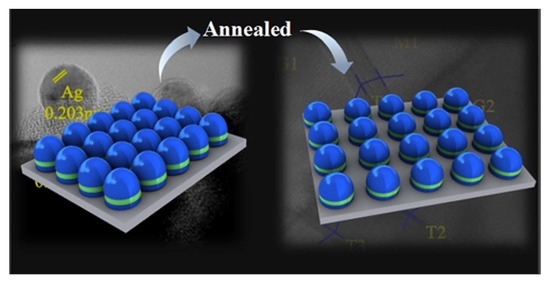
Ag Nanotwin-Assisted Grain Growth-Induced by Stress in SiO2/Ag/SiO2 Nanocap Arrays
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PS Colloidal Sphere Arrays
2.3. Preparation and Annealing of PS 500 nm/SiO2 12 nm/Ag 60 nm/SiO2 12 nm Nanocap Arrays
2.4. Characterization of Substrates
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rupert, T.J.; Gianola, D.S.; Gan, Y.; Hemker, K.J. Experimental observations of stress-driven grain boundary migration. Science 2009, 326, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhan, F.Y.; Gong, G.D.; Cao, Y.L.; Zhen, Q.; Hu, P.F. Room-temperature mechanochemical synthesis of silver nanoparticle homojunction assemblies for the surface-enhanced raman scattering substrate. RSC Adv. 2016, 6, 74662–74669. [Google Scholar] [CrossRef]
- Kumar, K.S.; Swygenhoven, H.V.; Sureshc, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Zhang, X.; Misra, A.; Wang, H.; Shen, T.D.; Nastasi, M.; Mitchell, T.E.; Hirth, J.P.; Hoagland, R.G.; Embury, J.D. Enhanced hardening in Cu/330 stainless steel multilayers by nanoscale twinning. Acta Mater. 2004, 52, 995–1002. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.F.; Chen, X.H.; Qian, L.H.; Lu, K. A Study on Ultrahigh Strength and High Electrical Conductivity in Copper. Sciences 2004, 304, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wang, Y.M.; Lu, Q.H.; Sui, M.L.; Lu, L.; Lu, K. Strain hardening and large tensile elongation in ultrahigh-strength nano-twinned copper. Appl. Phys. Lett. 2004, 85, 4932–4934. [Google Scholar] [CrossRef]
- Zhang, X.; Misra, A. Superior thermal stability of coherent twin boundaries in nanotwinned metals. Scr. Mater. 2012, 66, 860–865. [Google Scholar] [CrossRef]
- Lu, L.; Sui, M.L.; Lu, K. Superplastic extensibility of nanocrystalline copper at room temperature. Sciences 2000, 287, 1463–1466. [Google Scholar] [CrossRef]
- Shen, Y.F.; Lu, L.; Lu, Q.H.; Jin, Z.H.; Lu, K. Tensile properties of copper with nano-scale twins. Scr. Mater. 2005, 52, 989–994. [Google Scholar] [CrossRef]
- Lu, K.; Lu, L.; Suresh, S. Strengthening materials by engineering coherent internal boundaries at the nanoscale. Science 2009, 324, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.J.; Moldovan, D.; Yamakov, V.; Wolf, D.; Phillpot, S.R.; Gleiter, H. Stress-enhanced grain growth in a nanocrystalline material by molecular-dynamics simulation. Acta Mater. 2003, 51, 2097–2112. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Liao, X.Z.; Wu, X.L. Deformation twinning in nanocrystalline metals. J. Mater. Eng. Perform. 2005, 14, 467–472. [Google Scholar] [CrossRef]
- Gottstein, G.; Shvindlerman, L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Feng, X.M.; Ruan, F.X.; Hong, R.J.; Ye, J.S.; Hu, J.Q.; Hu, G.Q.; Yang, Z.L. Synthetically directed self-assembly and enhanced surface-enhanced Raman scattering property of twinned crystalline Ag/Ag homojunction nanoparticles. Langmuir 2011, 27, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Lu, L.; Lu, K. Electrical resistivity of ultrafine-grained copper with nanoscale growth twins. J. Appl. Phys. 2007, 102, 083708. [Google Scholar] [CrossRef]
- Anderoglu, O.; Misra, A.; Wang, H.; Zhang, X. Thermal stability of sputtered Cu films with nanoscale growth twins. J. Appl. Phys. 2008, 103, 094322. [Google Scholar] [CrossRef]
- Bufford, D.; Wang, H.; Zhang, X. High strength, epitaxial nanotwinned Ag films. Acta Mater. 2011, 59, 93–101. [Google Scholar] [CrossRef]
- Luo, X.M.; Zhu, X.F.; Zhang, G.P. Nanotwin-assisted grain growth in nanocrystalline gold films under cyclic loading. Nat. Commun. 2014, 5, 3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.; Zhang, Y.J.; Ding, X.; Cheng, Y.; Yang, J.H. Sphere surface-induced exchange bias effect of NiO/FeNi nanostructures. Solid State Commun. 2008, 148, 83–86. [Google Scholar] [CrossRef]
- Shanthil, M.; Fathima, H.; George Thomas, K. Cost-Effective Plasmonic Platforms: Glass Capillaries Decorated with Ag@SiO2 Nanoparticles on Inner Walls as SERS Substrates. ACS Appl. Mater. Interfaces 2017, 9, 19470–19477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Zhang, Q.P.; Park, S.Y.; Choe, A.; Fan, Z.Y.; Ko, H. Particle-Film Plasmons on Periodic Silver Film over Nanosphere (AgFON): A Hybrid Plasmonic Nanoarchitecture for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Bi, M.H.; Cui, L.; Zhou, Y.Z.; Du, X.W.; Qiao, S.Z.; Yang, J. 3D Aluminum Hybrid Plasmonic Nanostructures with Large Areas of Dense Hot Spots and Long-Term Stability. Adv. Funct. Mater. 2017, 1605703. [Google Scholar] [CrossRef]
- Chang, S.; Eichmann, S.L.; Huang, T.Y.; Yun, W.J.; Wang, W. Controlled Design and Fabrication of SERS-SEF Multifunctional Nanoparticles for Nanoprobe Applications: Morphology-Dependent SERS Phenomena. J. Phys. Chem. C 2017, 121, 8070–8076. [Google Scholar] [CrossRef]
- Jiang, R.B.; Qin, F.; Liu, Y.J.; Ling, X.Y.; Guo, J.; Tang, M.H.; Cheng, S.; Wang, J.F. Colloidal Gold Nanocups with Orientation-Dependent Plasmonic Properties. Adv. Mater. 2016, 28, 6322–6331. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Rana, U.; Malik, S. Facile Decoration of Polyaniline Fiber with Ag Nanoparticles for Recyclable SERS Substrate. ACS Appl. Mater. Interfaces 2015, 7, 10457–10465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Yan, C.; Chen, L.; Zhang, Y.J.; Yang, J.H. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Rupich, S.M.; Shevchenko, E.V.; Bodnarchuk, M.I.; Lee, B.; Talapin, D.V. Size-dependent multiple twinning in nanocrystal superlattices. J. Am. Chem. Soc. 2010, 132, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Liao, X.Z.; Wu, X.L.; Narayan, J. Grain size effect on deformation twinning and detwinning. J. Mater. Sci. 2013, 48, 4467–4475. [Google Scholar] [CrossRef] [Green Version]
- Meyers, M.A.; Vöhringe, O.; Lubarda, V.A. The onset of twinning in metals: A constitutive description. Acta Mater. 2001, 49, 4025–4039. [Google Scholar] [CrossRef]
- Stoney, G.G. The Tension of Metallic Films Deposited by Electrolysis. Proc. R. Soc. Lond. 1909, 82, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Freund, L.B.; Suresh, S. Thin Film Materials: Stress, Defect Formation, and Surface Evolution; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Coble, R.L. A Model for Boundary Diffusion Controlled Creep in Polycrystalline Materials. J. Appl. Phys. 1963, 34, 1679–1682. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Jiao, Y.; Tian, Y.; Wang, Y.G.; Jiang, Z.Y. Biomimetic Synthesis of TiO2–SiO2–Ag Nanocomposites with Enhanced Visible-Light Photocatalytic Activity. Appl. Mater. Interfaces 2013, 5, 3824–3832. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, M.; Yun, K.S. Ultrasonochemically Conjugated Metalloid/Triblock Copolymer Nanocomposite and Subsequent Thin Solid Laminate Growth for Surface and Interface Studies. Langmuir 2010, 26, 14216–14222. [Google Scholar] [CrossRef] [PubMed]
- Ganeev, R.A.; Ryasnyansky, A.I.; Stepanov, A.L.; Usmanov, T. Saturated absorption and nonlinear refraction of silicate glasses doped with silver nanoparticles at 532 nm. Opt. Quantum Electron. 2004, 36, 949–960. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.M.; Fan, C.Z.; Wang, J.Q.; He, J.N.; Liang, E.J.; Chao, M.J. Realization of high sensitive SERS substrates with one-pot fabrication of Ag-Fe3O4 nanocomposites. J. Colloid Interface Sci. 2015, 438, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced Raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wen, J.H.; Zhang, M.N.; Wang, D.H.; Wang, Y.X.; Chen, L.; Zhang, Y.J.; Yang, J.H.; Du, Y.W. Design of Hybrid Nanostructural Arrays to Manipulate SERS-active Substrates by Nanosphere Lithography. ACS Appl. Mater. Interfaces 2017, 9, 7710–7716. [Google Scholar] [CrossRef] [PubMed]




| Wavenumber (cm−1) | Band Assignment |
|---|---|
| 1584 | υ(CC) ring |
| 1481 | υ(CC) + δ(CH) |
| 1360 | νs(COO-) |
| 1182 | δ(CH) |
| 1133 | δ(CH) |
| 1077 | υ(CC) ring |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, Y.; Zhang, Y.; Chen, L.; Liu, Y.; Yang, J. Ag Nanotwin-Assisted Grain Growth-Induced by Stress in SiO2/Ag/SiO2 Nanocap Arrays. Nanomaterials 2018, 8, 436. https://doi.org/10.3390/nano8060436
Zhang F, Wang Y, Zhang Y, Chen L, Liu Y, Yang J. Ag Nanotwin-Assisted Grain Growth-Induced by Stress in SiO2/Ag/SiO2 Nanocap Arrays. Nanomaterials. 2018; 8(6):436. https://doi.org/10.3390/nano8060436
Chicago/Turabian StyleZhang, Fan, Yaxin Wang, Yongjun Zhang, Lei Chen, Yang Liu, and Jinghai Yang. 2018. "Ag Nanotwin-Assisted Grain Growth-Induced by Stress in SiO2/Ag/SiO2 Nanocap Arrays" Nanomaterials 8, no. 6: 436. https://doi.org/10.3390/nano8060436