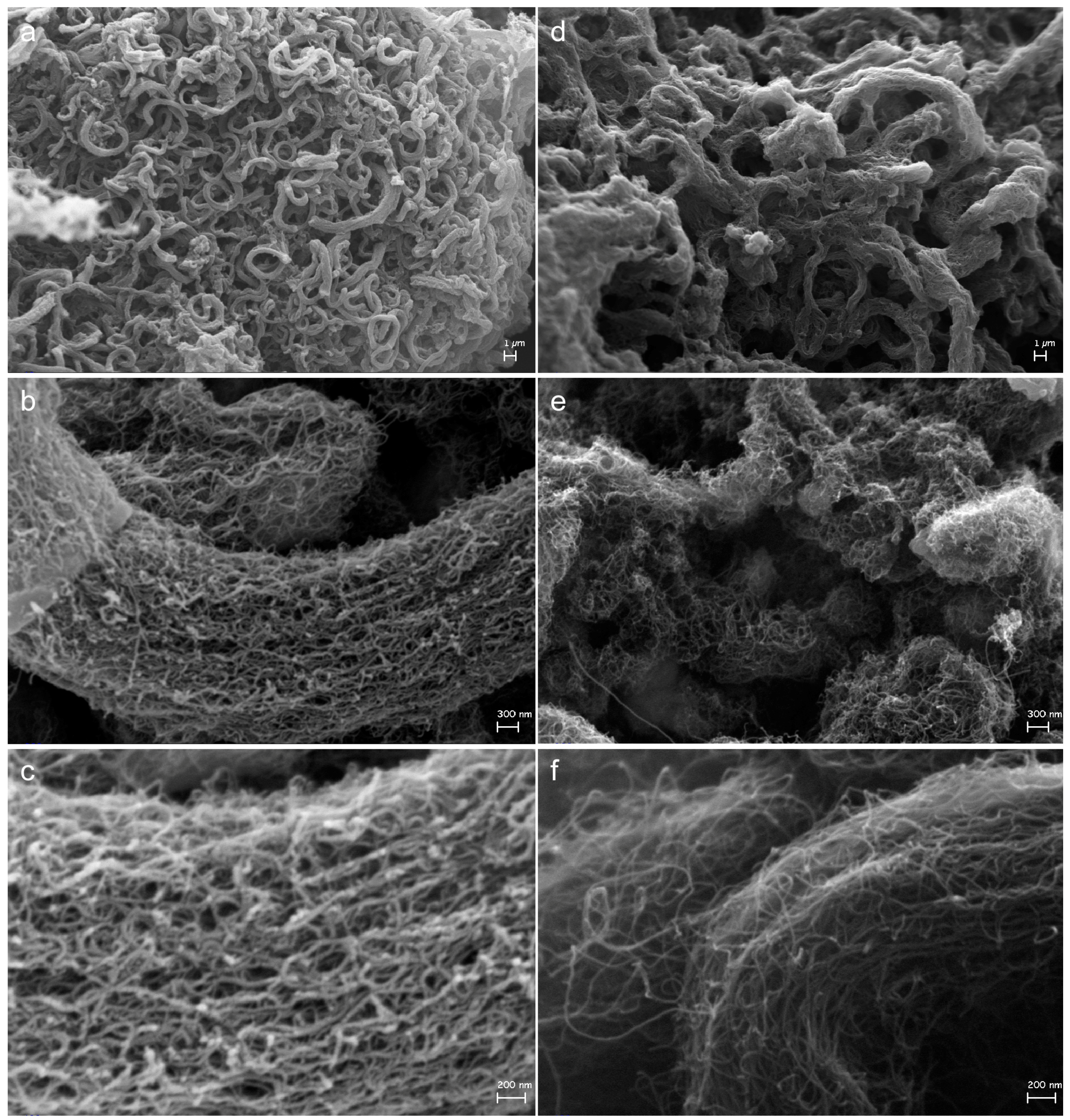
2.3. Effect on Morphology
2.4. Effect on Conductive Properties
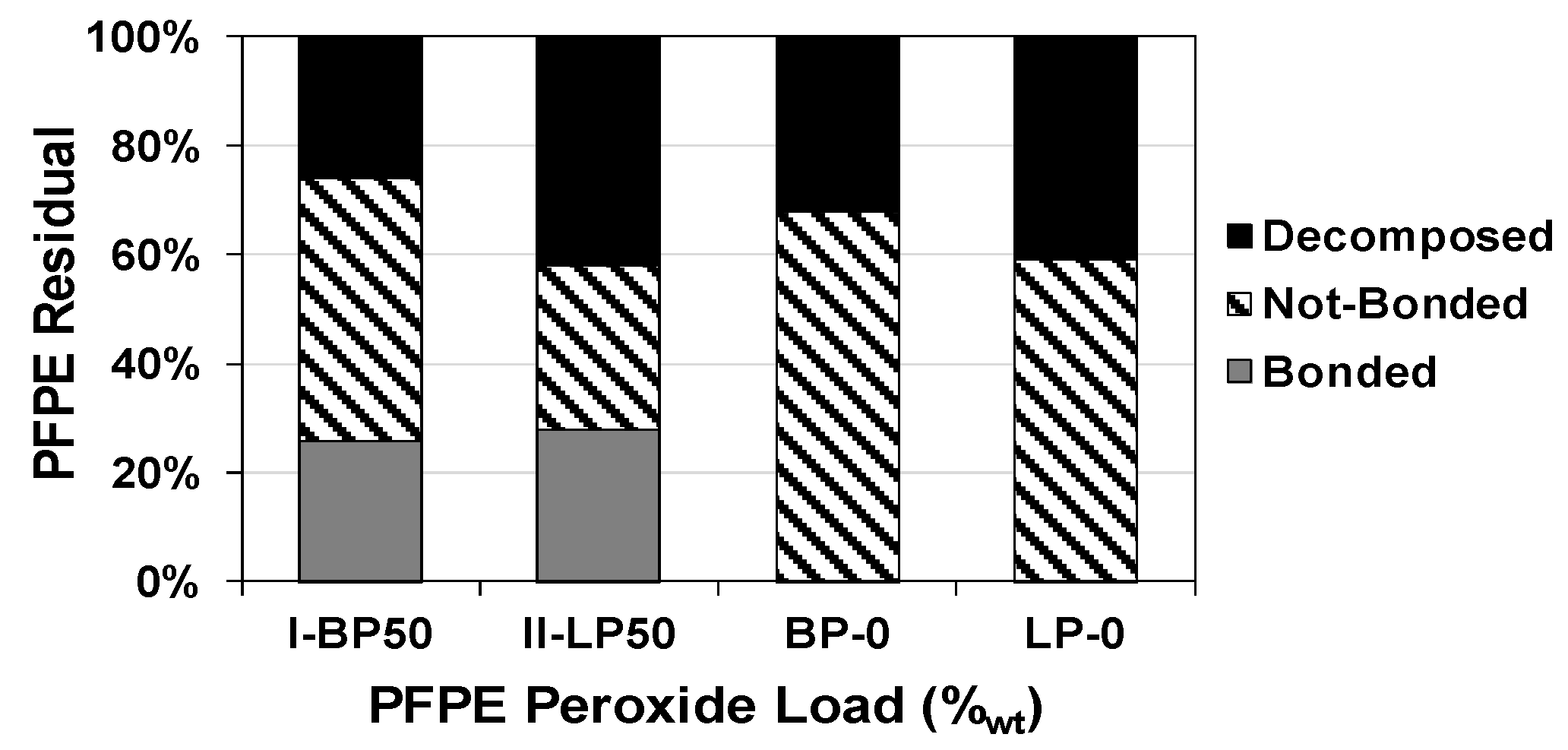
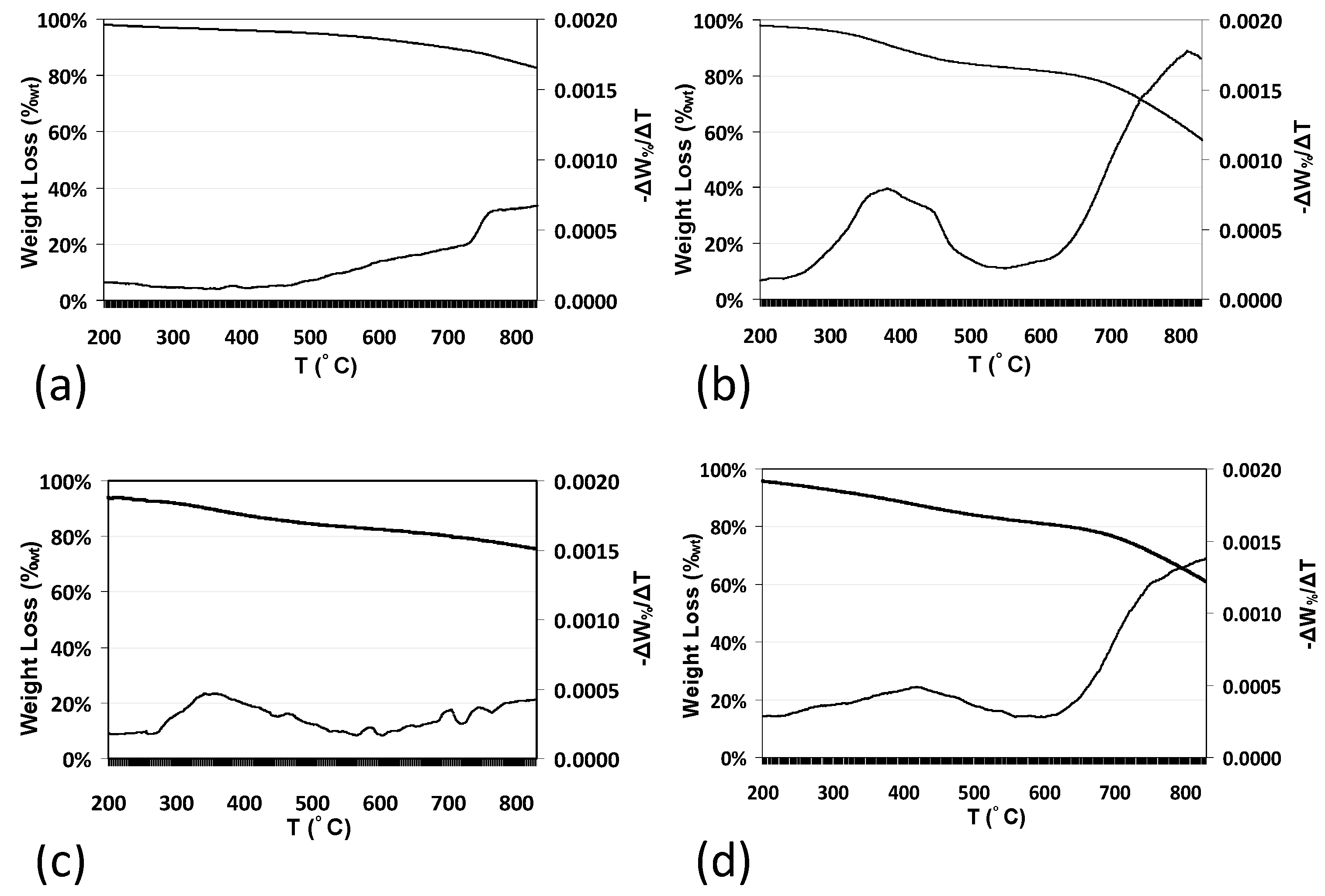
2.5. Effect on Thermal Stability
3. Materials and Methods
3.1. Materials
- -
- branched PFPE peroxide with general formula TO[CF2CF(CF3)O]m[CF(CF3)O]n(CF2O)p(O)vT: average molecular weight (AMW) around 2550 amu, equivalent molecular weight (EMW) around 1275 g/eq, ratio between perfluoro-iso-propylene oxide (C3, i.e., (CF3)CFCF2O and CF2CF(CF3)O randomly distributed), perfluoro(methyl)methylene oxide (C2, i.e., CF(CF3)O) and perfluoromethylene oxide (C1, i.e., CF2O) units 17.6:1.4:1, peroxidic content 0.286 wt % determined by iodometric titration [52];
- -
- branched PFPE fluid: Fomblin® YHVAC 18/8 by Solvay Specialty Polymers Inc., MWA around 2800 amu, ratio between perfluoro-iso-propylene oxide, (C3, i.e., CF2CF(CF3)O and CF2CF(CF3)O randomly distributed), and perfluoromethylene oxide, (C1, i.e., CF2O), units around 15, no peroxidic moieties along the polymer chain;
- -
- linear PFPE peroxide with general formula TO(CF2CF2O)m(CF2O)n(O)vTO: AMW around 29,000 amu, EMW around 1200 g/eq, ratio between perfluoroethylene oxide, (C2, i.e., CF2CF2O), and perfluoromethylene oxide, (C1, i.e., CF2O), units (m/n) around 1.15, peroxidic content 1.3 wt %;
- -
- linear PFPE fluid: Fomblin® M03 by Solvay Specialty Polymers Inc. (Woodburn, OR, USA), AMW around 4000 amu, ratio between perfluoroethylene oxide, (C2, i.e., CF2CF2O) and perfluoromethylene oxide, (C1, i.e., CF2O), units (m/n) around 1, no peroxidic moieties along the polymer chain (v = 0).
3.2. PFPE-Functionalization of MW-CNTs
3.3. Characterizations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes: Preparation and Properties, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 191–249. [Google Scholar]
- Engels, V.; Geng, J.; Jones, G.M.; Elliott, J.A.; Wheatley, A.E.H.; Boss, S.R. Cobalt catalyzed carbon nanotube growth on graphitic paper supports. Curr. Nanosci. 2011, 7, 315–322. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.M.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- De Heer, W.A.; Bacsa, W.S.; Chatelain, A.; Gergin, T.; Humphrey-Baker, R.; Forro, L.; Ugarte, D. Aligned carbon nanotube films: Production and optical and electronic properties. Science 1995, 268, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Delaney, P.; Choi, H.J.; Ihm, J.; Cohen, M.L. Broken symmetry and pseudogaps in ropes of carbon nanotubes. Nature 1998, 391, 466–468. [Google Scholar] [CrossRef] [Green Version]
- Orinakova, R.; Orinak, A. Recent applications of carbon nanotubes in hydrogen production and storage. Fuel 2011, 90, 3123–3140. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, K.H.; Heo, J.K.; Lee, Y.H. Fabrication of supercapacitor electrodes using fluorinated single-walled carbon nanotubes. J. Phys. Chem. 2003, 107, 8812–8815. [Google Scholar] [CrossRef]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential applications of carbon nanotubes. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications (Topics in Applied Physics); Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: New York, NY, USA, 2008; pp. 13–61. [Google Scholar]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall functionalization of carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Martínez-Hernández, A.L.; Velasco-Santos, C.; Castaño, V.M. Carbon nanotubes composites: Processing, grafting and mechanical and thermal properties. Curr. Nanosci. 2010, 6, 12–39. [Google Scholar] [CrossRef]
- Khabashesku, V.N.; Billups, W.E.; Margrave, J.L. Fluorination of single-wall carbon nanotubes and subsequent derivatization reactions. Acc. Chem. Res. 2002, 35, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, B.; Hamon, M.A.; Kamaras, K.; Itkis, M.E.; Haddon, R.C. Sidewall functionalization of single-walled carbon nanotubes by addition of dichlorocarbene. J. Am. Chem. Soc. 2003, 125, 14893–14900. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Saini, R.K.; Liang, F.; Sadana, A.K.; Billups, W.E. Functionalization of carbon nanotubes by free radicals. Org. Lett. 2003, 5, 1471–1473. [Google Scholar] [CrossRef] [PubMed]
- Tressaud, A.; Shirasaki, T.; Nanse, G.; Papirer, E. Fluorinated carbon blacks: Influence of the morphology of the starting material on the fluorination mechanism. Carbon 2002, 40, 217–220. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, T.H.; Lee, B.K.; Rho, J.S.; An, K.H.; Lee, Y.H. Surface properties of fluorinated single-walled carbon nanotubes. J. Fluor. Chem. 2003, 120, 99–104. [Google Scholar] [CrossRef]
- Chamssedine, F.; Guérin, K.; Dubois, M.; Disa, E.; Petit, E.; Fawal, Z.E.; Hamwi, A. Fluorination of single walled carbon nanotubes at low temperature: Towards the reversible fluorine storage into carbon nanotubes. J. Fluor. Chem. 2011, 132, 1072–1078. [Google Scholar] [CrossRef]
- Zhang, W.; Guérin, K.; Dubois, M.; Fawal, Z.E.; Ivanov, D.A.; Vidal, L.; Hamwi, A. Carbon nanofibres fluorinated using TbF4 as fluorinating agent. Part 1: Structural properties. Carbon 2008, 46, 1010–1016. [Google Scholar] [CrossRef]
- Umemoto, T. Recent advances in perfluoroalkylation methodology. In Fluorine-Containing Synthons; Soloshonok, V.A., Ed.; American Chemical Society: Denver, CO, USA, 2005; pp. 2–15. [Google Scholar]
- Wang, Y.Q.; Sherwood, P.M.A. Studies of carbon nanotubes and fluorinated nanotubes by X-ray and ultraviolet photoelectron spectroscopy. Chem. Mater. 2004, 16, 5427–5436. [Google Scholar] [CrossRef]
- Valentini, L.; Puglia, D.; Carniato, F.; Boccaleri, E.; Marchese, L.; Kenny, J.M. Use of plasma fluorinated single-walled carbon nanotubes for the preparation of nanocomposites with oxymatrix. Compos. Sci. Technol. 2008, 68, 1008–1014. [Google Scholar] [CrossRef]
- Tressaud, A.; Durand, E.; Labrugère, C. Surface modification of several carbon-based materials: Comparison between CF4 rf plasma and direct F2-gas fluorination routes. J. Fluor. Chem. 2004, 125, 1639–1648. [Google Scholar] [CrossRef]
- Guerin, K.; Dubois, M.; Houdayer, A.; Hamwi, A. Applicative performances of fluorinated carbons through fluorination routes: A review. J. Fluor. Chem. 2012, 134, 11–17. [Google Scholar] [CrossRef]
- Hudlicky, M.; Pavlath, A.E. Chemistry of Organic Fluorine Chemistry II: A Critical Review (ACS Monograph 187); American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Sansotera, M.; Navarrini, W.; Gola, M.; Dotelli, G.; Gallo Stampino, P.; Bianchi, C.L. Conductivity and superhydrophobic effect on PFPE-modified porous carbonaceous materials. Int. J. Hydrogen Energy 2012, 37, 6277–6284. [Google Scholar] [CrossRef]
- Sianesi, D.; Marchionni, G.; Pasquale, R.J.D. Perfluoropolyethers (PFPEs) from perfluoroolefin photooxidation. In Organo Fluorine Chemistry: Principles and Commercial Applications (Topics in Applied Chemistry); Banks, R.E., Smart, B.E., Tatlow, J.C., Eds.; Plenum Press: New York, NY, USA, 1994; pp. 431–461. [Google Scholar]
- Persico, F.; Sansotera, M.; Diamanti, M.V.; Magagnin, L.; Venturini, F.; Navarrini, W. Effect of amorphous fluorinated coatings on photocatalytic properties of anodized titanium surfaces. Thin Solid Films 2013, 545, 210–216. [Google Scholar] [CrossRef]
- Avataneo, M.; Navarrini, W.; De Patto, U.; Marchionni, G. Novel perfluoropolyethers containing 2,2,4-trifluoro-5-trifluoromethoxy-1,3-dioxole blocks: Synthesis and characterization. J. Fluor. Chem. 2009, 130, 933–937. [Google Scholar] [CrossRef]
- Navarrini, W.; Diamanti, M.V.; Sansotera, M.; Persico, F.; Menghua, W.; Magagnin, L.; Radice, S. UV-resistant amorphous fluorinated coating for anodized titanium surfaces. Prog. Org. Coat. 2012, 74, 794–800. [Google Scholar] [CrossRef]
- Demir, T.; Wei, L.; Nitta, N.; Yushin, G.; Brown, P.J.; Luzinov, I. Toward a long-chain perfluoroalkyl replacement: Water and oil repellency of polyethylene terephthalate (PET) films modified with perfluoropolyether-based polyesters. ACS Appl. Mater. Interfaces 2017, 9, 24318–24330. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.B.; Schwark, D.W.; Hirt, D.E. Surface characterization of linear low-density polyethylene films modified with fluorinated additives. Langmuir 2003, 19, 5851–5860. [Google Scholar] [CrossRef]
- Guarda, P.A.; Barchiesi, E.; Fontana, G.; Petricci, S.; Pianca, M.; Marchionni, G. Peroxidic perfluoropolyether from tetrafluoroethylene oxidation: Micro structural analysis by NMR spectroscopy and mechaniostic considerations. J. Fluor. Chem. 2005, 126, 141–153. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef] [PubMed]
- Sansotera, M.; Gola, M.; Navarrini, W. Perfluoropolyether-functionalized carbon-based materials and their applications. In New Fluorinated Carbons: Fundamentals and Applications; Boltalina, O.V., Nakajima, T., Tressaud, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 361–392. [Google Scholar]
- Sansotera, M.; Gola, M.; Dotelli, G.; Navarrini, W. The role of perfluoropolyethers in the development of polymeric proton exchange membrane fuel cells. In Fluorinated Polymers Volume 2: Applications; Ameduri, B., Sawada, H., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 158–178. [Google Scholar]
- Zhao, F.; Franz, S.; Vicenzo, A.; Cavallotti, P.L.; Sansotera, M.; Navarrini, W. Electrodeposition of nanostructured columnar cobalt for self-lubricant coatings. Electrochim. Acta 2011, 56, 9644–9651. [Google Scholar] [CrossRef]
- Yan, K.-K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic electrospun nanofiber membrane coated by carbon nanotubes network for membrane distillation. Desalination 2018, 437, 26–33. [Google Scholar] [CrossRef]
- Wang, C.-F.; Hung, S.-W.; Kuo, S.-W.; Chang, C.-J. Combining hierarchical surface roughness with fluorinated surface chemistry to preserve superhydrophobicity after organic contamination. Appl. Surf. Sci. 2014, 320, 658–663. [Google Scholar] [CrossRef]
- Hattori, Y.; Watanabe, Y.; Kawasaki, S.; Okino, F.; Pradhan, B.K.; Kyotani, T.; Tomita, A.; Touhara, H. Carbon-alloying of the rear surfaces of nanotubes by direct fluorination. Carbon 1999, 37, 1033–1038. [Google Scholar] [CrossRef]
- Li, G.; Kaneko, K.; Ozeki, S.; Okino, F.; Touhara, H. Water rejective nature of fluorinated microporous carbon fibers. Langmuir 1995, 11, 716–717. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Gola, M.; Bianchi, C.L.; Wormald, P.; Famulari, A.; Avataneo, M. Peroxidic perfluoropolyether for the covalent binding of perfluoropolyether chains on carbon black surface. J. Fluor. Chem. 2012, 132, 1254–1261. [Google Scholar] [CrossRef]
- Sansotera, M.; Bianchi, C.L.; Lecardi, G.; Marchionni, G.; Metrangolo, P.; Resnati, G.; Navarrini, W. Highly hydrophobic carbon black obtained by covalent linkage of perfluorocarbon and perfluoropolyether chains on the carbon surface. Chem. Mater. 2009, 21, 4498–4504. [Google Scholar] [CrossRef]
- Hirsch, G.; Vostrowsky, O. Functionalization of carbon nanotubes. In Functional Organic Materials: Syntheses, Strategies and Applications; Muller, T.J.J., Bunz, U.H.F., Eds.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2007; pp. 1–57. [Google Scholar]
- Galimberti, M.; Barchiesi, E.; Navarrini, W. α-Branched perfluorodiacyl peroxides: Preparation and characterization. J. Fluor. Chem. 2005, 126, 587–593. [Google Scholar] [CrossRef]
- Thommes, M.; Kohn, R.; Froba, M. Systematic sorption studies on surface and pore size characteristics of different MCM-48 silica materials. In Studies in Surface Science and Catalysis, Vol. 128—Characterisation of Porous Solids V, Proceedings of the 5th International Symposium on the Characterisation of Porous Solids (COPS-V), Heidelberg, Germany, 30 May–2 June 1999; Unger, K.K., Kreysa, G., Baselt, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 259–268. [Google Scholar]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.L.; Guarda, P.A.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon 2013, 59, 150–159. [Google Scholar] [CrossRef]
- Poquillon, D.; Viguier, B.; Andrieu, E. Experimental data about mechanical behavior during compression tests for various matted fibres. J. Mater. Sci. 2005, 40, 5963–5970. [Google Scholar] [CrossRef] [Green Version]
- Slobodian, P.; Riha, P.; Olejnik, R.; Saha, P. Electromechanical properties of carbon nanotube networks under compression. Meas. Sci. Technol. 2011, 22, 1–7. [Google Scholar] [CrossRef]
- Touhara, H.; Okino, F. Property control of carbon materials by fluorination. Carbon 2000, 38, 241–267. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Magagnin, L.; Bianchi, C.L.; Sanguineti, A.; Metrangolo, P.; Resnati, G. Hydrophobic carbonaceous materials obtained by covalent bonding of perfluorocarbon and perfluoropolyether chains. J. Mater. Chem. 2010, 20, 8607–8616. [Google Scholar] [CrossRef]
- Navarrini, W.; Bianchi, C.L.; Magagnin, L.; Nobili, L.; Carignano, G.; Metrangolo, P.; Resnati, G.; Sansotera, M. Low surface energy coatings covalently bonded on diamond-like carbon films. Diam. Relat. Mater. 2010, 19, 336–341. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, J.; Macias-Garcia, A.; Alexandre-Franco, M.F.; Gomez-Serrano, V. Electrical conductivity of carbon blacks under compression. Carbon 2005, 43, 741–747. [Google Scholar] [CrossRef]

| Specimen | Contact Angle | Surface Composition 1 (at %) | Specific Surface Area 2 (m2/g) | Micropore Area 3 (m2/g) | |||
|---|---|---|---|---|---|---|---|
| Static | Hysteresis | F | O | C | |||
| MW-CNTs | n.s. 4 | - | - | 1.3 | 98.7 | 389 | 31 |
| I-BP50 | 174° | 4.0° | 9.2 | 2.1 | 88.7 | 231 | 0 |
| II-LP50 | 159° | 5.3° | 4.2 | 2.4 | 93.4 | 308 | 0 |
| III-F | 172° | 4.2° | 14.2 | 2.0 | 83.8 | 277 | 26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansotera, M.; Talaeemashhadi, S.; Gambarotti, C.; Pirola, C.; Longhi, M.; Ortenzi, M.A.; Navarrini, W.; Bianchi, C.L. Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes. Nanomaterials 2018, 8, 176. https://doi.org/10.3390/nano8030176
Sansotera M, Talaeemashhadi S, Gambarotti C, Pirola C, Longhi M, Ortenzi MA, Navarrini W, Bianchi CL. Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes. Nanomaterials. 2018; 8(3):176. https://doi.org/10.3390/nano8030176
Chicago/Turabian StyleSansotera, Maurizio, Sadaf Talaeemashhadi, Cristian Gambarotti, Carlo Pirola, Mariangela Longhi, Marco A. Ortenzi, Walter Navarrini, and Claudia L. Bianchi. 2018. "Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes" Nanomaterials 8, no. 3: 176. https://doi.org/10.3390/nano8030176