Biosynthesis of Silver Nanoparticles Using Ligustrum Ovalifolium Fruits and Their Cytotoxic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Extract
2.3. Determination of Total Phenolic Content
2.4. Determination of Antioxidant Activity
2.5. Synthesis of AgNPs
2.6. Characterization of the AgNPs
2.7. Cytotoxic Activity
3. Results and Discussion
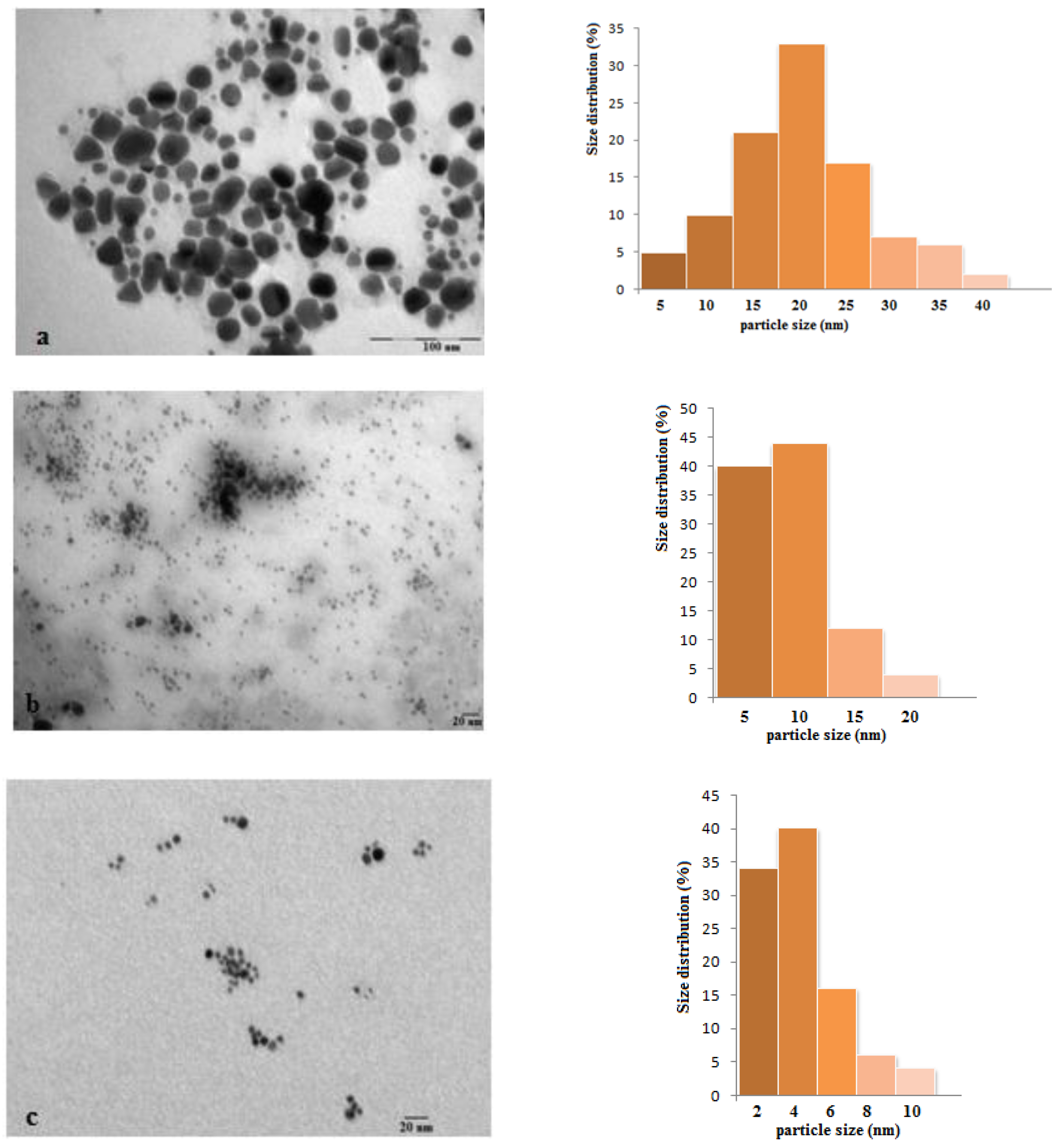
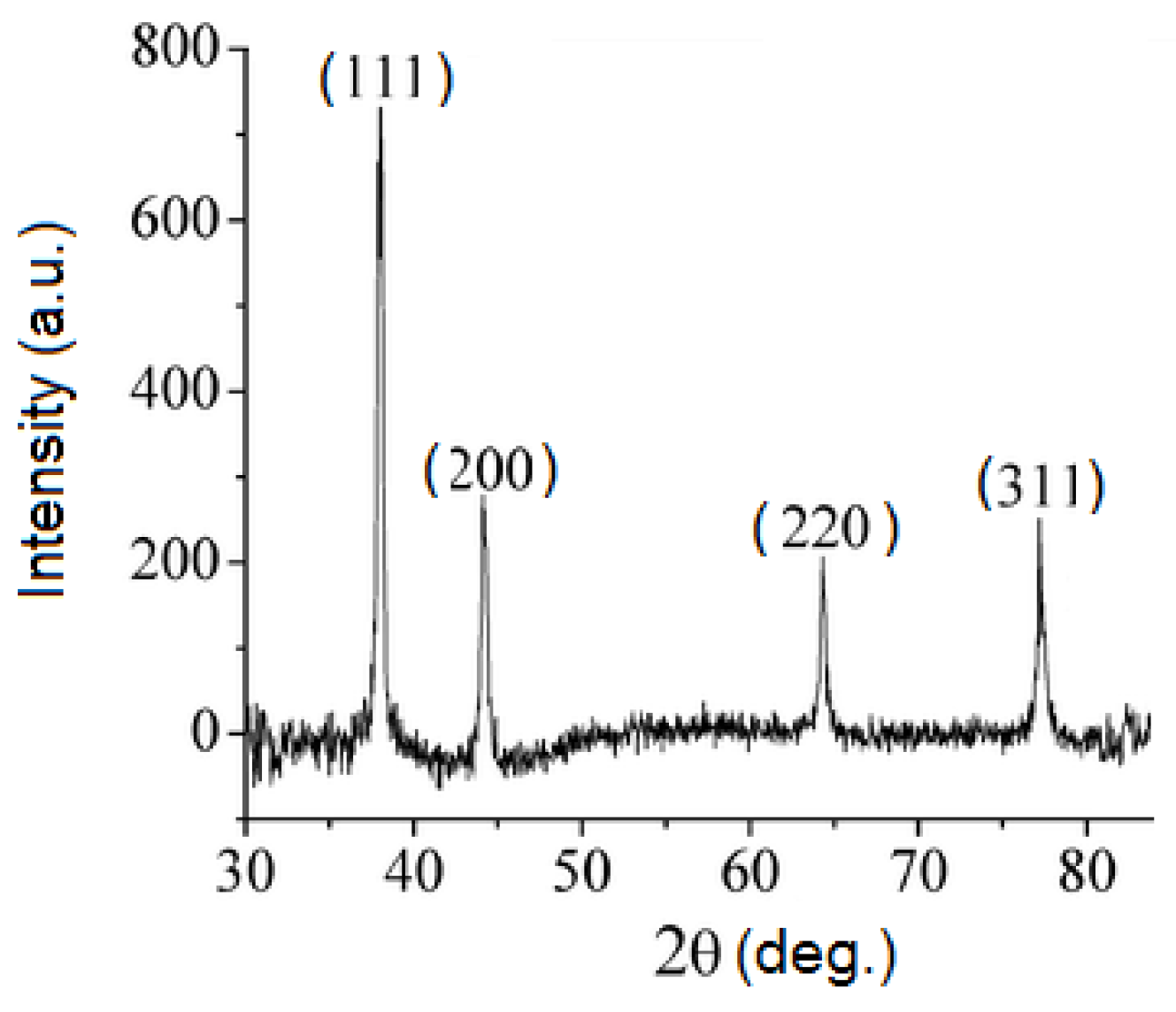
3.1. Synthesis and Characterization of Silver Nanoparticles
3.2. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Janardhanan, R.; Karuppaiah, M.; Hebalkar, N.; Rao, T.N. Synthesis and surface chemistry of nano silver particles. Polyhedron 2009, 28, 2522–2530. [Google Scholar] [CrossRef]
- Thakkar, N.; Mhatre, S.S.; Parich, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Hsu, C.C.; Yin, M.C. Antioxidative characteristics of aqueous and ethanol extracts of glossy privet fruit. Food Chem. 2009, 112, 914–918. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruit extract. Mater. Sci. Eng. C 2017, 79, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, L.; Decea, N.; Kiss, M.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosynthesized gold nanoparticles on diabetic rats. Colloids Surf. B 2017, 150, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.E.; Al-Qahtani, A.; al-Mutairi, A.; Al-Shamri, B.; Aabed, K. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; David, L.; Achim, M.; Clichici, S.; Filip, G.A. A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruit extract and their antioxidant activity. J. Mol. Liq. 2016, 221, 271–278. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Vijaya, J.J.; Kaviyarasu, K.; Kombaiah, K.; Kennedy, L.J.; Ramalingam, R.J.; Munusamy, M.A.; Al-Lohedan, H.A. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Cao, C.; Zhang, X.; Zhan, Y.; Wang, X.; Yang, X.; Zhou, K.; Xiao, X.; Yuan, B. Woolfberry fruit (Licium barbarum) extract mediated novel route for the green synthesis of silver nanoparticles. Opt. Int. J. Light Electron Opt. 2017, 130, 162–170. [Google Scholar] [CrossRef]
- Filip, A.G.; Potara, M.; Florea, A.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; David, L.; Moldovan, B.; Olenic, L.; et al. Comparative evaluation by scanning confocal Raman spectroscopy and transmission electron microscopy of therapeutic effects of noble metal nanoparticles in experimental acute inflammation. RSC Adv. 2015, 5, 67435–67448. [Google Scholar] [CrossRef]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharovschi, S.; Bolfa, P.; David, L. Antioxidant Activity of Cornelian Cherry (Cornus mas L.) Fruits Extracts and the in Vivo Evaluation of their Anti-inflammatory Effects. J. Func. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Danila, O.O.; Berghian, A.S.; Dionisie, V.; Gheban, D.; Olteanu, D.; Tabaran, F.; Baldea, I.; Katona, G.; Moldovan, B.; Clichici, S.; et al. The effects of silver nanoparticles on behaviour, apoptosis and nitro-oxidative stress in offspring Wistar rats. Nanomedicine 2017, 12, 1455–1473. [Google Scholar] [CrossRef] [PubMed]
- Ajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications and toxicity effects. Int. Nano Lett. 2012, 2. [Google Scholar] [CrossRef]
- Singh, P.; Kin, Y.J.; Zhang, D.; Yan, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, H.; Chen, Z.S.; Chen, G. Silver nanoparticles: synthesis, properties and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barrasa, J.; Lopez-de-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: synthesis through chemical methods in solution and biological applications. Cent. Eur. J. Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shan, A.; Liu, T.; Zhang, C.; Zhang, Z. In vitro immunomodulatory effects of an oleanolic acid-enriched extract of Ligustrum lucidum fruit (Ligustrum lucidum supercritical CO2 extract) on piglet immunocytes. Int. Immunopharmacol. 2012, 14, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.K.; Wu, W.K.; Pak, W.F.; Ko, K.M. Hepatoprotective action of an oleanolic acid-enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother. Res. 2001, 15, 589–591. [Google Scholar]
- Lee, S.I.; Oh, S.H.; Park, K.Y.; Park, B.H.; Kim, J.S.; Kim, S.D. Antihyperglycemic effects of fruits of privet (Ligustrum obtusifolium) in streptozotocin induced diabetic rats fed a high fat diet. J. Med. Food 2009, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Yen, F.L.; Ng, L.T.; Lin, C.C. Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J. Ethnopharmacol. 2007, 111, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.C.; Kim, J.W.; Kwong, C.H.; Kim, T.H.; Kim, Y.K. Fructus ligustri lucidi extracts induce human glioma cells death through regulation of Akt/mTOR pathway in vitro and reduce glioma tumor browth in U87MG xenograft mouse model. Phytother. Res. 2011, 25, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; He, M.L.; Dong, Q.; Xie, W.D.; Chen, Y.C.; Lim, M.C.; Leung, P.C.; Zhang, Y.O.; Kung, H.F. Aqueous extract of fructus Ligustri lucidi enhances the sensitivy of human colorectal carcinoma DLD-1 cells to doxorubicin-induced apoptosis via Tbx3 suppression. Integr. Cancer Ther. 2011, 10, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Du, Q.; Deng, S.; An, H.-M.; Pan, C.-H.; Shen, K.-P.; Xu, L.; Wei, M.-M.; Wang, S.-S. Ligustrum lucidum Ait. fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21. Oncol. Rep. 2014, 32, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Zeisser-Labouebe, M.; Lange, N.; Gurniy, R.; Delie, F. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int. J. Pharm. 2006, 326, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Met. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Kiss, A.K.; Mank, M.; Melzig, M.F. Dual inhibition of metallopeptidases ACE and NEP by extracts and iridioids from Ligustrum vulgare L. J. Ethnopharmacol. 2008, 120, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Poopathi, S.H.; De Britto, L.J.; Praba, V.L.; Mani, C.; Praveen, M. Synthesis of silver nanoparticles from Azadirachta indica—A most effective method for mosquito control. Environ. Sci. Pollut. Res. 2015, 22, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, S.; Ravi, S.; Kathiravan, V.; Velmurugan, S. Rapid biological synthesis of silver nanoparticles using Leucas martinicensis leaf extract for catalytic and antibacterial activity. Environ. Sci. Pollut. Res. Sci. 2014, 21, 11439–11446. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.R.; Castilla, C.L.; Gomez, N.B.; Garcia, A.; Marcos, R.; Carmona, E.R. Leaf extract from the endemic plant Peumus boldus as an effective bioproduct for the green synthesis of silver nanoparticles. Mater. Lett. 2016, 183, 255–260. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Mkhize, Z. Eco-friendly synthesis of AgNPs using Verbascum thapsus extract and its photocatalytic activity. Mater. Lett. 2016, 185, 452–455. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Wang, J.; Yang, Q.; Yao, B.; Zhao, Y.; Zhao, A.; Sun, W.; Zhang, Q. Synthesis, characterization and evaluation cytotoxic activity of silver nanoparticles synthesized by Chinese herbal Cornus officinalis via environment friendly approach. Environ. Toxicol. Pharmacol. 2017, 56, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea Arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Gengan, R.M.; Anand, K.; Phulukdaree, A.; Chuturgoon, A. A549 cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids Surf. B 2013, 105, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.R.; Nagy, A.; Brown, R.P.; Zhang, Q.; Malhan, S.G.; Goering, P.L. Silver nanoparticles: significance of physico-chemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous response of ovarian cancer cells to silver nanoparticles as a single agent and in combination with cisplatin. J. Nanomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Young, X.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, G.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Murugesan, K. Plant mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: Assessment of their antibacterial and anticancer activity. Karbala Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, B.; Sincari, V.; Perde-Schrepler, M.; David, L. Biosynthesis of Silver Nanoparticles Using Ligustrum Ovalifolium Fruits and Their Cytotoxic Effects. Nanomaterials 2018, 8, 627. https://doi.org/10.3390/nano8080627
Moldovan B, Sincari V, Perde-Schrepler M, David L. Biosynthesis of Silver Nanoparticles Using Ligustrum Ovalifolium Fruits and Their Cytotoxic Effects. Nanomaterials. 2018; 8(8):627. https://doi.org/10.3390/nano8080627
Chicago/Turabian StyleMoldovan, Bianca, Vladislav Sincari, Maria Perde-Schrepler, and Luminita David. 2018. "Biosynthesis of Silver Nanoparticles Using Ligustrum Ovalifolium Fruits and Their Cytotoxic Effects" Nanomaterials 8, no. 8: 627. https://doi.org/10.3390/nano8080627




