Microfabricated Tactile Sensors for Biomedical Applications: A Review
Abstract
:1. Introduction
2. Principles of Measurement
2.1. Piezoresistive Sensors
2.2. Piezoelectric Sensors
2.3. Capacitive Sensors
3. Microfabrication Process
- (1)
- photolithography: it is the process used for pattern transfer into the material. The pattern, designed by means of a CAD software, is transferred onto a glass mask, which has on the surface a photodefinable opaque material with the shape of the desired pattern. A substrate, spin-coated with photoresist (a photoresistive organic polymer), is placed in contact with the mask and they are hit by UV light, used to make the photoresist soluble into the opaque material. Lastly, mask and substrate are separated, and the photoresist is removed from the new system [41];
- (2)
- stencil lithography: is a relatively new process used to produce patterns through a shadow mask and evaporation of material in a vacuum, and based on the method of physical vapor deposition. The main advantages of this method are the sub-micrometer resolution and its applicability with fragile substrates, like biological macromolecules [42,43];
- (3)
- (4)
- etching: it is the process of selectively removing materials in fixed patterns, using both liquid chemical substances (wet etching) and gas-phase chemistry (dry etching). Furthermore, etching can be either isotropic or anisotropic: in the first case, the etching acts equally in all direction of the space, whereas in the second case the effect is directional. Dry etching is commonly used to achieve anisotropic outcomes [45];
- (5)
- bonding: the process of permanently binding together two substrates, in particular solid-state materials with smooth and flat substances, usually used for packaging. Many techniques have been developed to perform bonding, such as the fusion bonding, which employs chemical reaction between the bonding surfaces of several materials, and the anodic bonding, which is a thermally activated process supported by electrical field. Micromechanical sandwiched silicon systems are usually fabricated through high-temperature bonding (>700 °C), whereas silicon wafer and glass substrate are bonded together by means of middle temperature (200–500 °C) [46].
4. Application in Medicine
4.1. Prosthetic Hands
4.1.1. Piezoresistive Sensors
4.1.2. Piezoelectric Sensors
4.1.3. Capacitive Sensors

4.2. Microsurgical Force Sensors
4.2.1. Piezoelectric Sensors

4.2.2. Piezoresistive and Capacitive Sensors
4.3. Biomechanical Analysis
Piezoresistive Sensors
4.4. Multimodal Sensors

4.5. New Frontiers
5. Discussion and Conclusions
| Sensing Principle | Author, Year, Reference | Microfabrication Process | Design | Application | Metrological Properties |
|---|---|---|---|---|---|
| Piezoresistive sensors | Beebe et al., 1995–1998 [59,60] | Silicon direct bonding and bulk micromachining | Silicon piezoresistive diaphragm | Human finger force measurement |
|
| Dario, Carrozza et al., 2005–2009 [61,62,63,64,90,94] | Subtractive dry etching | Silicon-based three-axial force sensor | Robotic tactile sensing; MIS |
| |
| Dargahi et al., 2010–2011 [88,89] | - | PVDF membrane | MIS |
| |
| Hseih et al., 2000, [92] | Silicon bulk micromachining | Micro shear-stress sensor | Biomechanical analysis |
| |
| Alfaro et al., 2009, [93] | CMOS process, maskless dryetching | Piezoresistive strain gauges | Biomechanical analysis |
| |
| Wahab et al., 2008, [95] | Silicon bulk processing (designed only) | Wheatstone bridge configuration | Biomechanical analysis |
| |
| Ando et al., 1994 [65] | Etching | PVDF electrodes housed in silica | Artificial tactile sensing for touch and slip |
| |
| Dargahi et al. [66,81,82,83] | Photolithography and anisotropic etching | Silicon, tooth-like pattered layer transfers force to PVDF film | Endoscopic grasper |
| |
| Ezhilvalavan et al., 2006 [84] | Deep reactive ion, ion beam and wet-chemical etching | PZT force sensors with top and bottom electrodes forming capacitor | MIS | Only electrical characterization, e.g., leakage current 10−7 A/cm2 (applied electric field of 200 kV·cm−1) | |
| Li et al., 2008 [86]Sharma et al.,2012 [85] | Mold-transfer method | PVDF-TrFE copolymer | MIS |
| |
| Capacitive sensors | Gray and Fearing 1996 [69] | - | Rubber layer on polysilicon capacitor | General biomedical purposes |
|
| Lee et al., 2005–2006 [70,71] | Bonding | PDMS layer | Robotic skin |
| |
| Muhammad et al., 2011 [73,75] | Bonded and Etched-Back Silicon-On-Insulator wafers, Deep Reactive Ion Etching | PDMS-coated capacitive sensor | Robotic finger |
| |
| Multimodal sensors | Castelli 2002 [52] | - | Capacitive sensors for force and temperature | Robotic tactile skin |
|
| Egel et al., 2005 [97] | Etching, lift-off pattering | Strain gauge for force measurement, RTD for temperature measurement | Robotic tactile skin | - | |
| Optical-based sensors | Su et al., 2011 [104] Liu et al., 2012 [105] | - | Fabry-Perot interferometer | MIS |
|
| Cowie et al., 2007 [107] | - | Fiber Bragg gratings | General biomedical purposes |
| |
| De Rossi et al., 2001 [109] | - | Light intensity modulation | Biomechanical analysis |
| |
| Ahmadi et al., 2010 [89] | - | Light intensity modulation | MIS | - | |
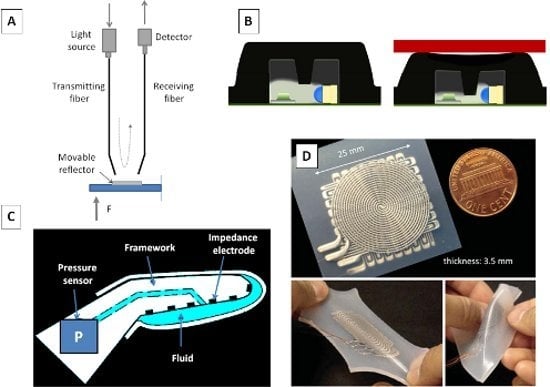
| Fluidic sensors | Fishel et al., 2008 [56] | - | Pressure sensor housed into a fluid-filled fingertip | Biomimetic Fingertips | - |
| Ponce Wong et al., 2012 [111] | Soft lithography | Galinstan-filled microchannels | Artificial skin |
| |
| Park et al., 2012 [112] | Silicon layered molding and casting process | Multilayered mircochannels in elastomer matrix | Fingertips | - | |
| Ionic Polymeric Metal Composite (IPMC) | Bonomo et al., 2008 [122] | - | Two IPMC membranes | MIS |
|
Acknowledgments
Conflicts of Interest
References
- Clot, J.; Stojiljkovic, Z. Integrated behaviour of artificial skin. IEEE Trans. Biomed. Eng. 1977, 24, 396–399. [Google Scholar]
- Hillis, W.D. Active Touch Sensing. S.M. Thesis, MIT EF & CS, Cambridge, MA, USA, January 1981. [Google Scholar]
- Harmon, L.D. Touch-sensing technology—A review. Soc. Manuf. Eng. 1980, 58. [Google Scholar]
- Harmon, L.D. A sense of touch begins to gather momentum. Sens. Rev. 1981, 1, 82–88. [Google Scholar] [CrossRef]
- Harmon, L.D. Automated tactile sensing. Int. J. Robot Res. 1982, 1, 3–32. [Google Scholar] [CrossRef]
- Tegin, J.; Wikander, J. Tactile sensing in intelligent robotic manipulation—A review. Ind. Robot 2005, 32, 64–70. [Google Scholar] [CrossRef]
- Chappell, P.H. Making sense of artificial hands. J. Med. Eng. Technol. 2011, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roham, H.; Najarian, S.; Hosseini, S.M.; Dargahi, J. Design and fabrication of a new tactile probe for measuring the modulus of elasticity of soft tissues. Sens. Rev. 2007, 27, 317–323. [Google Scholar] [CrossRef]
- Najarian, S.; Fallahnezhad, M.; Afshari, E. Advances in medical robotic systems with specific applications in surgery—A review. J. Med. Eng. Technol. 2011, 35, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, J.; Najarian, S.; Liu, B. Sensitivity analysis of a novel tactile probe for measurement of tissue softness with applications in biomedical robotics. J. Mater. Process Tech. 2007, 183, 176–182. [Google Scholar] [CrossRef]
- Dargahi, J.; Sedaghati, R.; Singh, H.; Najarian, S. Modeling and testing of an endoscopic piezoelectric-based tactile sensor. Mechatronics 2007, 17, 462–467. [Google Scholar] [CrossRef]
- Voldman, J.; Gray, M.L.; Schmidt, M.A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1999, 1, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Polla, D.L.; Erdman, A.G.; Robbins, W.P.; Markus, D.T.; Diaz-Diaz, J.; Rizq, R.; Nam, Y.; Brickner, H.T. Microdevices in Medicine 1. Annu. Rev. Biomed. Eng. 2000, 2, 551–576. [Google Scholar] [CrossRef] [PubMed]
- Mokwa, W. Medical implants based on microsystems. Meas. Sci. Technol. 2007, 18, R47. [Google Scholar] [CrossRef]
- Zhang, X. Silicon microsurgery-force sensor based on diffractive optical MEMS encoders. Sens. Rev. 2004, 24, 37–41. [Google Scholar] [CrossRef]
- Katuri, K.C.; Asrani, S.; Ramasubramanian, M.K. Intraocular pressure monitoring sensors. IEEE Sens. J. 2008, 8, 12–19. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jung, J.M.; Jeong, J.S.; Yang, S.S. Micro devices for a cerebrospinal fluid (CSF) shunt system. Sens. Actuator A Phys. 2004, 110, 68–76. [Google Scholar] [CrossRef]
- Hedrich, F.; Kliche, K.; Storz, M.; Billat, S.; Ashauer, M.; Zengerle, R. Thermal flow sensors for MEMS spirometric devices. Sens. Actuator A Phys. 2010, 162, 373–378. [Google Scholar] [CrossRef]
- Silvestri, S.; Schena, E. Micromachined flow sensors in biomedical applications. Micromachines 2012, 3, 225–243. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Peppas, N.A. Microfabricated drug delivery devices. Int. J. Pharm. 2005, 306, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Yobas, L.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 2007, 11, 883–892. [Google Scholar] [CrossRef]
- Lin, H.K.; Zheng, S.; Williams, A.J.; Balic, M.; Groshen, S.; Scher, H.I.; Fleisher, M.; Stadler, W.; Datar, R.H.; Tai, Y.C.; Cote, R.J. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010, 16, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Nicholls, H.R. Review Article Tactile sensing for mechatronics—A state of the art survey. Mechatronics 1999, 9, 1–31. [Google Scholar] [CrossRef]
- Eltaib, M.E.H.; Hewit, J.R. Tactile sensing technology for minimal access surgery––A review. Mechatronics 2003, 13, 1163–1177. [Google Scholar] [CrossRef]
- Tiwana, M.I.; Redmond, S.J.; Lovell, N.H. A review of tactile sensing technologies with applications in biomedical engineering. Sens. Actuator A Phys. 2012, 179, 17–31. [Google Scholar] [CrossRef]
- Lucarotti, C.; Oddo, C.M.; Vitiello, N.; Carrozza, M.C. Synthetic and bio-artificial tactile sensing: A review. Sensors 2013, 13, 1435–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, B.J.; Cutkosky, M.R.; Kovacs, G.T.A. A traction stress sensor array for use in high-resolution robotic tactile imaging. J. Microelectromech. Syst. 2000, 9, 425–434. [Google Scholar] [CrossRef]
- Liu, C. Piezoresistive Sensors. In Foundaments of MEMS, 2nd ed.; Pearson Prentice Hall: Upper Saddler River, NJ, USA, 2006; pp. 207–244. [Google Scholar]
- Barlian, A.A.; Park, W.T.; Mallon, J.R.; Rastegar, A.J.; Pruitt, B.L. Review: Semiconductor piezoresistance for microsystems. Proc. IEEE 2009, 97, 513–552. [Google Scholar] [CrossRef]
- Curie, J.; Curie, P. Piezoelectric and allied phenomena in rochelle salt. Comput. Rend. Acad. Sci. Paris 1880, 91, 294–297. [Google Scholar]
- Caliò, R.; Rongala, U.B.; Camboni, D.; Milazzo, M.; Stefanini, C.; de Petris, G.; Oddo, C.M. Piezoelectric energy harvesting solutions. Sensors 2014, 14, 4755–4790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddiss, E.; Chau, T. Electroactive polymeric sensors in hand prostheses: Bending response of an ionic polymer metal composite. Med. Eng. Phys. 2006, 28, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Omote, K.; Ohigashi, H.; Koga, K. Temperature dependence of elastic, dielectric, and piezoelectric properties of “single crystallineˮ films of vinylidene fluoride trifluoroethylene copolymer. J. Appl. Phys. 1997, 81, 2760–2769. [Google Scholar] [CrossRef]
- Sirohi, J.; Chopra, I. Fundamental understanding of piezoelectric strain sensors. J. Intel. Mater. Syst. Struct. 2000, 11, 246–257. [Google Scholar] [CrossRef]
- Takashima, K.; Horie, S.; Mukai, T.; Ishida, K.; Matsushige, K. Piezoelectric properties of vinylidene fluoride oligomer for use in medical tactile sensor applications. Sens. Actuator A Phys. 2008, 144, 90–96. [Google Scholar] [CrossRef]
- Franssila, S. Introduction to Microfabrication, 2nd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2010. [Google Scholar]
- Hu, H.; Han, Y.; Song, A.; Chen, S.; Wang, C.; Wang, Z. A Finger-shaped tactile sensor for fabric surfaces evaluation by 2-dimensional active sliding touch. Sensors 2014, 14, 4899–4913. [Google Scholar] [CrossRef] [PubMed]
- Kristel. Sensor Datasheet. Available online: http://www.kistler.com/us/en/innovation/measuredparameters/force (accessed on 4 July 2014).
- Kristel. Amplifier Datasheet. Available online: http://www.kistler.com/us/en/product/amplifier/5171A1 (accessed on 4 July 2014).
- Beeby, S. MEMS Mechanical Sensors; Artech House: Norwood, MA, USA, 2004; pp. 137–139. [Google Scholar]
- Madou, M.J. Photolithography. In Fundamentals of Microfabrication and Nanotechnology: Manufacturing Techniques for Microfabrication and Nanotechnology, 3rd ed.; Taylor and Francis Group, LLC; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Rogers, J.A.; Lee, H.H. Combinations of top-down and bottom-up nanofabrication techniques and their application to create functional devices. In Unconventional Nanopatterning Techniques and Applications; John Wiley & Sons, Inc.: Hoboker, NJ, USA, 2009; pp. 379–418. [Google Scholar]
- Takano, N.; Doeswijk, L.M.; van den Boogaart, M.A.; Auerswald, J.; Knapp, H.F.; Dubochet, O.; Hessler, T.; Brugger, J. Fabrication of metallic patterns by microstencil lithography on polymer surfaces suitable as microelectrodes in integrated microfluidic systems. J. Micromech. Microeng. 2006, 16, 1606–1613. [Google Scholar] [CrossRef]
- Seshan, K. Handbook of thin film deposition, equipment and processing. In Handbook of Thin Film Deposition, 3rd ed.; Elsevier Inc.: Oxford, UK, 2012. [Google Scholar]
- Madou, M.J. Pattern Transfer with Additive Techniques. In Fundamentals of Microfabrication and Nanotechnology: Manufacturing Techniques for Microfabrication and Nanotechnology, 3rd ed.; Taylor and Francis Group, LLC; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Dziuban, J.A. Bonding. In Bonding in Microsystem Technology; Springer Science & Business Media: Rotterdam, The Netherland, 2007. [Google Scholar]
- Johansson, R.S.; Vallbo, Å.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300. [Google Scholar] [PubMed]
- Vallbo, Å.B.; Johansson, R.S. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol. 1984, 3, 3–14. [Google Scholar] [PubMed]
- Maeno, T.; Kobayashi, K.; Yamazaki, N. Relationship between the structure of human finger tissue and the location of tactile receptors. JSME Int. J.-Ser. C 1998, 41, 94–100. [Google Scholar] [CrossRef]
- Dahiya, R.S.; Metta, G.; Valle, M.; Sandini, G. Tactile sensing—From humans to humanoids. IEEE Trans. Robot. 2010, 26, 1–20. [Google Scholar] [CrossRef]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F. An integrated tactile-thermal robot sensor with capacitive tactile array. IEEE Trans. Ind. Appl. 2002, 38, 85–90. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Cheng, M.-Y.; Chang, W.-Y.; Tsao, L.-C.; Yang, S.-A.; Shih, W.-P.; Chang, F.-Y.; Chang, S.-H.; Fan, K.-C. An integrated flexible temperature and tactile sensing array using PI-copper films. Sens. Actuator A Phys. 2008, 143, 143–153. [Google Scholar] [CrossRef]
- Francomano, M.T.; Accoto, D.; Guglielmelli, E. Artificial sense of slip—A review. IEEE Sens. J. 2013, 13, 2489–2498. [Google Scholar] [CrossRef]
- Francomano, M.T.; Accoto, D.; Morganti, E.; Lorenzelli, L.; Guglielmelli, E. A Microfabricated Flexible Slip Sensor. In Proceedings of the 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1919–1924.
- Fishel, J.A.; Santos, V.J.; Loeb, G.E. A Robust Micro-Vibration Sensor for Biomimetic Fingertips. In Proceedings of the 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Scottsdale, AZ, USA, 19–22 October 2008; pp. 659–663.
- Oddo, C.M.; Controzzi, M.; Beccai, L.; Cipriani, C.; Carrozza, M.C. Roughness encoding for discrimination of surfaces in artificial active-touch. IEEE Trans. Rob. 2008, 27, 522–533. [Google Scholar] [CrossRef]
- Mukaibo, Y.; Shirado, H.; Konyo, M.; Maeno, T. Development of a Texture Sensor Emulating the Tissue Structure and Perceptual Mechanism of Human Fingers. In Proceedings of IEEE International Conference on Robotics and Automation (ICRA), Barcelona, Spain, 18–22 April 2005; pp. 2565–2570.
- Beebe, D.J.; Hsieh, A.S.; Denton, D.D.; Radwin, R.G. A silicon force sensor for robotics and medicine. Sens. Actuator A Phys. 1995, 50, 55–65. [Google Scholar] [CrossRef]
- Beebe, D.J.; Denton, D.D.; Radwin, R.G.; Webster, J.G. A silicon-based tactile sensor for finger-mounted applications. IEEE Trans. Biomed. Eng. 1998, 45, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Beccai, L.; Roccella, S.; Arena, A.; Valvo, F.; Valdastri, P.; Menciassi, A.; Chiara Carrozza, M.; Dario, P. Design and fabrication of a hybrid silicon three-axial force sensor for biomechanical applications. Sens. Actuator A Phys. 2005, 120, 370–382. [Google Scholar] [CrossRef]
- Oddo, C.M.; Beccai, L.; Muscolo, G.G.; Carrozza, M.C. Biomimetic MEMS-Based Tactile Sensor Array with Fingerprints Integrated in a Robotic Fingertip for Artificial Roughness Encoding. In Proceedings of the 2009 IEEE International Conference on Robotics and Biomimetics (ROBIO), Guilin, China, 19–23 December 2009; pp. 894–900.
- Oddo, C.M.; Beccai, L.; Felder, M.; Giovacchini, F.; Carrozza, M.C. Artificial roughness encoding with a bio-inspired MEMS-based tactile sensor array. Sensors 2009, 9, 3161–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, L.; Oddo, C.M.; Controzzi, M.; Cipriani, C.; Förster, A.; Carrozza, M.C.; Schmidhuber, J. Learning tactile skills through curious exploration. Front. Neurorobotics 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Shinoda, H.; Ando, S. Ultrasonic Emission Tactile Sensor for Contact Localization and Characterization. In Proceedings of the 1994 IEEE International Conference on Robotics and Automation, San Diego, CA, USA, 8–13 May 1994; pp. 2536–2543.
- Dargahi, J. A piezoelectric tactile sensor with three sensing elements for robotic, endoscopic and prosthetic applications. Sens. Actuator A Phys. 2000, 80, 23–30. [Google Scholar] [CrossRef]
- Choi, B.J.; Chun, J.; Choi, H.R. Development of Anthropomorphic Robot Hand with Tactile Sensor: SKKU Hand II. In Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems, Beijing, China, 9–15 October 2006; pp. 3779–3784.
- Choi, B.; Choi, H.R.; Kang, S. Development of Tactile Sensor for Detecting Contact Force and Slip. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2005), Edmond, Canada, 2–6 August 2005; pp. 2638–2643.
- Gray, B.L.; Fearing, R.S. A Surface Micromachined Microtactile Sensor Array. In Proceedings of the IEEE International Conference on Robotics and Automation, Minneapolis, MN, USA, 22–28 April 1996; pp. 1–6.
- Lee, H.K.; Chang, S.I.; Kim, S.J.; Yun, K.S.; Yoon, E.; Kim, K.H. A Modular Expandable Tactile Sensor Using Flexible Polymer. In Proceedings of the 18th IEEE International Conference on Micro Electro Mechanical Systems, Miami Beach, FL, USA, 30 January–3 February 2005; pp. 642–645.
- Lee, H.K.; Chang, S.I.; Yoon, E. A flexible polymer tactile sensor: Fabrication and modular expandability for large area deployment. J. Microelectromech. Syst. 2006, 15, 1681–1686. [Google Scholar] [CrossRef]
- Schmitz, A.; Maggiali, M.; Natale, L.; Bonino, B.; Metta, G. Tactile Sensor for the Fingertips of the Humanoid Robot iCub. In Proceedings of the International Conference on Intelligent Robots and Systems (IROS), Taipei, Taiwan, 18–22 October 2010; pp. 2212–2217.
- Muhammad, H.B.; Oddo, C.M.; Beccai, L.; Recchiuto, C.; Anthony, C.J.; Adams, M.J.; Carrozza, M.C.; Hukins, D.W.L.; Ward, M.C.L. Development of a bioinspired MEMS based capacitive tactile sensor for a robotic finger. Sens. Actuator A Phys. 2011, 165, 221–229. [Google Scholar] [CrossRef]
- Cannata, G.; Maggiali, M.; Metta, G.; Sandini, G. An Embedded Artificial Skin for Humanoid Robots. In Proceedings of the IEEE International Conference on Multisensor Fusion and Integration for Intelligent Systems, Seoul, South Korea, 20–22 August 2008; pp. 434–438.
- Muhammad, H.B.; Recchiuto, C.; Oddo, C.M.; Beccai, L.; Anthony, C.J.; Adams, M.J.; Carrozza, M.C.; Ward, M.C.L. A capacitive tactile sensor array for surface texture discrimination. Microelectron. Eng. 2011, 88, 1811–1813. [Google Scholar] [CrossRef]
- Puangmali, P.; Althoefer, K.; Seneviratne, L.D.; Murphy, D.; Dasgupta, P. State-of-the-art in force and tactile sensing for minimally invasive surgery. IEEE J. Sens. 2008, 8, 371–381. [Google Scholar] [CrossRef]
- Hamed, A.; Tang, S.C.; Ren, H.; Squires, A.; Payne, C.; Masamune, K.; Tang, G.; Mohammadpour, J.; Tse, Z.T.H. Advances in haptics, tactile sensing, and manipulation for robot-assisted minimally invasive surgery, noninvasive surgery, and diagnosis. J. Robot. 2012, 2012. [Google Scholar] [CrossRef]
- Rebello, K.J. Applications of MEMS in surgery. Proc. IEEE 2004, 92, 43–55. [Google Scholar] [CrossRef]
- Konstantinova, J.; Jiang, A.; Althoefer, K.; Dasgupta, P.; Nanayakkara, T. Implementation of Tactile Sensing for Palpation in Robot-Assisted Minimally Invasive Surgery. IEEE J. Sens. 2013, 14, 2490–2501. [Google Scholar] [CrossRef]
- Eklund, A.; Bergh, A.; Lindahl, O.A. A catheter tactile sensor for measuring hardness of soft tissue: Measurement in a silicone model and in an in vitro human prostate model. Med. Biol. Eng. Comp. 1999, 37, 618–624. [Google Scholar] [CrossRef]
- Dargahi, J.; Parameswaran, M.; Payandeh, S. A micromachined piezoelectric tactile sensor for an endoscopic grasper-theory, fabrication and experiments. J. Microelectromech. Syst. 2000, 9, 329–335. [Google Scholar] [CrossRef]
- Sedaghati, R.; Dargahi, J.; Singh, H. Design and modeling of an endoscopic piezoelectric tactile sensor. Int. J. Solids Struct. 2005, 42, 5872–5886. [Google Scholar] [CrossRef]
- Qasaimeh, M.A.; Sokhanvar, S.; Dargahi, J.; Kahrizi, M. PVDF-based microfabricated tactile sensor for minimally invasive surgery. J. Microelectromech. Syst. 2009, 18, 195–207. [Google Scholar] [CrossRef]
- Ezhilvalavan, S.; Zhang, Z.; Loh, J.; Ying, J.Y. Microfabrication of PZT force sensors for minimally invasive surgical tools. J. Phys. Conf. Ser. 2006, 34. [Google Scholar] [CrossRef]
- Sharma, T.; Je, S.S.; Gill, B.; Zhang, J.X. Patterning piezoelectric thin film PVDF-TrFE based pressure sensor for catheter application. Sens. Actuator A Phys. 2012, 177, 87–92. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.M.; Lee, S.; Gorton, A.; Schulz, M.J.; Ahn, C.H. Flexible dome and bump shape piezoelectric tactile sensors using PVDF-TrFE copolymer. J. Microelectromech. Syst. 2008, 17, 334–341. [Google Scholar] [CrossRef]
- Kalantari, M.; Ramezanifard, M.; Ahmadi, R.; Dargahi, J.; Kovecses, J. Design, Fabrication, and Testing of a Piezoresistive Hardness Sensor in Minimally Invasive Surgery. In Proceedings of the 2010 IEEE Haptics Symposium, Waltham, MA, USA, 25–26 March 2010; pp. 431–437.
- Atieh, A.; Ahmadi, R.; Kalantari, M.; Dargahi, J.; Packirisamy, M. Piezoresistive Based Tactile Sensor for Use in Minimally Invasive Surgery. In Proceedings of the 2011 IEEE 37th Annual Northeast Bioengineering Conference (NEBEC), Troy, NY, USA, 1–3 April 2011; pp. 1–2.
- Ahmadi, R.; Dargahi, J.; Packirisamy, M.; Cecere, R. A New Hybrid Catheter-Tip Tactile Sensor with Relative Hardness Measuring Capability for Use in Catheter-Based Heart Surgery. In Proceedings of IEEE Sensors, Kona, HI, USA, 1–4 November 2010; pp. 1592–1595.
- Valdastri, P.; Harada, K.; Menciassi, A.; Beccai, L.; Stefanini, C.; Fujie, M.; Dario, P. Integration of a miniaturised triaxial force sensor in a minimally invasive surgical tool. IEEE Trans. Biomed. Eng. 2006, 53, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Urry, S. Plantar pressure-measurement sensors. Measur. Sci. Technol. 1999, 10, R16–R32. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Fang, Y.K.; Ju, M.S.; Chen, G.S.; Ho, J.J.; Yang, C.H.; Wu, P.M.; Wu, G.S.; Chen, T.Y.-F. A contact-type piezoresistive micro-shear stress sensor for above-knee prosthesis application. J. Microelectromech. Syst. 2001, 10, 121–127. [Google Scholar] [CrossRef]
- Alfaro, F.; Weiss, L.; Campbell, P.; Miller, M.; Fedder, G.K. Design of a multi-axis implantable MEMS sensor for intraosseous bone stress monitoring. J. Micromech. Microeng. 2009, 19. [Google Scholar] [CrossRef]
- Valdastri, P.; Roccella, S.; Beccai, L.; Cattin, E.; Menciassi, A.; Carrozza, M.C.; Dario, P. Characterization of a novel hybrid silicon three-axial force sensor. Sens. Actuator A Phys. 2005, 123, 249–257. [Google Scholar] [CrossRef]
- Wahab, Y.; Zayegh, A.; Veljanovski, R.; Begg, R.K. Micro-Sensor for Foot Pressure Measurement. In Proceedings of the TENCON 2008—2008 IEEE Region 10 Conference, Hyderabad, India, 19–21 November 2008; pp. 1–5.
- Yang, Y.J.; Cheng, M.Y.; Shih, S.C.; Huang, X.H.; Tsao, C.M.; Chang, F.Y.; Fan, K.C. A 32 × 32 temperature and tactile sensing array using PI-copper films. Int. J. Adv. Manuf. Tech. 2010, 46, 945–956. [Google Scholar] [CrossRef]
- Engel, J.; Chen, J.; Fan, Z.; Liu, C. Polymer micromachined multimodal tactile sensors. Sens. Actuator A Phys. 2005, 117, 50–61. [Google Scholar] [CrossRef]
- Taffoni, F.; Formica, D.; Saccomandi, P.; Pino, G.D.; Schena, E. Optical fiber-based MR-compatible sensors for medical applications: An overview. Sensors 2013, 13, 14105–14120. [Google Scholar] [CrossRef]
- Puangmali, P.; Dasgupta, P.; Seneviratne, L.D.; Althoefer, K. Miniaturized Triaxial Optical Fiber Force Sensor for MRI-Guided Minimally Invasive Surgery. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Anchorage, AK, USA, 3–8 May 2010; pp. 2592–2597.
- Polygerinos, P.; Ataollahi, A.; Schaeffter, T.; Razavi, R.; Seneviratne, L.D.; Althoefer, K. MRI-compatible intensity-modulated force sensor for cardiac catheterization procedures. IEEE Trans. Biomed. Eng. 2011, 58, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peirs, J.; Clijnen, J.; Reynaerts, D.; Brussel, H.V.; Herijgers, P.; Corteville, B.; Boone, S. A micro optical force sensor for force feedback during minimally invasive robotic surgery. Sens. Actuator A Phys. 2004, 115, 447–455. [Google Scholar] [CrossRef]
- Yip, M.C.; Yuen, S.G.; Howe, R.D. A robust uniaxial force sensor for minimally invasive surgery. IEEE Trans. Biomed. Eng. 2010, 57, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Abushagur, A.A.; Arsad, N.; Reaz, M.I.; Bakar, A. Advances in bio-tactile sensors for minimally invasive surgery using the Fibre Bragg grating force sensor technique: A survey. Sensors 2014, 14, 6633–6665. [Google Scholar]
- Su, H.; Zervas, M.; Furlong, C.; Fischer, G.A. Miniature MRI-Compatible Fiber-Optic Force Sensor Utilizing Fabry-Perot Interferometer. In MEMS and Nanotechnology; Proulx, T., Ed.; Springer: New York, NY, USA, 2011; Volume 4, pp. 131–136. [Google Scholar]
- Liu, X.; Iordachita, I.I.; He, X.; Taylor, R.H.; Kang, J.U. Miniature fiber-optic force sensor based on low-coherence Fabry-Perot interferometry for vitreoretinal microsurgery. Biomed. Opt. Express 2012, 3, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Chung, J.H.; Lee, J.J. Tactile sensor arrays using fiber Bragg grating sensors. Sens. Actuator A Phys. 2006, 126, 312–327. [Google Scholar] [CrossRef]
- Cowie, B.M.; Webb, D.J.; Tam, B.; Slack, P.; Brett, P.N. Fibre Bragg grating sensors for distributive tactile sensing. Measur. Sci. Technol. 2007, 18, 138–146. [Google Scholar] [CrossRef]
- Accoto, D.; Schena, E.; Cidda, M.; Francomano, M.; Saccomandi, P.; Silvestri, S. A Micro Opto-Mechanical Displacement Sensor Based on Micro-Diffraction Gratings: Design and Characterization. In Proceedings of the 35th Annual International Conference of the IEEE in Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4714–4717.
- De Rossi, S.M.; Lenzi, T.; Vitiello, N.; Donati, M.; Persichetti, A.; Giovacchini, F.; Vecchi, F.; Carrozza, M.C. Development of An In-Shoe Pressure-Sensitive Device for Gait Analysis. In Proceedings of the 35th Annual International Conference of the IEEE in Engineering in Medicine and Biology Society (EMBC), Boston, MA, USA, 30 August–3 September 2011; pp. 5637–5640.
- Wettels, N.; Santos, V.J.; Johansson, R.S.; Loeb, G.E. Biomimetic tactile sensor array. Adv. Robot. 2008, 22, 829–849. [Google Scholar] [CrossRef]
- Ponce Wong, R.D.; Posner, J.D.; Santos, V.J. Flexible microfluidic normal force sensor skin for tactile feedback. Sens. Actuator A Phys. 2012, 179, 62–69. [Google Scholar] [CrossRef]
- Park, Y.L.; Chen, B.R.; Wood, R.J. Design and fabrication of soft artificial skin using embedded microchannels and liquid conductors. IEEE Sens. J. 2012, 12, 2711–2718. [Google Scholar] [CrossRef]
- Spigler, G.; Oddo, C.M.; Carrozza, M.C. Soft-Neuromorphic Artificial Touch for Applications in Neuro-Robotics. In Proceedings of the 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1913–1918.
- Bucci, L.D.; Chou, T.S.; Krichmar, J.L. Sensory Decoding in a Tactile, Interactive Neurorobot. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, 31 May–7 June 2014.
- Krishna, G.M.; Rajanna, K. Tactile sensor based on piezoelectric resonance. Sens. J. IEEE 2004, 4, 691–697. [Google Scholar] [CrossRef]
- Lindahl, O.A.; Constantinou, C.E.; Eklund, A.; Murayama, Y.; Hallberg, P.; Omata, S. Tactile resonance sensors in medicine. J. Med. Eng. Technol. 2009, 33, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Y.; Constantinou, C.E.; Omata, S. Micro-mechanical sensing platform for the characterization of the elastic properties of the ovum via uniaxial measurement. J. Biomech. 2004, 37, 67–72. [Google Scholar] [CrossRef]
- Lindberg, P.; Andersson, B.; Bergh, A.; Ljungberg, B.; Lindahl, O. Prostate cancer detection with an improved resonance sensor system: Parameter evaluation in a silicone model and on human prostate tissue in vitro. Med. Biol. Eng. Comput. 2006, 44, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Shahinpoor, M.; Kwang, J.K. Ionic polymer-metal composites: IV. Industrial and medical applications. Smart Mater. Struct. 2005, 14, 197–214. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Bar-Cohen, Y.; Simpson, J.O.; Smith, J. Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles—A review. Smart Mater. Struct. 1998, 7, R15–R30. [Google Scholar] [CrossRef]
- Ferrara, L.; Shahinpoor, M.; Kim, K.J.; Schreyer, H.B.; Keshavarzi, A.; Benzel, E.; Lantz, J.W. Use of Ionic Polymer-Metal Composites (IPMCs) as a Pressure Transducer in the Human Spine. In Proceedings of the International Society for Optics and Photonics, 1999 Symposium on Smart Structures and Materials, Newport Beach, CA, USA, 28 May 1999; pp. 394–401.
- Bonomo, C.; Brunetto, P.; Fortuna, L.; Giannone, P.; Graziani, S.; Strazzeri, S. A tactile sensor for biomedical applications based on IPMCs. Sens. J. IEEE 2008, 8, 1486–1493. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccomandi, P.; Schena, E.; Oddo, C.M.; Zollo, L.; Silvestri, S.; Guglielmelli, E. Microfabricated Tactile Sensors for Biomedical Applications: A Review. Biosensors 2014, 4, 422-448. https://doi.org/10.3390/bios4040422
Saccomandi P, Schena E, Oddo CM, Zollo L, Silvestri S, Guglielmelli E. Microfabricated Tactile Sensors for Biomedical Applications: A Review. Biosensors. 2014; 4(4):422-448. https://doi.org/10.3390/bios4040422
Chicago/Turabian StyleSaccomandi, Paola, Emiliano Schena, Calogero Maria Oddo, Loredana Zollo, Sergio Silvestri, and Eugenio Guglielmelli. 2014. "Microfabricated Tactile Sensors for Biomedical Applications: A Review" Biosensors 4, no. 4: 422-448. https://doi.org/10.3390/bios4040422