In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HTT/Graphene Nanohybrids
2.3. Characterization
2.4. Photocatalytic Experiment of Alcohol Oxidation
2.5. Radical Scavenging Experiments
3. Results and Discussion
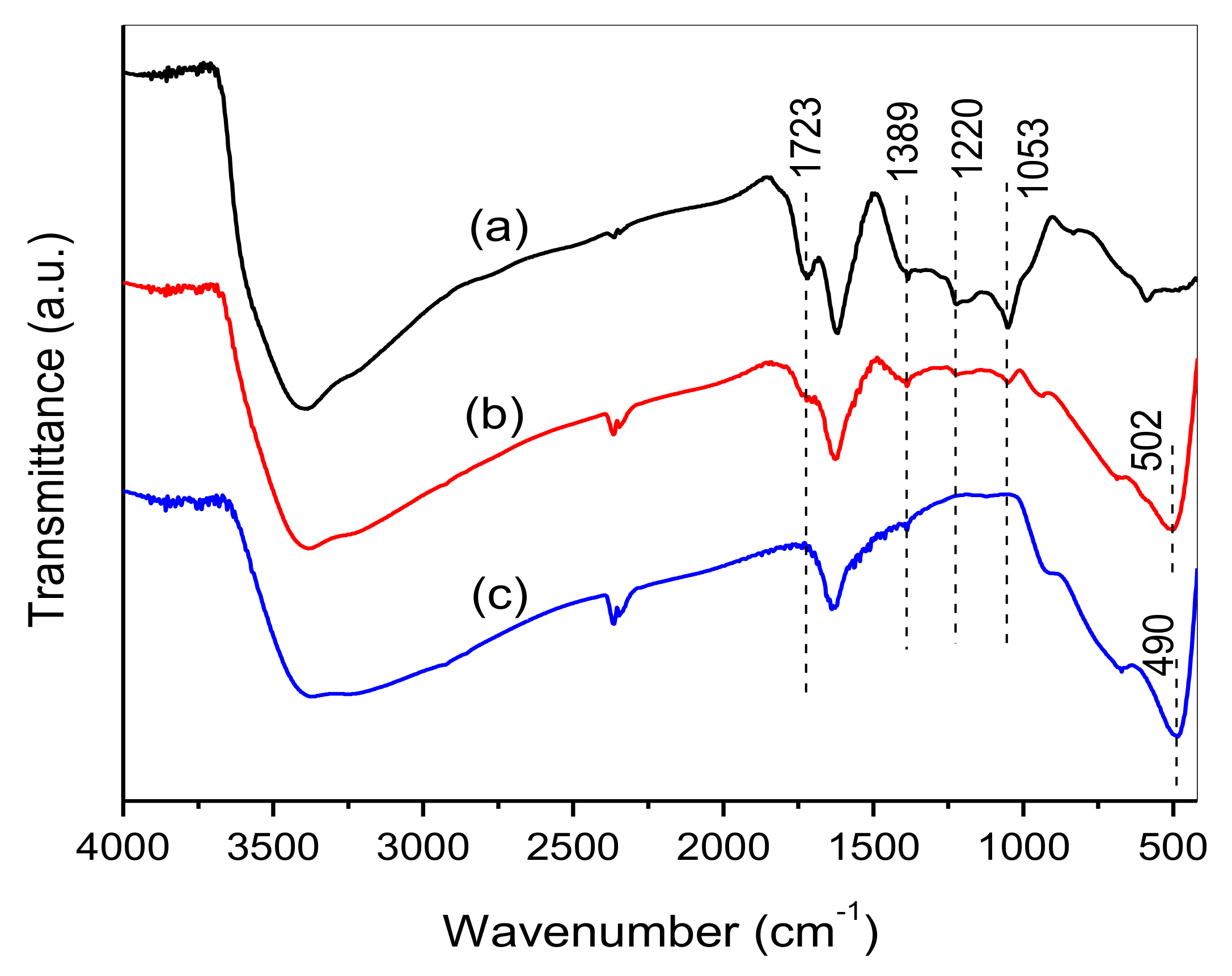
3.1. XRD, FTIR and Raman Spectra
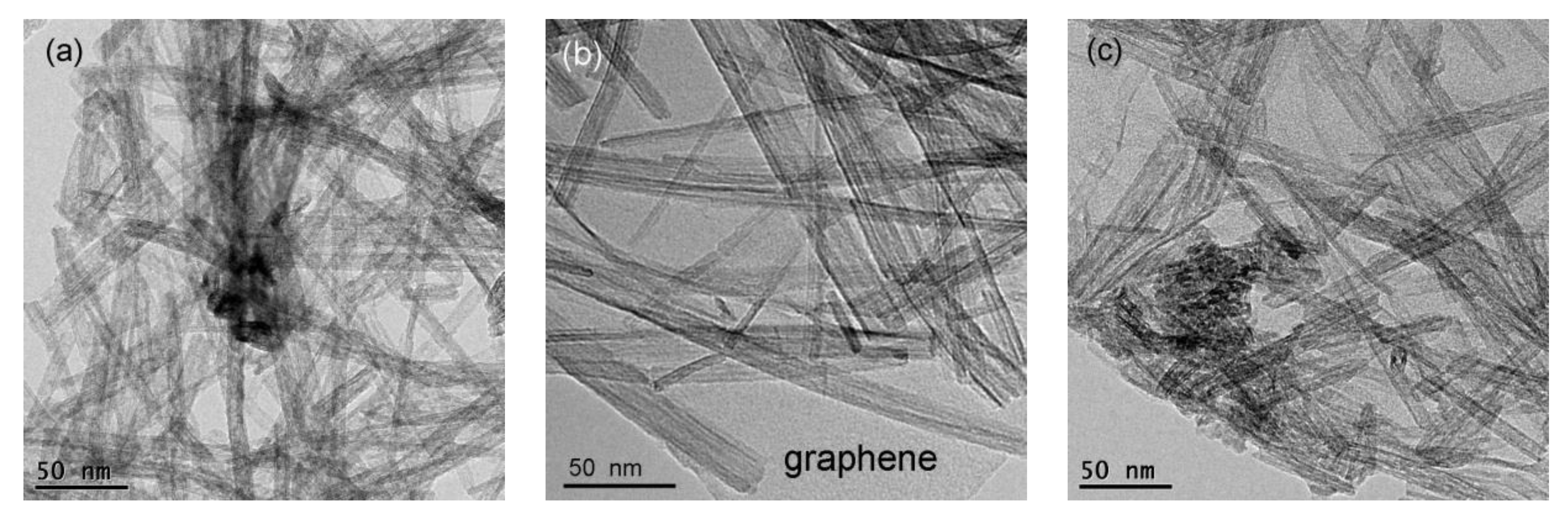
3.2. TEM and Brunauer–Emmett–Teller (BET) Surface Area Analyses
3.3. XPS Analysis
3.4. Enhanced Visible Light Catalytic Activities
3.5. Stability of Composite Photocatalysts
3.6. Visible Light Absorption Generated by Benzyl Alcohol Surface Adsorption
3.7. Improved Charge Transfer via the Chemically Bonded Interface between HTT and Graphene
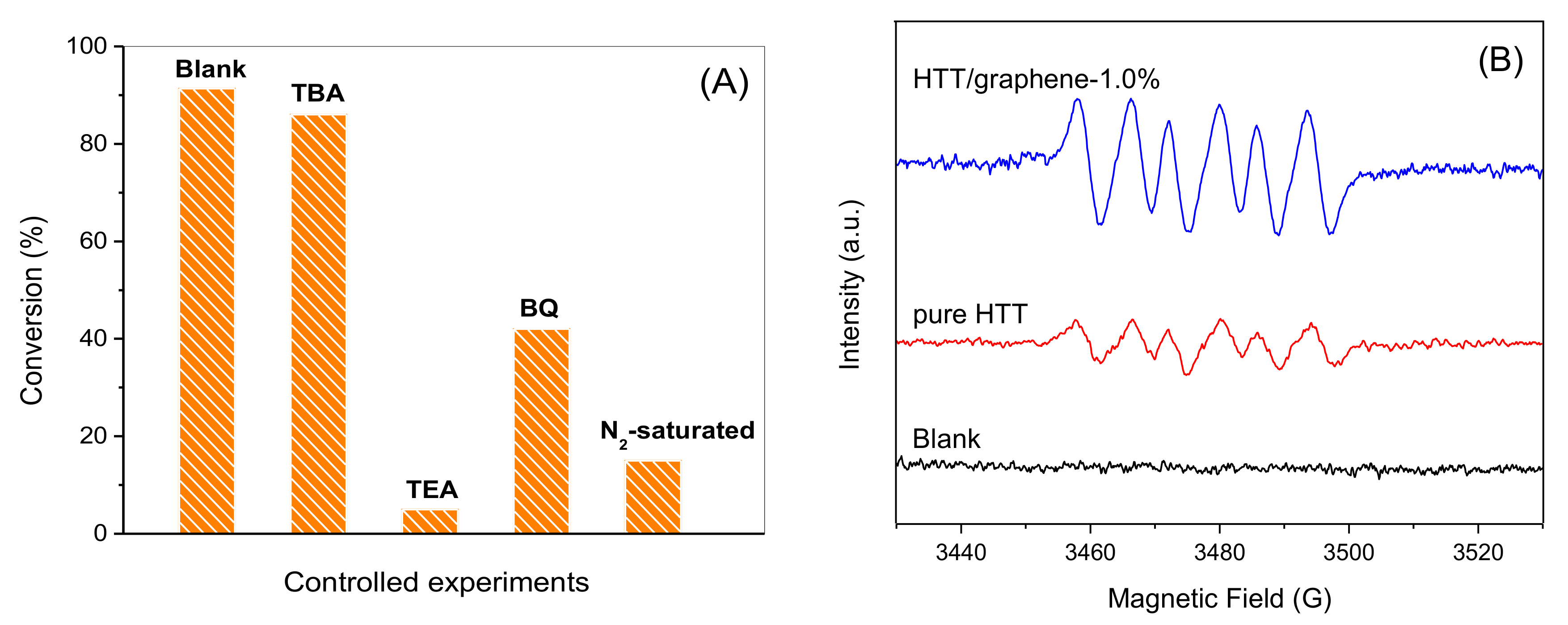
3.8. Radical Trapping Experiments
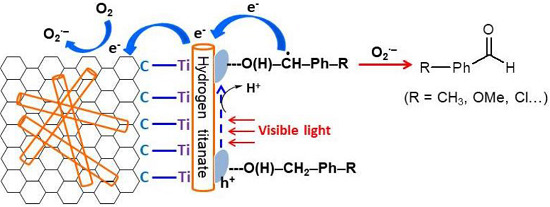
3.9. Mechanism of Improved Visible Light Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, C.C.; Ma, W.H.; Zhao, J.C. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Lang, X.J.; Ma, W.H.; Chen, C.C.; Ji, H.W.; Zhao, J.C. Selective aerobic oxidation mediated by TiO2 photocatalysis. Acc. Chem. Res. 2014, 47, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Li, Z.J.; Sun, L.Q.; Zhang, X.L.; Raziq, F.; Zada, A.; Qu, Y.; Jing, L.Q. Coupling of nanocrystalline anatase TiO2 to aporous nanosized LaFeO3 for efficient visible-light photocatalytic degradation of pollutants. Nanomaterials 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Liu, H.W.; Zheng, Z.F.; Yuan, Y.; Zhao, J.C.; Waclawik, E.R.; Ke, X.B.; Zhu, H.Y. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Wang, Z.L.; Wang, W.D.; Huang, W.X. Engineering highly active TiO2 photocatalysts via the surface-phase junction strategy employing a titanate nanotube precursor. J. Catal. 2014, 310, 16–23. [Google Scholar] [CrossRef]
- Zheng, Z.K.; Huang, B.B.; Lu, J.B.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y.; Whangbo, M.H. Hydrogenated titania: Synergy of surface modification and morphology improvement for enhanced photocatalytic activity. Chem. Commun. 2012, 28, 5733–5735. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharyya, K.; Ayyub, P.; Tyagi, A.K. Photocatalytic properties of one-dimensional nanostructured titanates. J. Phys. Chem. C 2010, 114, 9424–9430. [Google Scholar] [CrossRef]
- Yu, J.G.; Xiang, Q.J.; Zhou, M.H. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B-Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Zheng, Z.F.; Liu, H.W.; Ye, J.P.; Zhao, J.C.; Waclawika, E.R.; Zhu, H.Y. Structure and contribution to photocatalytic activity of the interfaces in nanofibers with mixed anatase and TiO2(B) phases. J. Mol. Catal. A-Chem. 2010, 316, 75–82. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; Guillard, C.; Berhault, G.; Ghorbel, A. Effect of Na content and thermal treatment of titanate nanotubes on the photocatalytic degradation of formic acid. Appl. Catal. B-Environ. 2013, 138, 401–415. [Google Scholar] [CrossRef]
- Tang, Z.R.; Li, F.; Zhang, Y.H.; Fu, X.Z.; Xu, Y.-J. Composites of titanate nanotube and carbon nanotube as photocatalyst with high mineralization ratio for gas-phase degradation of volatile aromatic pollutant. J. Phys. Chem. C 2011, 115, 7880–7886. [Google Scholar] [CrossRef]
- Kim, I.Y.; Lee, J.M.; Kim, T.W.; Kim, H.N.; Kim, H.; Choi, W.; Hwang, S.-J. A strong electronic coupling between graphene nanosheets and layered titanate nanoplates: A soft-chemical route to highly porous nanocomposites with improved photocatalytic activity. Small 2012, 8, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.G.; Han, K.; Ye, H.Q.; Zhu, C.Y.; Gao, Y.P.; Liu, Y.; Zhou, Y.H. Graphene oxide/triethanolamine modified titanate nanowires as photocatalytic membrane for water treatment. Chem. Eng. J. 2017, 320, 74–80. [Google Scholar] [CrossRef]
- Kim, S.; Han, K.I.; Lee, I.G.; Park, W.K.; Yoon, Y.; Yoo, C.S.; Yang, W.S.; Hwang, W.S. A gallium oxide-graphene oxide hybrid composite for enhanced photocatalytic reaction. Nanomaterials 2016, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.Q.; Peng, W.C. 2D transition metal dichalcogenides and graphene-based ternary composites for photocatalytic hydrogen evolution and pollutants degradation. Nanomaterials 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, M.-Q.; Tang, Z.-R.; Xu, Y.-J. CdS-graphene nanocomposites as visible light photocatalyst for redox reactions in water: A green route for selective transformation and environmental remediation. J. Catal. 2013, 303, 60–69. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.J.; Li, Y.M.; Wang, Y.; Li, J.H. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Guo, J.J.; Zhu, S.M.; Mao, L.; Ma, J.; Zhang, D. A high-performance Bi2WO6-graphene photocatalyst for visible light-induced H2 and O2 generation. Nanoscale 2014, 6, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.D.; Zeng, D.W.; Huang, Q.W.; Tian, S.Q.; Xie, C.S. Chemically bonded graphene/BiOCl nanocomposites as high-performance photocatalysts. Phys. Chem. Chem. Phys. 2012, 14, 10572–10578. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.W.; Tian, S.Q.; Zeng, D.W.; Wang, X.X.; Song, W.L.; Li, Y.Y.; Xiao, W.; Xie, C.S. Enhanced photocatalytic activity of chemically bonded TiO2/graphene composites based on the effective interfacial charge transfer through the C-Ti bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: A promising route for 21st century organic chemistry. Chem. Commun. 2007, 43, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Q.; Chen, C.; Zang, L.; Ma, W.; Zhao, J. Oxygen atom transfer in the photocatalytic oxidation of alcohols by TiO2: Oxygen isotope studies. Angew. Chem. Int. Ed. 2009, 48, 6081–6084. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hashimoto, K.J.; Kominami, H. Preparation of Au/CeO2 exhibiting strong surface plasmon resonance effective for selective or chemoselective oxidation of alcohols to aldehydes or ketones in aqueous suspensions under irradiation by green light. J. Am. Chem. Soc. 2012, 134, 14526–14533. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.J.; Wen, L.R.; Lin, S.; Bi, J.H.; Feng, P.Y.; Fu, X.Z.; Wu, L. Monolayer HNb3O8 for selective photocatalytic oxidation of benzylic alcohols with visible light response. Angew. Chem. Int. Ed. 2014, 53, 2951–2955. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Yang, J.; Shen, X.X.; Wei, J.P.; Zhang, L.N.; Zhao, D.; Yao, B.H. Selective oxidation of alcohols on hydrogen titanate nanotubes under visible light irradiation: Relationship between nanostructure and catalytic activity. Catal. Sci. Technol. 2016, 6, 7604–7614. [Google Scholar] [CrossRef]
- Yu, L.H.; Lin, Y.M.; Li, D.Z. Visible-light-induced aerobic oxidation of alcohols in a green catalytic system of carbonate-like species doped TiO2. Appl. Catal. B-Environ. 2017, 216, 88–94. [Google Scholar] [CrossRef]
- Andryushina, N.S.; Stroyuk, O.L. Influence of colloidal graphene oxide on photocatalytic activity of nanocrystalline TiO2 in gas-phase ethanol and benzene oxidation. Appl. Catal. B-Environ. 2014, 148, 543–549. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Feng, J.F.; Chen, Y.M.; Gao, S.Y.; Cao, R. Visible-light-driven selective oxidation of alcohols using a dye-sensitized TiO2-polyoxometalate catalyst. J. Catal. 2017, 351, 59–66. [Google Scholar] [CrossRef]
- Zhai, Q.Q.; Bo, T.; Hu, G.X. High photoactive and visible-light responsive graphene/titanate nanotubes photocatalysts: Preparation and characterization. J. Hazard. Mater. 2011, 198, 78–86. [Google Scholar]
- Dang, H.F.; Dong, X.F.; Dong, Y.C.; Huang, J.S. Facile and green synthesis of titanate nanotube/graphene nanocomposites for photocatalytic H2 generation from water. Int. J. Hydrog. Energy 2013, 38, 9178–9185. [Google Scholar] [CrossRef]
- Zou, J.P.; Ma, J.; Huang, Q.; Luo, S.L.; Yu, J.; Luo, X.B.; Dai, W.L.; Sun, J.; Guo, G.C.; Au, C.T.; et al. Graphene oxide as structure-directing and morphology-controlling agent for the syntheses of heterostructured graphene-Bi2MoO6/Bi3.64Mo0.36O6.55 composites with high photocatalytic activity. Appl. Catal. B-Environ. 2014, 156, 447–455. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Synthesis and characterization of ion-exchangeable titanate nanotubes. Chem.-Eur. J. 2003, 9, 2229–2238. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.H.; Zhao, X.L.; Dai, J.; Mo, S.R. Synthesis of uniform Bi2WO6-reduced graphene oxide nanocomposites with significantly enhanced photocatalytic reduction activity. J. Phys. Chem. C 2015, 119, 3068–3078. [Google Scholar] [CrossRef]
- Manna, A.K.; Pati, S.K. Tuning the electronic structure of graphene by molecular charge transfer: A computational study. Chem.-Asian J. 2009, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin Film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Shishido, T.; Teramura, K.; Tanaka, T. A unique photo-activation mechanism by “in situ doping” for photo-assisted selective NO reduction with ammonia over TiO2 and photooxidation of alcohols over Nb2O5. Catal. Sci. Technol. 2011, 1, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Ma, W.; Zhao, Y.; Chen, C.; Ji, H.; Zhao, J. Visible-light-induced selective photocatalytic aerobic oxidation of amines into imines on TiO2. Chem.-Eur. J. 2012, 18, 2624–2631. [Google Scholar] [CrossRef]
- Yang, M.Q.; Zhang, N.; Xu, Y.J. Synthesis of fullerene-, carbon nanotube-, and graphene-TiO2 nanocomposite photocatalysts for selective oxidation: A comparative study. ACS Appl. Mater. Inter. 2013, 5, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, H.; Liu, Y.; Li, J.H. Graphene and its derivatives for the development of solar cells, photoelectrochemical, and photocatalytic applications. Energy Environ. Sci. 2013, 6, 1362–1387. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G. Graphene-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2013, 4, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Wang, F.C.; Fu, X.Z.; Zheng, Y. A green and facile self-assembly preparation of gold nanoparticles/ZnO nanocomposite for photocatalytic and photoelectrochemical applications. J. Mater. Chem. 2012, 22, 2868–2877. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Jiang, J.; Zhang, L.Z. Selective oxidation of benzyl alcohol into benzaldehyde over semiconductors under visible light: The case of Bi12O17Cl2 nanobelts. Appl. Catal. B-Environ. 2013, 142, 487–493. [Google Scholar] [CrossRef]










| Catalyst | BET Surface Area/m2·g−1 | Conv./% | Rate Constant k/h−1 | SBET Normalized Rate Constants k |
|---|---|---|---|---|
| pure HTT | 225.1 | 27.6 | 0.053 | 0.24 × 10−3 |
| HTT/graphene-0.2% | 228.9 | 57.1 | 0.141 | 0.61 × 10−3 |
| HTT/graphene-0.4% | 234.4 | 78.0 | 0.252 | 1.08 × 10−3 |
| HTT/graphene-1.0% | 240.5 | 91.8 | 0.411 | 1.71 × 10−3 |
| HTT/graphene-2.0% | 244.7 | 69.2 | 0.195 | 0.80 × 10−3 |
| HTT/graphene-4.0% | 248.0 | 43.8 | 0.097 | 0.39 × 10−3 |
| HTT/graphene-1.0%-mixed | 233.6 | 32.3 | 0.078 | 0.33 × 10−3 |
| Entry | Substrate | Product | Time (h) | Conv. (%) | Sel. (%) |
|---|---|---|---|---|---|
| 1 | 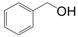 | 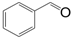 | 6 | 91.8 | 96 |
| 2 |  | 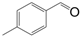 | 5 | 92.5 | 97 |
| 3 |  | 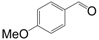 | 4.5 | 97.6 | 96 |
| 4 | 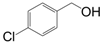 | 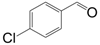 | 7 | 89.2 | 97 |
| 5 |  | 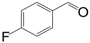 | 9 | 84.9 | 98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; You, J.; Dai, J.; Chen, Y.; Li, Y. In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity. Nanomaterials 2018, 8, 229. https://doi.org/10.3390/nano8040229
Yang J, You J, Dai J, Chen Y, Li Y. In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity. Nanomaterials. 2018; 8(4):229. https://doi.org/10.3390/nano8040229
Chicago/Turabian StyleYang, Juan, Jun You, Jun Dai, Yumei Chen, and Yao Li. 2018. "In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity" Nanomaterials 8, no. 4: 229. https://doi.org/10.3390/nano8040229