Optical Gratings Coated with Thin Si3N4 Layer for Efficient Immunosensing by Optical Waveguide Lightmode Spectroscopy
Abstract
:1. Introduction
| Si3N4 | SiO2 | TiO2 | Ta2O5 | HfO2 | |
|---|---|---|---|---|---|
| Refractive index | 2.02 [16] | 1.46 [16] | 2.58 [17] | 2.10 [12] | 2.08 [18] |
| Leakage current (A/cm2) at 2 V | 1 × 10−15 [19] | 1 × 10−8 [19] | >1 × 10−7 [20] | 9 × 10−8 [21] | 2 × 10−9 [18] |
| Dielectric constant | 7.5 [14] | 3.9 [14] | 80–30 [20] | 26.0 [21] | 25.0 [18] |
2. Experimental Section
2.1. Materials and Reagents
2.2. OWLS Transducer and Measuring Principle

2.3. Silicon Nitride Deposition and Characterization
2.4. Atomic Force Microscopy Surface Characterization
2.5. Immunosensing Analytical Procedure
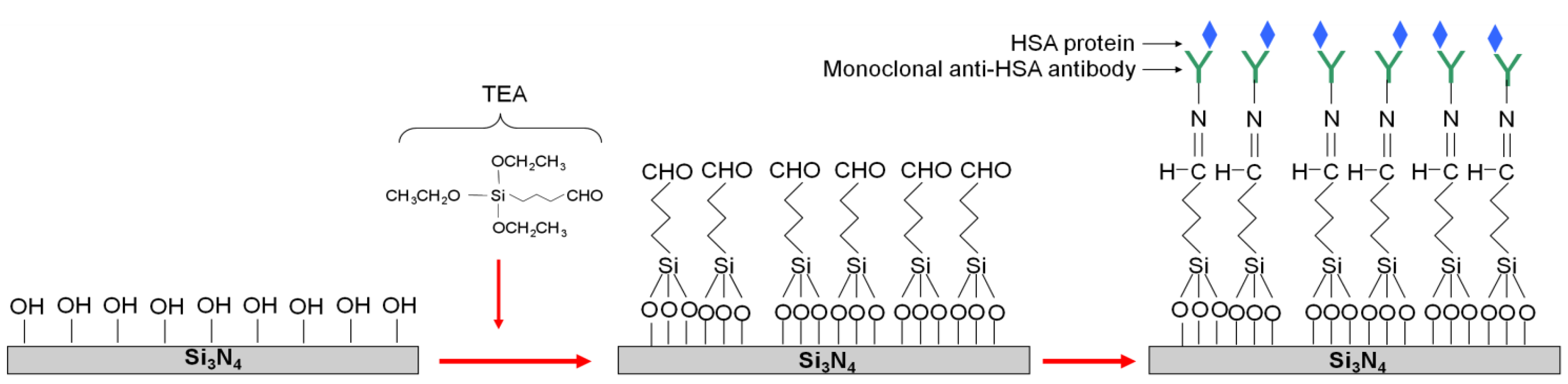
3. Results and Discussion
3.1. Silicon Nitride Thin Layer Characterization

3.2. Silicon Nitride-Coated OWLS Sensor Chip: Atomic Force Microscopy and OWLS Performance Characterization


3.3. Immunosensor: Proof of Concept
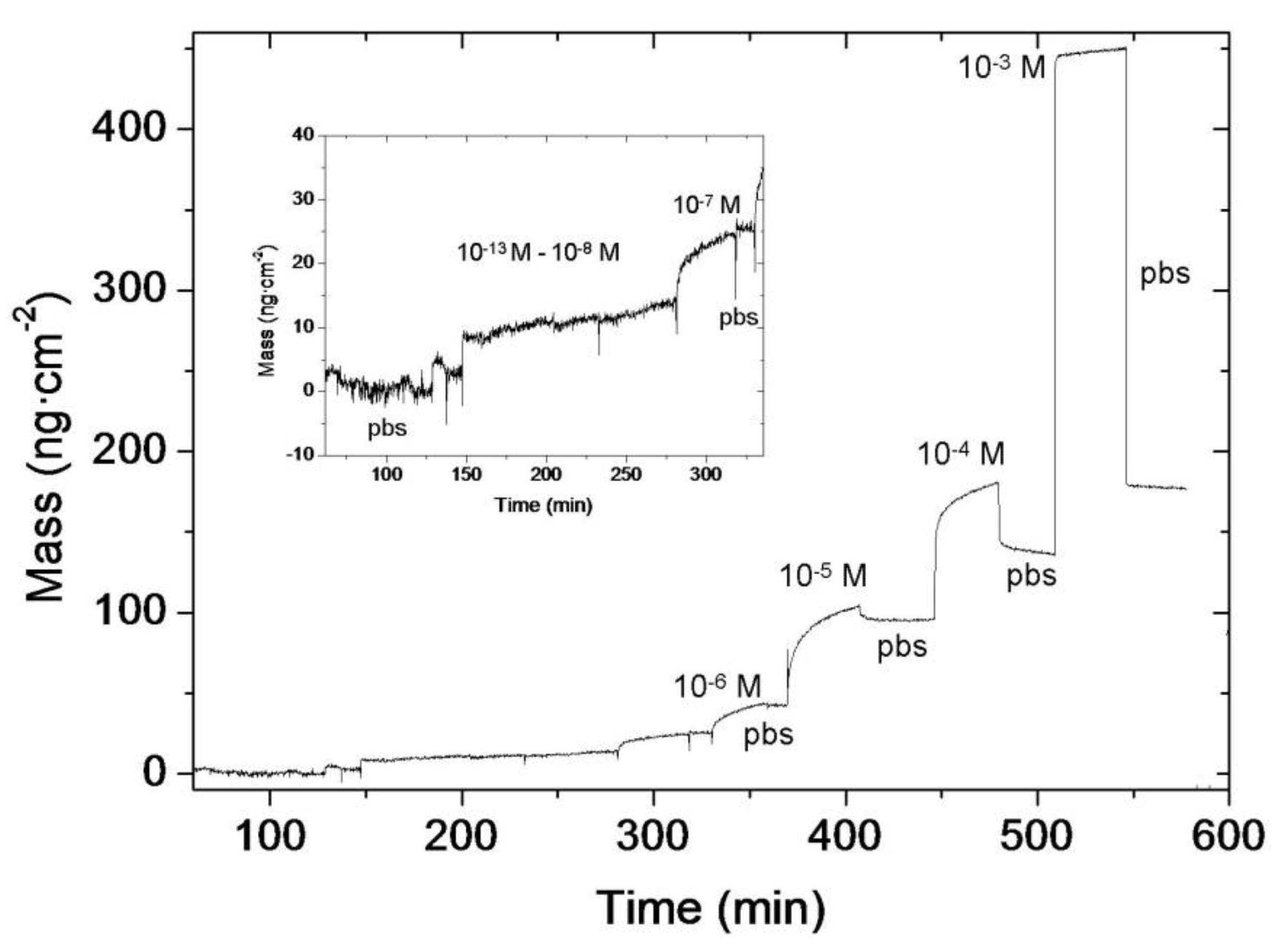

4. Conclusions
Acknowledgments
References
- Plaxco, K.W.; Soh, H.T. Switch-based biosensors: A new approach towards real-time, in vivo molecular detection. Trends Biotechnol. 2011, 29, 1–5. [Google Scholar] [CrossRef]
- Killard, A.J.; Deasy, B.; O’Kennedy, R.; Smyth, M.R. Antibodies: Production, functions and applications in biosensors. TrAC Trends Anal. Chem. 1995, 14, 257–266. [Google Scholar]
- Grieshaber, D.; Reimhult, E.; Vörös, J. Enzymatic biosensors towards a multiplexed electronic detection system for early cancer diagnostics. In Proceedings of the 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS ’07), Bangkok, Thailand, 16-19 January 2007; pp. 402–405.
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [Green Version]
- Adányi, N.; Váradi, M.; Kim, N.; Szendrö, I. Development of new immunosensors for determination of contaminants in food. Curr. Appl. Phys. 2006, 6, 279–286. [Google Scholar] [CrossRef]
- Lukosz, W. Integrated optical chemical and direct biochemical sensors. Sens. Actuat. B Chem. 1995, 29, 37–50. [Google Scholar] [CrossRef]
- Erickson, D.; Mandal, S.; Yang, A.; Cordovez, B. Nanobiosensors: Optofluidic, electrical and mechanical approaches to biomolecular detection at the nanoscale. Microfluid. Nanofluid. 2008, 4, 33–52. [Google Scholar] [CrossRef]
- Bearinger, J.P.; Vörös, J.; Hubbell, J.A.; Textor, M. Electrochemical optical waveguide lightmode spectroscopy (EC-OWLS): A pilot study using evanescent-field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)-coated waveguide chips. Biotechnol. Bioeng. 2003, 82, 465–473. [Google Scholar] [CrossRef]
- Diéguez, L.; Darwish, N.; Mir, M.; Martínez, E.; Moreno, M.; Samitier, J. Effect of the refractive index of buffer solutions in evanescent optical biosensors. Sens. Lett. 2009, 7, 851–855. [Google Scholar] [CrossRef]
- Vörös, J.; Ramsden, J.J.; Csúcs, G.; Szendro, I.; de Paul, S.M.; Textor, M.; Spencer, N.D. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Kurrat, R.; Textor, M.; Ramsden, J.J.; Boni, P.; Spencer, N.D. Instrumental improvements in optical waveguide light mode spectroscopy for the study of biomolecule adsorption. Rev. Sci. Instrum. 1997, 68, 2172–1. [Google Scholar]
- Schmitt, K.; Oehse, K.; Sulz, G.; Hoffmann, C. Evanescent field sensors based on tantalum pentoxide waveguides—A review. Sensors 2008, 8, 711–738. [Google Scholar] [CrossRef]
- Diéguez, L.; Darwish, N.; Graf, N.; Vörös, J.; Zambelli, T. Electrochemical tuning of the stability of PLL/DNA multilayers. Soft Matter 2009, 5, 2415–2421. [Google Scholar] [CrossRef]
- Ma, T.P. Making silicon nitride film a viable gate dielectric. IEEE Trans. Electron. Devices 1998, 45, 680–690. [Google Scholar] [CrossRef]
- Schmitt, K.; Hoffmann, C. High-refractive-index waveguide platforms for chemical and biosensing. Springer Ser. Chem. Sens. Biosens. 2010, 7, 21–54. [Google Scholar] [CrossRef]
- Palik, E. Handbook of Optical Constants of Solids; Academic Press: Orlando, FL, USA, 1985; p. 999. [Google Scholar]
- Yamada, Y.; Uyama, H.; Watanabe, S.; Nozoye, H. Deposition at low substrate temperatures of high-quality TiO2 films by radical beam-assisted evaporation. Appl. Opt. 1999, 38, 6638–6641. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, C.; Lu, Y.F.; Wu, Y.H.; Liew, T.; Li, M.F.; Cho, B.J.; Choi, W.K.; Yakovlev, N. Physical and electrical characterization of HfO2 metal-insulator-metal capacitors for Si analog circuit applications. J. Appl. Phys. 2003, 94, 551–557. [Google Scholar]
- Wang, X.W.; Shi, Y.; Ma, T.P.; Cui, G.J.; Tamagawa, T.; Golz, J.W.; Halpen, B.L.; Schmitt, J.J. Extending gate dielectric scaling limit by use of nitride or oxynitride. In Proceedings of the 1995 Symposium on VLSI Technology, Kyoto, Japan, 6-8 June 1995; pp. 109–110.
- Seo, M.; Rha, S.H.; Kim, S.K.; Han, J.H.; Lee, W.; Han, S.; Hwang, C.S. The mechanism for the suppression of leakage current in high dielectric TiO2 thin films by adopting ultra-thin HfO2 films for memory application. J. Appl. Phys. 2011, 110, 024105–1. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications. Mater. Sci. Eng. R Rep. 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Hofmann, O.; Voirin, G.; Niedermann, P.; Manz, A. Three-dimensional microfluidic confinement for efficient sample delivery to biosensor surfaces. Application to immunoassays on planar optical waveguides. Anal. Chem. 2002, 74, 5243–5250. [Google Scholar] [CrossRef]
- Busse, S.; Kaeshammer, J.; Kraemer, S.; Mittler, S. Gold and thiol surface functionalized integrated optical Mach-Zehnder interferometer for sensing purposes. Sens. Actuat. B 1999, B60, 148–154. [Google Scholar]
- Nabok, V.A.; Starodub, F.N.; Ray, K.A.; Hassan, K.A. Biochemical and biomolecular sensing. In Registration of Immunoglobuline AB/AG Reaction with Planar Polarization Interferometer; Robert, A.L., Ed.; SPIE: Boston, MA, USA, 2000; pp. 1–9. [Google Scholar]
- Brandenburg, A.; Gombert, A. Grating couplers as chemical sensors: A new optical configuration. Sens. Actuat. B: Chem. 1997, 17, 35–40. [Google Scholar]
- Tiefenthaler, K.; Lukosz, W. Sensitivity of grating couplers as integrated-optical chemical sensors. J. Opt. Soc. Am. B 1989, 6, 209–220. [Google Scholar] [CrossRef]
- Ramsden, J. Review of new experimental techniques for investigating random sequential adsorption. J. Stat. Phys. 1993, 73, 853–877. [Google Scholar] [CrossRef]
- Feijter, J.A.D.; Veer, J.B.F.A. Ellipsometry as a tool to study the adsorption behavior of synthetic and biopolymers at the air-water interface. Biopolymers 1978, 17, 1759–1772. [Google Scholar] [CrossRef]
- Caballero, D.; Samitier, J.; Bausells, J.; Errachid, A. Direct patterning of anti-human serum albumin antibodies on aldehyde-terminated silicon nitride surfaces for hsa protein detection. Small 2009, 5, 1531–1534. [Google Scholar] [CrossRef]
- Caballero, D.; Martinez, E.; Bausells, J.; Errachid, A.; Samitier, J. Impedimetric immunosensor for human serum albumin detection on a direct aldehyde-functionalized silicon nitride surface. Anal. Chim. Acta 2012, 720, 43–48. [Google Scholar]
- Ténégal, F.; Gheorghiu de la Rocque, A.; Dufour, G.; Sénémaud, C.; Doucey, B.; Bahloul-Hourlier, D.; Goursat, P.; Mayne, M.; Cauchetier, M. Structural determination of sintered Si3N4/SiC nanocomposite using the XPS differential charge effect. J. Electron Spectrosc. Relat. Phenom. 2000, 109, 241–248. [Google Scholar] [CrossRef]
- Peters, T.J. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1997; Volume 43, pp. 1–432. [Google Scholar]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Mol. Biol. 1998, 5, 827–835. [Google Scholar]
- Fleer, G. Adsorption from Solution at the Solid/Liquid Interface; Porfitt, G.D., Rochester, C.H., Eds.; Academic Press: London, UK, 1983. [Google Scholar]
- Hartmann, W.K.; Saptharishi, N.; Yang, X.Y.; Mitra, G.; Soman, G. Characterization and analysis of thermal denaturation of antibodies by size exclusion high-performance liquid chromatography with quadruple detection. Anal. Biochem. 2004, 325, 227–239. [Google Scholar]
- Lin, S.; Lu, C.-C.; Chien, H.-F.; Hsu, S.-M. An on-line quantitative immunoassay system based on a quartz crystal microbalance. J. Immunol. Methods 2000, 239, 121–124. [Google Scholar] [CrossRef]
- Darwish, N.; Caballero, D.; Moreno, M.; Errachid, A.; Samitier, J. Multi-analytic grating coupler biosensor for differential binding analysis. Sens. Actuat. B: Chem. 2010, 144, 413–417. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Diéguez, L.; Caballero, D.; Calderer, J.; Moreno, M.; Martínez, E.; Samitier, J. Optical Gratings Coated with Thin Si3N4 Layer for Efficient Immunosensing by Optical Waveguide Lightmode Spectroscopy. Biosensors 2012, 2, 114-126. https://doi.org/10.3390/bios2020114
Diéguez L, Caballero D, Calderer J, Moreno M, Martínez E, Samitier J. Optical Gratings Coated with Thin Si3N4 Layer for Efficient Immunosensing by Optical Waveguide Lightmode Spectroscopy. Biosensors. 2012; 2(2):114-126. https://doi.org/10.3390/bios2020114
Chicago/Turabian StyleDiéguez, Lorena, David Caballero, Josep Calderer, Mauricio Moreno, Elena Martínez, and Josep Samitier. 2012. "Optical Gratings Coated with Thin Si3N4 Layer for Efficient Immunosensing by Optical Waveguide Lightmode Spectroscopy" Biosensors 2, no. 2: 114-126. https://doi.org/10.3390/bios2020114





