Development of an Electrochemical-Based Aspartate Aminotransferase Nanoparticle Ir-C Biosensor for Screening of Liver Diseases
Abstract
:1. Introduction


2. Experimental Section
2.1. Materials and Reagents
2.2. Fabrication Prototype of Thick-Film Screen-Printable biosensor

2.3. Experimental Measurement Procedure
3. Results and Discussion
3.1. Quantification of L-Glutamate Concentration with Enzymatically Generated H2O2


3.2. Determination of Aspartate Aminotransferase (AST)
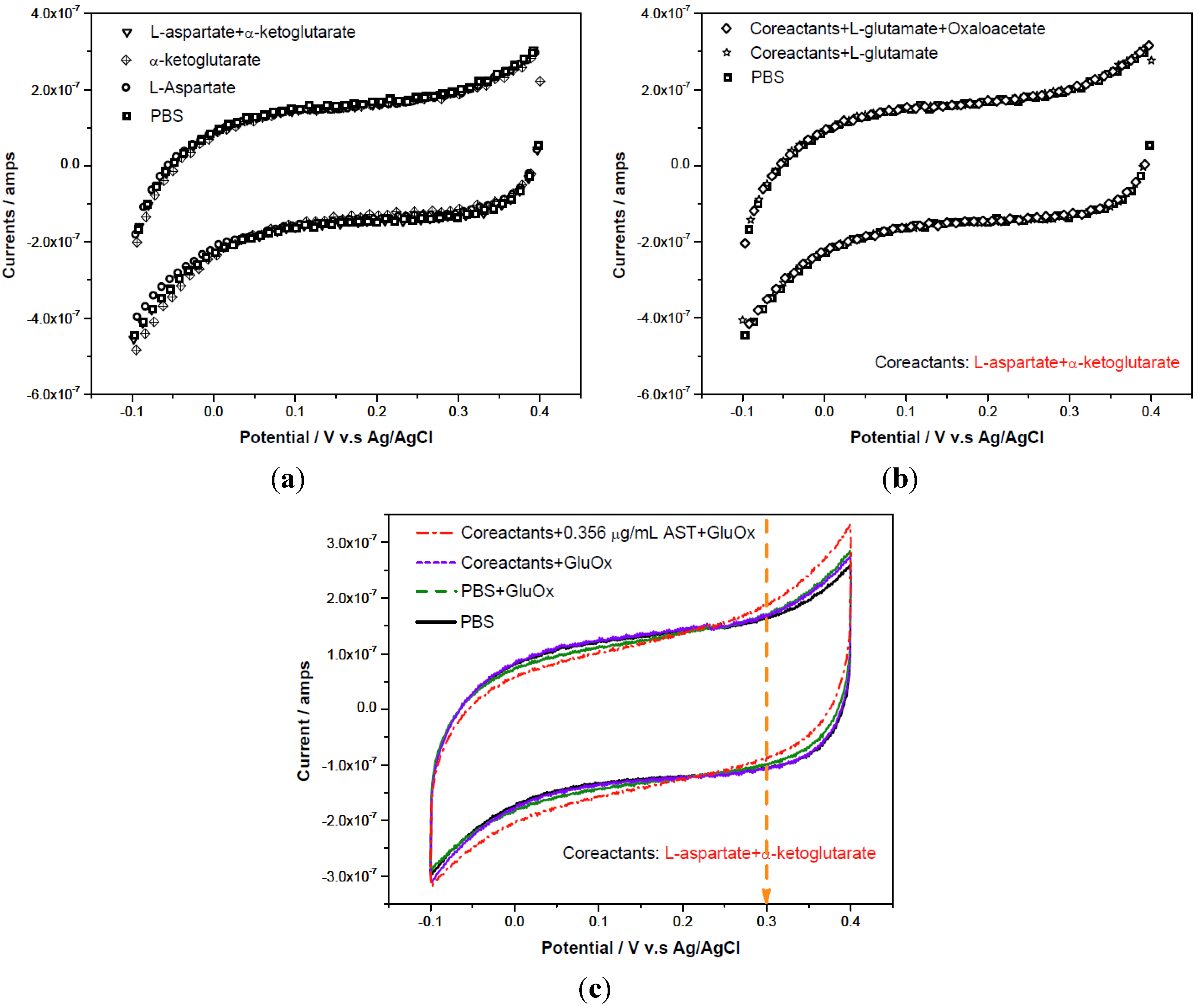
3.2.1. Cyclic Voltammetric Study on AST
3.2.2. AST Detection with Amperometric Method
 = 0.71 μg/mL) and maximum catalytic current (imax = 20.4 nA) were obtained. At low AST concentrations (between 0 to 0.445 μg/mL) the calibration curve showed linearly with sensitivity of 18.5 nA/[μg/mL].
= 0.71 μg/mL) and maximum catalytic current (imax = 20.4 nA) were obtained. At low AST concentrations (between 0 to 0.445 μg/mL) the calibration curve showed linearly with sensitivity of 18.5 nA/[μg/mL].


4. Conclusions
Acknowledgments
References
- Dafour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar]
- Collins, R.D. Differential Diagnosis in Primary Care; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 57–58. [Google Scholar]
- Schiff, E.R.; Maddrey, W.C.; Sorrell, M.F. Schiff’s Diseases of the Liver; John Wiley and Sons: Oxford, UK, 2011; pp. 18–23. [Google Scholar]
- Huang, X.J.; Choi, Y.K.; Im, H.S.; Oktay, Y.; Euisik, Y.; Kim, H.S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Howie-Esquivel, J.; White, M. Biomarkers in acute cardiovascular disease. J. Cardiovasc. Nurs. 2008, 23, 124–131. [Google Scholar]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castellanos, M.; Blanco, M.; Rodríguez-Yáñez, M.; Serena, J.; Leira, R.; Castillo, J. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in ischemic stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1387–1393. [Google Scholar] [CrossRef]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Argibay, B.; Agulla, J.; Pérez-Mato, M.; Rodríguez-González, R.; Brea, D.; Castillo, J. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J. Cereb. Blood Flow Metab. 2011, 31, 1378–1386. [Google Scholar] [CrossRef]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37°C: Part 5. Clin. Chem. Lab. Med. 2002, 40, 725–733. [Google Scholar] [CrossRef]
- Han, Y.D.; Song, S.Y.; Lee, J.H.; Lee, D.S.; Yoon, H.C. Multienzyme-modified biosensing surface for the electrochemical analysis of aspartate transaminase and alanine transaminase in human plasma. Anal. Bioanal. Chem. 2011, 400, 797–805. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, Q.; Liu, H.; Liu, C.; Caia, X. The study of a disposable eagerntless biosensor for fast test of aspartate aminotransferase. Electroanalysis 2008, 20, 1135–1141. [Google Scholar] [CrossRef]
- Ohgami, N.; Upadhyay, S.; Kabata, A.; Morimoto, K.; Kusakabe, H.; Suzuki, H. Determination of the activities of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase in a microfluidic system. Biosens. Bioelectron. 2007, 22, 1330–1336. [Google Scholar] [CrossRef]
- Song, M.J.; Yun, D.H.; Hong, S.I. An electrochemical biosensor array for rapid detection of alanine aminotransferase and aspartate aminotransferase. Biosci. Biotechnol. Biochem. 2009, 73, 474–478. [Google Scholar] [CrossRef]
- Mizutani, F.; Sato, Y.; Sawaguchi, T.; Yabuki, S.; Iijima, S. Rapid measurement of transaminase activities using an amperometric L-glutamate-sensing electrode based on a glutamate oxidase-polyion complex-bilayer membrane. Sens. Actuat. 1998, 52, 23–29. [Google Scholar] [CrossRef]
- Ren, H.X.; Huang, X.J.; Kim, J.H.; Choi, Y.K.; Gu, N. Pt/Au bimetallic hierarchical structure with micro/nano-array via photolithography and electrochemical synthesis: from design to GOT and GPT biosensors. Talanta 2009, 78, 1371–1377. [Google Scholar] [CrossRef]
- Chang, K.S.; Hsu, W.L.; Chen, H.Y.; Chang, C.K.; Chen, C.Y. Determination of glutamate pyruvate transaminase activity in clinical specimens using a biosensor composed of immobilized l-glutamate oxidase in a photo-crosslinkable polymer membrane on a palladium-deposited screen-printed carbon electrode. Anal. Chim. Acta 2003, 481, 199–208. [Google Scholar] [CrossRef]
- Elzanowska, H.; Abu-Irhayem, E.; Skrzynecka, B.; Birss, V.I. Hydrogen peroxide detection at electrochemically and sol-gel derived Ir oxide films. Electroanalysis 2004, 16, 478–490. [Google Scholar]
- Shen, J.; Dudik, L.; Liu, C.C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sens. Actuat. 2007, 1, 106–113. [Google Scholar]
- Chang, K.S.; Chang, C.K.; Chou, S.F.; Chen, C.Y. Sequential measurement of aminotransferase activities by amperometric biosensors. Biosens. Bioelectron. 2007, 22, 2914–2920. [Google Scholar] [CrossRef]
- You, T.; Niwa, O.; Kurita, R.; Iwasaki, Y.; Hayashi, K.; Suzuki, K.; Hirono, S. Reductive H2O2 detection at nanoparticle iridium/carbon film electrode and its application as L-glutamate enzyme sensor. Electroanalysis 2004, 16, 54–59. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.H.; Liu, C.C. An electrochemical biosensor of the ketone 3-β-hydroxybutyrate for potential diabetic patient management. Sens. Actuat. 2008, 2, 818–825. [Google Scholar]
- Bartling, B.; Li, L.; Liu, C.C. Determination of total bile acid levels using a thick-film screen-printed Ir/C sensor for the detection of liver disease. Analyst 2009, 134, 973–979. [Google Scholar] [CrossRef]
- Liao, W.Y.; Liu, C.C.; Wang, C. Detection of lipoprotein-associated phospholipase A2 using a nano-iridium particle catalyst-based biosensor. Sens. Actuat. 2008, 2, 993–999. [Google Scholar]
- Hsueh, C.J.; Wang, J.H.; Dai, L.; Liu, C.C. Determination of alanine aminotransferase with an electrochemical nano Ir-C Biosensor for the screening of liver diseases. Biosensors 2011, 1, 101–117. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hsueh, C.-J.; Wang, J.H.; Dai, L.; Liu, C.-C. Development of an Electrochemical-Based Aspartate Aminotransferase Nanoparticle Ir-C Biosensor for Screening of Liver Diseases. Biosensors 2012, 2, 234-244. https://doi.org/10.3390/bios2020234
Hsueh C-J, Wang JH, Dai L, Liu C-C. Development of an Electrochemical-Based Aspartate Aminotransferase Nanoparticle Ir-C Biosensor for Screening of Liver Diseases. Biosensors. 2012; 2(2):234-244. https://doi.org/10.3390/bios2020234
Chicago/Turabian StyleHsueh, Chang-Jung, Joanne H. Wang, Liming Dai, and Chung-Chiun Liu. 2012. "Development of an Electrochemical-Based Aspartate Aminotransferase Nanoparticle Ir-C Biosensor for Screening of Liver Diseases" Biosensors 2, no. 2: 234-244. https://doi.org/10.3390/bios2020234



