State-of-the-Art Methods for Skeletal Muscle Glycogen Analysis in Athletes—The Need for Novel Non-Invasive Techniques
Abstract
:1. Introduction
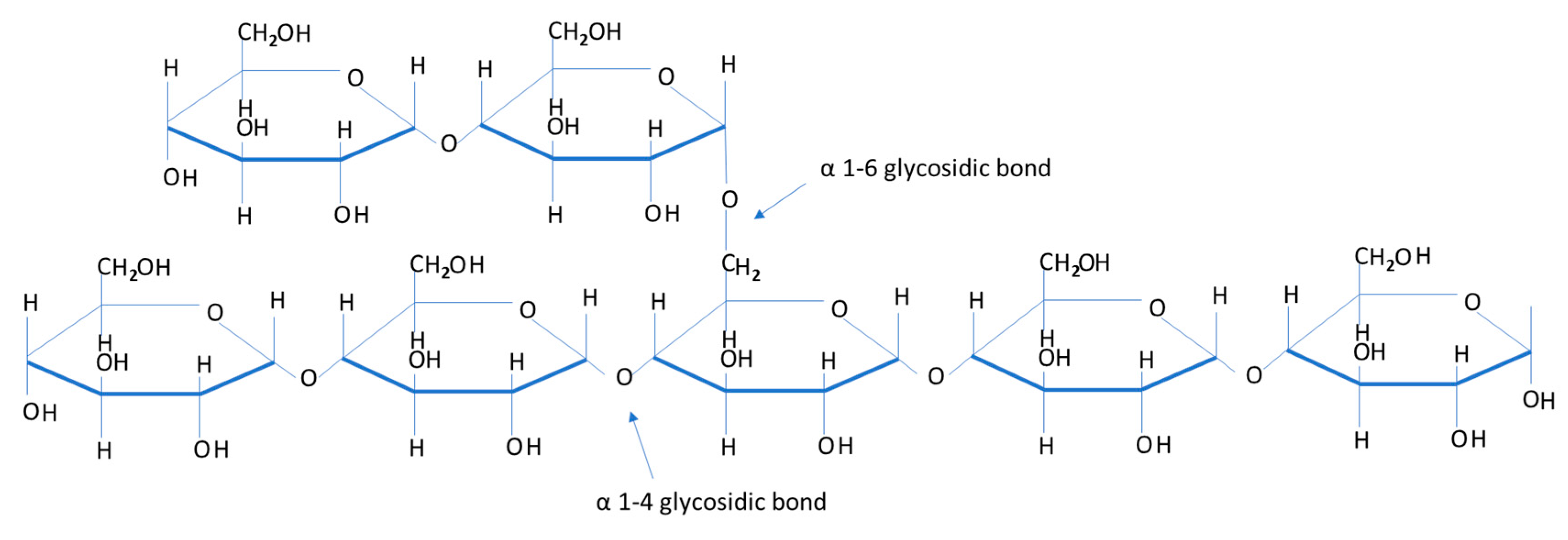
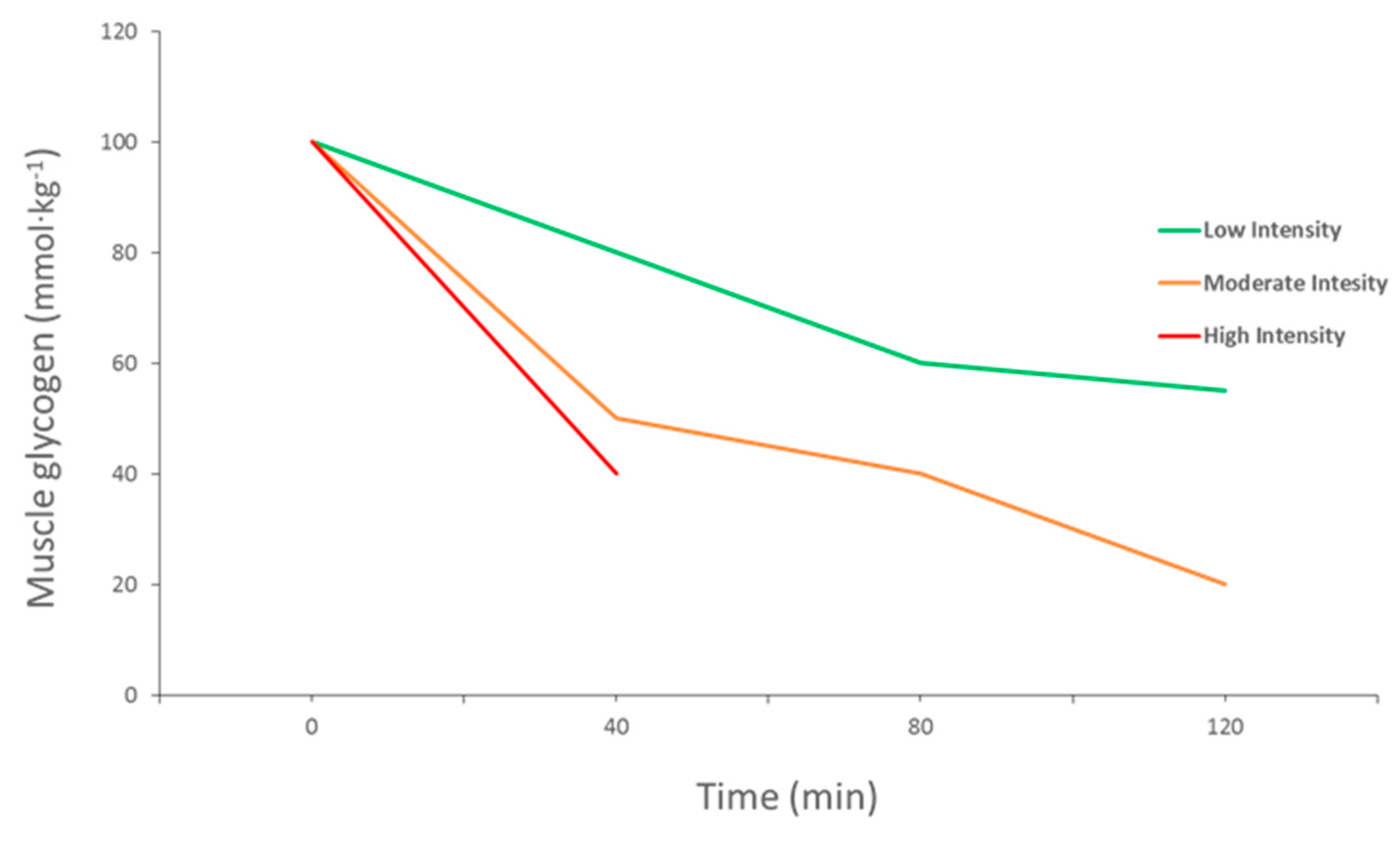
2. Importance of Muscle Glycogen
3. Current Athlete Recommendations
4. Methods of Measuring Muscle Glycogen in Athletes
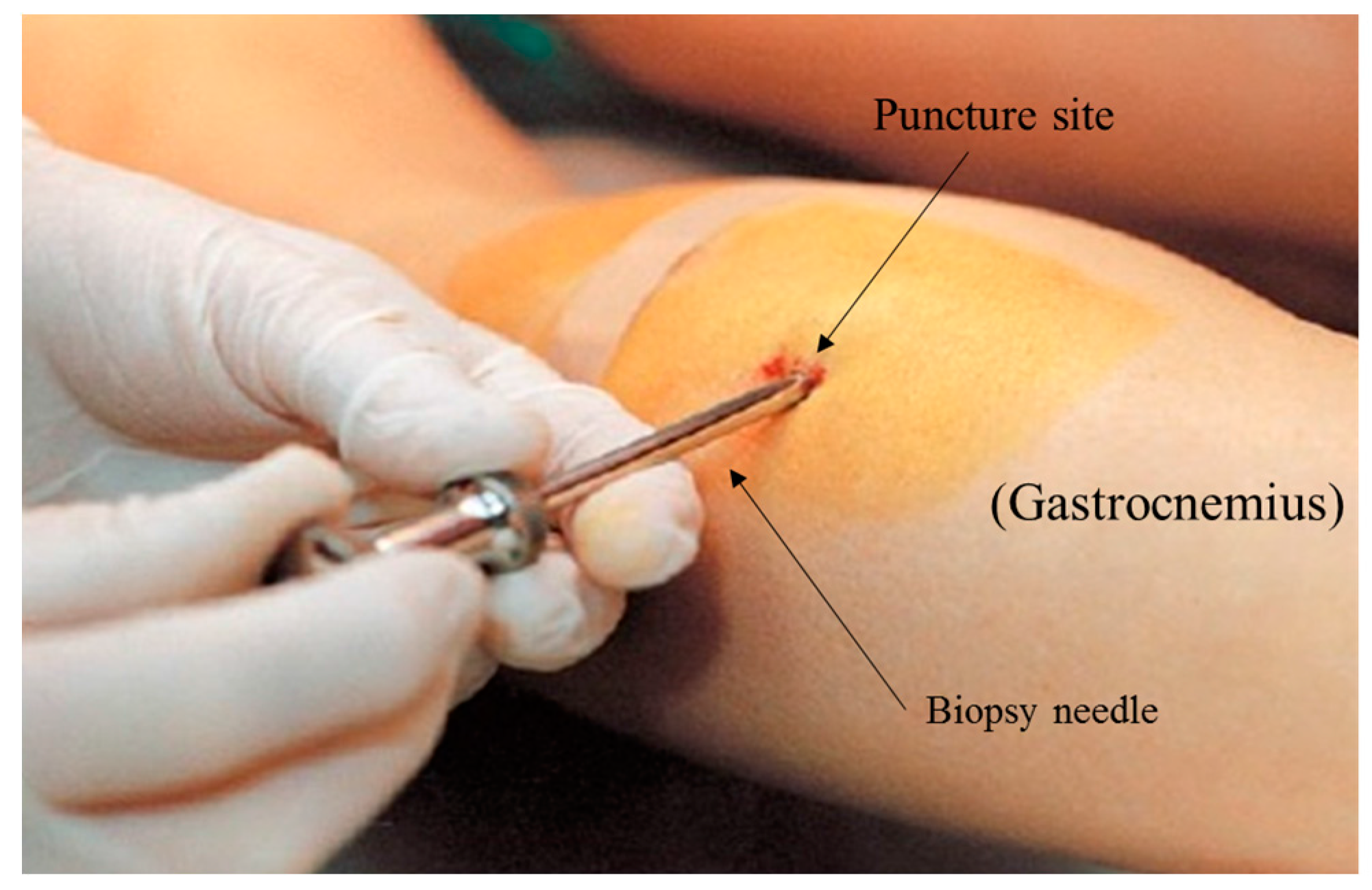
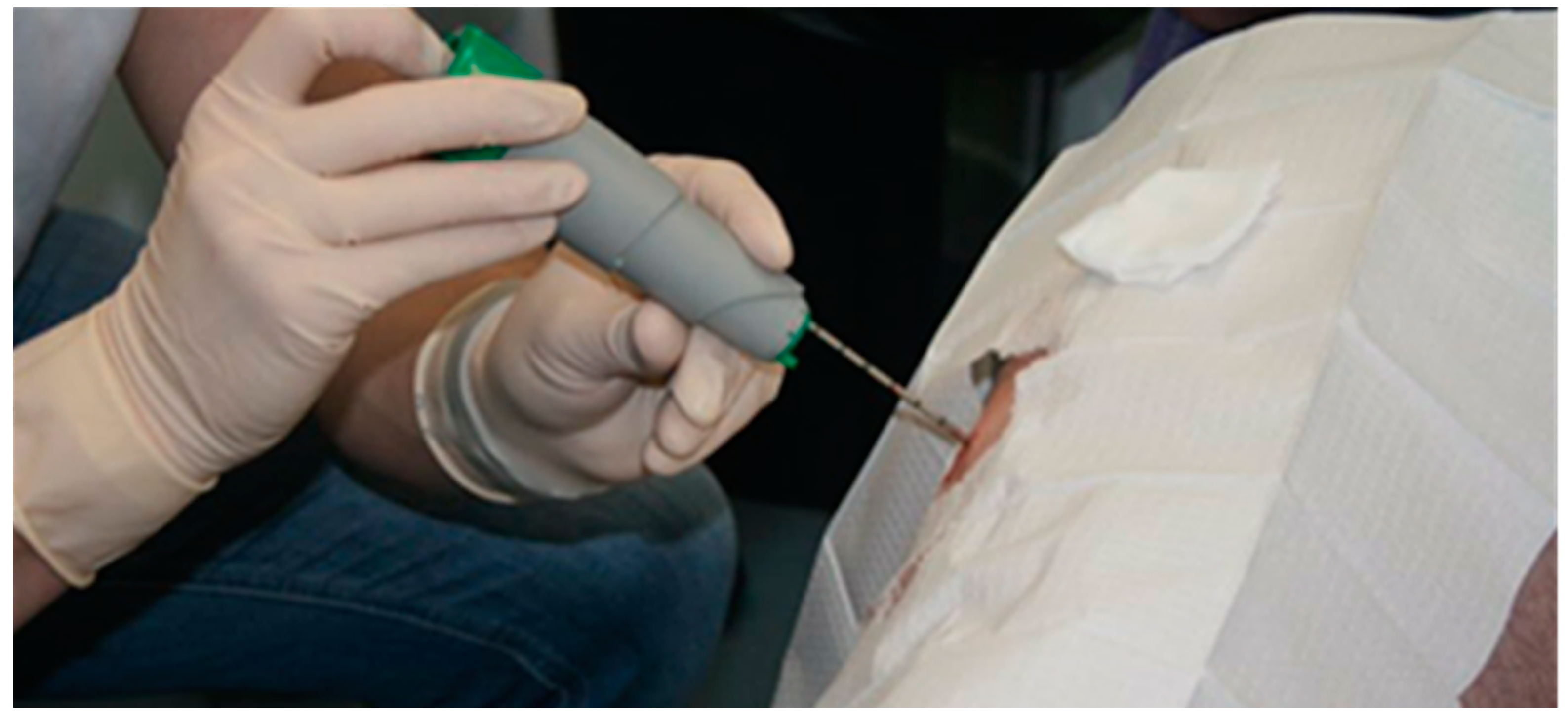
4.1. The Elusive Gold Standard
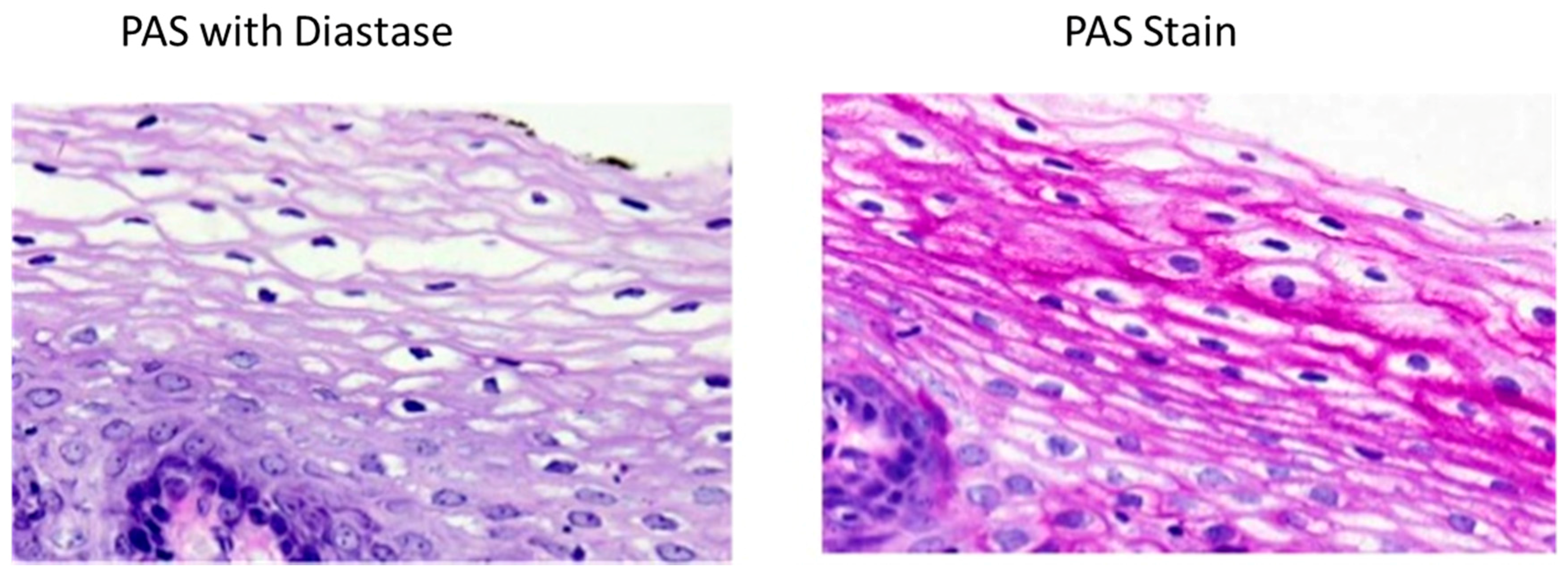
4.2. Histochemical Methods
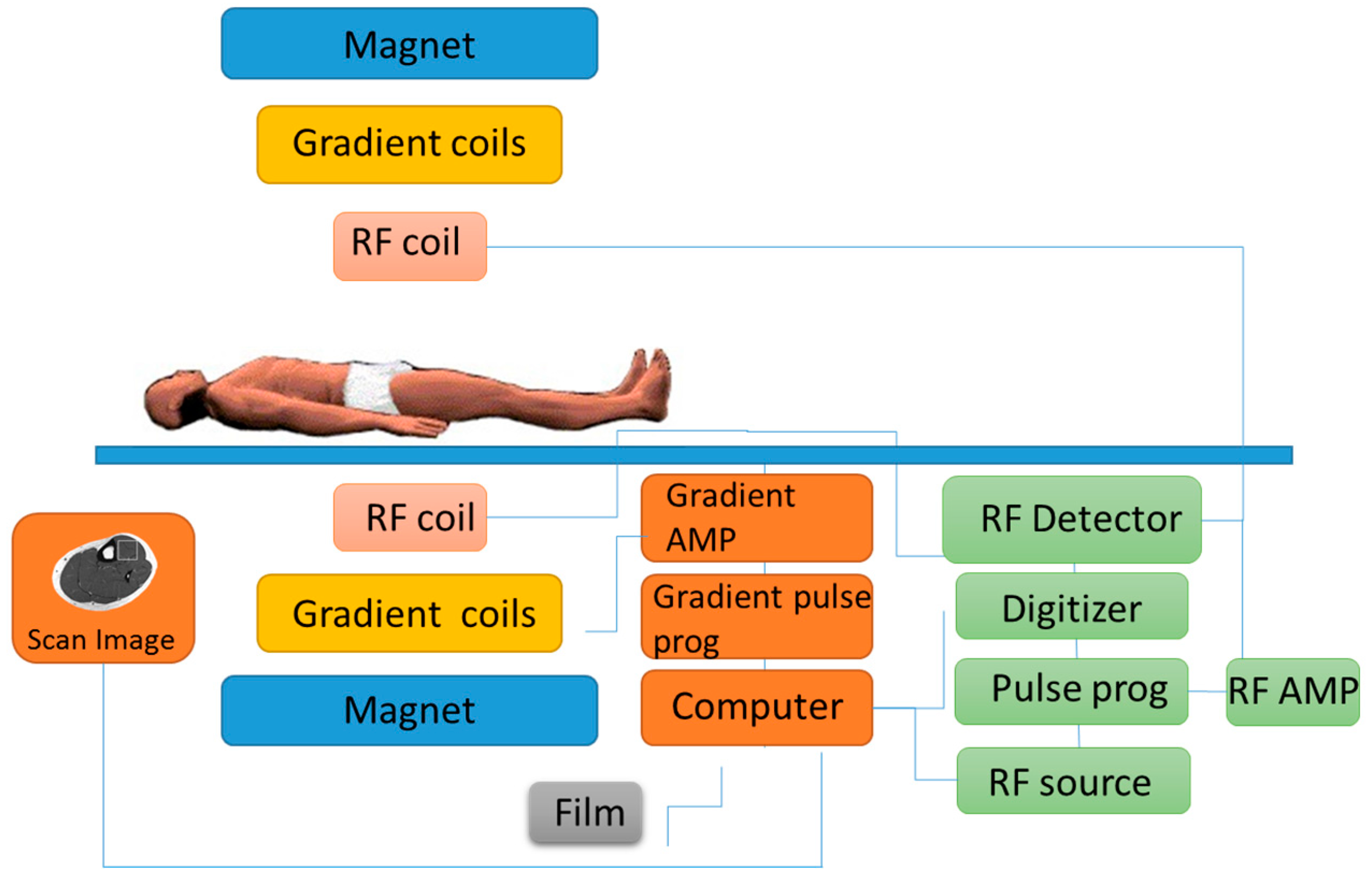
4.3. Magnetic Resonance Spectroscopy
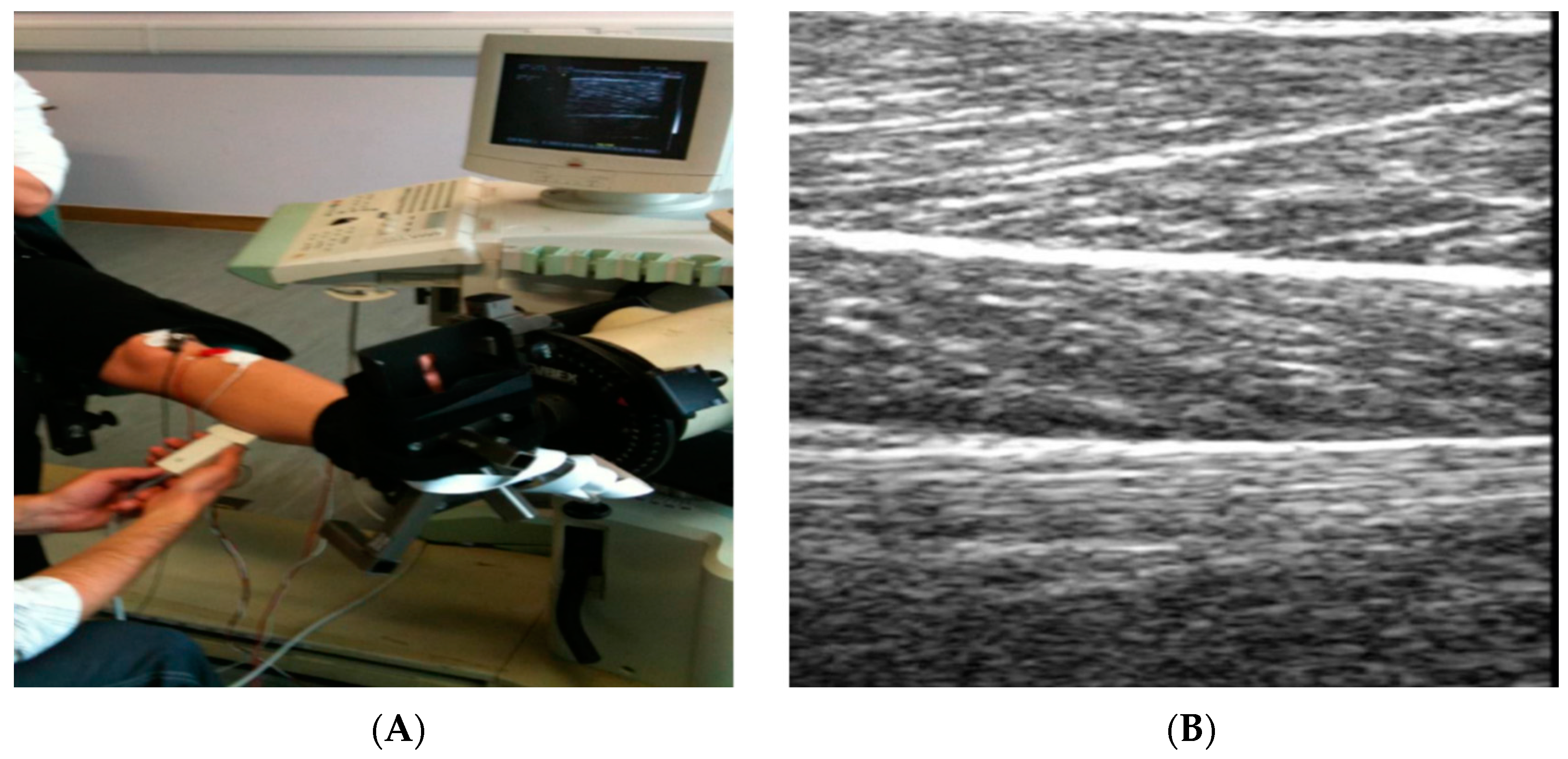
4.4. Musculoskeletal High Frequency Ultrasound
4.5. Advances in Sensor Technology to Detect Physiological Markers
5. Conclusions and Future Research Directions
Conflicts of Interest
References
- Loader, J.; Montgomery, P.; Williams, M.; Lorenzen, C.; Kemp, J. Classifying training drills based on movement demands in australian football. Int. J. Sports Sci. Coach. 2012, 7, 57–68. [Google Scholar] [CrossRef]
- Mooney, M.G.; Hunter, J.R.; O’Brien, B.J.; Berry, J.T.; Young, W.B. Reliability and validity of a novel intermittent peak running speed test for australian football. J. Strength Cond. Res. 2011, 25, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.D.; Twist, C.; Lamb, K.L.; Nicholas, C.W. Heart rate responses to small-sided games among elite junior rugby league players. J. Strength Cond. Res. 2010, 24, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; van Loon, L.J.; Hawley, J.A. Post-exercise muscle glycogen resynthesis in humans. J. Appl. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Leckey, J.J.; Burke, L.M.; Morton, J.P.; Hawley, J.A. Altering fatty acid availability does not impair prolonged, continuous running to fatigue: Evidence for carbohydrate dependence. J. Appl. Physiol. 2016, 120, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Torrens, S.L.; Areta, J.L.; Parr, E.B.; Hawley, J.A. Carbohydrate dependence during prolonged simulated cycling time trials. Eur. J. Appl. Physiol. 2016, 116, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Baba, O. Production of monoclonal antibody that recognizes glycogen and its application for immunohistochemistry. Kokubyo Gakkai Zasshi 1993, 60, 264–287. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.; Tupling, A.R.; Houston, M. Biochemistry Primer for Exercise Science; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Ahlborg, B.; Bergström, J.; Ekelund, L.G.; Hultman, E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiol. 1967, 70, 129–142. [Google Scholar] [CrossRef]
- Maehlum, S.; Hermansen, L. Muscle glycogen concentration during recovery after prolonged severe exercise in fasting subjects. Scand. J. Clin. Lab. Investig. 1978, 38, 557–560. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Hargreaves, M. Carbohydrate nutrition and fatigue. Sports Med. 1992, 13, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.; Piehl, K.; Saltin, B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J. Physiol. 1974, 241, 45. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.K.; Paton, C.D.; Garnham, A.P.; Burke, L.M.; Carey, A.L.; Hawley, J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008, 105, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Maclaren, D.; Morton, J. Biochemistry for Sport and Exercise Metabolism: Translate by Gaeini A; Samt Publication: Tehran, Iran, 2012. [Google Scholar]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, L.; Hultman, E.; Saltin, B. Muscle glycogen during prolonged severe exercise. Acta Physiol. Scand. 1967, 71, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Balsom, P.D.; Gaitanos, G.; Söderlund, K.; Ekblom, B. High-intensity exercise and muscle glycogen availability in humans. Acta Physiol. Scand. 1999, 165, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Marquet, L.-A.; Brisswalter, J.; Louis, J.; Tiollier, E.; Burke, L.M.; Hawley, J.A.; Hausswirth, C. Enhanced endurance performance by periodization of cho intake: “Sleep low” strategy. Med. Sci. Sports Exerc. 2016, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Marquet, L.-A.; Tiollier, E.; Bermon, S.; Hausswirth, C.; Brisswalter, J. The impact of sleeping with reduced glycogen stores on immunity and sleep in triathletes. Eur. J. Appl. Physiol. 2016, 116, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Hargreaves, M.; Baar, K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1343–E1351. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.; Kay, J.; Phyn, C.; Meier, S.; Lee, J.; Roche, J. Short communication: Effects of dietary nonstructural carbohydrates pre-and postpartum on reproduction of grazing dairy cows. J. Dairy Sci. 2010, 93, 4292–4296. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Schabort, E.J.; Noakes, T.D.; Dennis, S.C. Carbohydrate-loading and exercise performance. Sports Med. 1997, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bussau, V.A.; Fairchild, T.J.; Rao, A.; Steele, P.; Fournier, P.A. Carbohydrate loading in human muscle: An improved 1 day protocol. Eur. J. Appl. Physiol. 2002, 87, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Louhelainen, J.; Iqbal, Z.; Cochran, A.J.; Gibala, M.J.; Gregson, W.; Close, G.L.; Drust, B.; Morton, J.P. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: Implications for mitochondrial biogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R450–R458. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Croft, L.; Bartlett, J.D.; MacLaren, D.P.; Reilly, T.; Evans, L.; McArdle, A.; Drust, B. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J. Appl. Physiol. 2009, 106, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Venables, M.C.; Mann, C.H.; Martin, C.; Philp, A.; Baar, K.; Jeukendrup, A.E. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 2010, 42, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zwetsloot, K.A.; Triplett, N.T.; Meaney, M.P.; Farris, G.E.; Nieman, D.C. Human skeletal muscle biopsy procedures using the modified bergström technique. J. Visual. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Investig. 1975, 35, 609–616. [Google Scholar] [CrossRef]
- Bergström, J. Muscle Electrolytes in Man Determined by Neutron Activation Analysis on Needle Biopsy Specimens; Univ. Forl.: Jönköping, Sweden, 1962. [Google Scholar]
- Evans, W.; Phinney, S.; Young, V. Suction applied to a muscle biopsy maximizes sample size. Med. Sci. Sports Exerc. 1981, 14, 101–102. [Google Scholar]
- Hennessey, J.V.; Chromiak, J.A.; Dellaventura, S.; Guertin, J.; Maclean, D.B. Increase in percutaneous muscle biopsy yield with a suction-enhancement technique. J. Appl. Physiol. 1997, 82, 1739–1742. [Google Scholar] [PubMed]
- Bradley, W.J.; Morehen, J.C.; Haigh, J.; Clarke, J.; Donovan, T.F.; Twist, C.; Cotton, C.; Shepherd, S.; Cocks, M.; Sharma, A. Muscle glycogen utilisation during rugby match play: Effects of pre-game carbohydrate. J. Sci. Med. Sport 2016, 19, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; Pearce, E.; Smith, K.; Lach, B. Suction-modified bergström muscle biopsy technique: Experience with 13,500 procedures. Muscle Nerve 2011, 43, 716–725. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.; Saris, W.H.; Kruijshoop, M.; Wagenmakers, A.J. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar] [PubMed]
- Prats, C.; Gomez-Cabello, A.; Nordby, P.; Andersen, J.L.; Helge, J.W.; Dela, F.; Baba, O.; Ploug, T. An optimized histochemical method to assess skeletal muscle glycogen and lipid stores reveals two metabolically distinct populations of type i muscle fibers. PLoS ONE 2013, 8, e77774. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, T.J.; Fournier, P.A. Gilycogen determiniationi using periodic acid-schiff: Arifact of muscle preparation. Med. Sci. Sports Exerc. 2004, 36, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Tsuruta, S.; Yasuda, M.; Nakamura, T.; Shinoda, E.; Furuyashiki, T.; Kakutani, R.; Takata, H.; Kato, Y.; Ashida, H. Comparative analysis of carbohydrate-binding specificities of two anti-glycogen monoclonal antibodies using elisa and surface plasmon resonance. Carbohydr. Res. 2012, 350, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Befroy, D.E.; Shulman, G.I. Magnetic resonance spectroscopy studies of human metabolism. Diabetes 2011, 60, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Avison, M.; Rothman, D.; Nadel, E.; Shulman, R. Detection of human muscle glycogen by natural abundance 13C-NMR. Proc. Natl. Acad. Sci. USA 1988, 85, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Kogan, F.; Hariharan, H.; Reddy, R. Chemical exchange saturation transfer (cest) imaging: Description of technique and potential clinical applications. Curr. Radiol. Rep. 2013, 1, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Price, T.B. Magnetic resonance technology in training and sports. Br. J. Sports Med. 2000, 34, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Gonzalez, J.T.; Beelen, M.; Cermak, N.M.; Smith, F.E.; Thelwall, P.E.; Taylor, R.; Trenell, M.I.; Stevenson, E.J.; van Loon, L.J. Sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion compared with glucose ingestion in trained athletes. J. Appl. Physiol. 2016, 120, 1328–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.; Price, T.B.; Rothman, D.L.; Shulman, R.G.; Shulman, G.I. Validation of 13C-NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn. Resonan. Med. 1992, 27, 13–20. [Google Scholar] [CrossRef]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13c nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jue, T.; Rothman, D.; Tavitian, B.; Shulman, R. Natural-abundance 13C-NMR study of glycogen repletion in human liver and muscle. Proc. Natl. Acad. Sci. USA 1989, 86, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Jue, T.; Rothman, D.L.; Shulman, G.I.; Tavitian, B.A.; DeFronzo, R.A.; Shulman, R.G. Direct observation of glycogen synthesis in human muscle with 13C-NMR. Proc. Natl. Acad. Sci. USA 1989, 86, 4489–4491. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Wei, Q.; Cortes, N. Dynamic ultrasound imaging applications to quantify musculoskeletal function. Exerc. Sport Sci. Rev. 2014, 42, 126. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.S.; Corrado, G. Ultrasound in sports medicine. Sports Med. 2012, 42, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.; Tatarinov, A.; Sarvazyan, N. Ultrasonic assessment of tissue hydration status. Ultrasonics 2005, 43, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Utter, A.C.; McAnulty, S.R.; Sarvazyan, A.; Query, M.C.; Landram, M.J. Evaluation of ultrasound velocity to assess the hydration status of wrestlers. J. Strength Cond. Res. 2010, 24, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Bone, J.L.; Ross, M.L.; Tomcik, K.A.; Jeacocke, N.A.; Hawley, J.A.; Burke, L.M. Ultrasound technology fails to provide indirect estimate of muscle glycogen concentration: 1891 board# 43 June 2, 2:00 pm–3:30 pm. Med. Sci. Sports Exerc. 2016, 48, 520. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Zwetsloot, K.A.; Meaney, M.P.; Farris, G.E. Ultrasonic assessment of exercise-induced change in skeletal muscle glycogen content. BMC Sports Sci. Med. Rehabil. 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Staelin, D.H.; Morgenthaler, A.W.; Kong, J.A. Electromagnetic Waves; Pearson Education India: Uttar Pradesh, India, 1994. [Google Scholar]
- White, J.F. High Frequency Techniques: An Introduction to RF and Microwave Design and Computer Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Mason, A.; Korostynska, O.; Ortoneda-Pedrola, M.; Shaw, A.; Al-Shamma’a, A. A resonant co-planar sensor at microwave frequencies for biomedical applications. Sens. Actuators A Phys. 2013, 202, 170–175. [Google Scholar] [CrossRef]
- Korostynska, O.; Blakey, R.; Mason, A.; Al-Shamma’a, A. Novel method for vegetable oil type verification based on real-time microwave sensing. Sens. Actuators A Phys. 2013, 202, 211–216. [Google Scholar] [CrossRef]
- Blakey, R.; Korostynska, O.; Mason, A.; Al-Shamma’a, A. Real-time microwave based sensing method for vegetable oil type verification. Procedia Eng. 2012, 47, 623–626. [Google Scholar] [CrossRef]
- Goh, J.; Mason, A.; Al-Shamma’a, A.; Wylie, S.; Field, M.; Browning, P. Lactate detection using a microwave cavity sensor for biomedical applications. In Proceedings of the 2011 Fifth International Conference on Sensing Technology (ICST), Palmerston North, New Zealand, 28 November–1 December 2011; pp. 436–441.
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.; van den Berg, A. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.H.; Mason, A.; Field, M.; Browning, P.; Al-Shamma’a, A. Using a microwave sensor as an online indicator of neurological impairment during surgical procedures. Sens. Lett. 2015, 13, 535–538. [Google Scholar] [CrossRef]
- Fok, M.; Bashir, M.; Fraser, H.; Strouther, N.; Mason, A. A novel microwave sensor to detect specific biomarkers in human cerebrospinal fluid and their relationship to cellular ischemia during thoracoabdominal aortic aneurysm repair. J. Med. Syst. 2015, 39, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Korostynska, O.; Mason, A.; Al-Shamma’a, A. Microwave sensors for the non-invasive monitoring of industrial and medical applications. Sens. Rev. 2014, 34, 182–191. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Liu, J.; Yang, Q.; Cai, X. A sensitive biosensor based on os-complex mediator and glucose oxidase for low concentration glucose determination. J. Electroanal. Chem. 2008, 619, 11–16. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the glucowatch G2 biographer and the continuous glucose monitoring system during hypoglycemia. Experience of the diabetes research in children network (DirecNet). Diabetes Care 2004, 27, 722. [Google Scholar]
- Do Amaral, C.E.F.; Wolf, B. Current development in non-invasive glucose monitoring. Med. Eng. Phys. 2008, 30, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Harman-Boehm, I.; Naidis, E.; Mayzel, Y.; Trieman, L. Validity of glucotrack®, a non-invasive glucose monitor, for variety of diabetics. Age 2011, 1, 61. [Google Scholar]
- Chuang, H.; Trieu, M.-Q.; Hurley, J.; Taylor, E.J.; England, M.R.; Nasraway, S.A. Pilot studies of transdermal continuous glucose measurement in outpatient diabetic patients and in patients during and after cardiac surgery. J. Diabetes Sci. Technol. 2008, 2, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]


| Glycogen Assessment Techniques | Portable? | Accuracy | Real-Time? | Non-Invasive? | Size? | Time of Measurement | Cost |
|---|---|---|---|---|---|---|---|
| Modified Bergström Muscle Biopsy/Assay | Yes | High | No | No | The Bergström needle (5 mm) | days | Low |
| Magnetic resonance spectroscopy | No | High | No | Yes | Height 200 cm approx. | min/h | High |
| Width 199 cm approx. | |||||||
| Musculoskeletal high frequency ultrasound | Yes | Low | Yes | Yes | Height 121 cm approx. | min | Moderate |
| Width 40 cm approx. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greene, J.; Louis, J.; Korostynska, O.; Mason, A. State-of-the-Art Methods for Skeletal Muscle Glycogen Analysis in Athletes—The Need for Novel Non-Invasive Techniques. Biosensors 2017, 7, 11. https://doi.org/10.3390/bios7010011
Greene J, Louis J, Korostynska O, Mason A. State-of-the-Art Methods for Skeletal Muscle Glycogen Analysis in Athletes—The Need for Novel Non-Invasive Techniques. Biosensors. 2017; 7(1):11. https://doi.org/10.3390/bios7010011
Chicago/Turabian StyleGreene, Jacob, Julien Louis, Olga Korostynska, and Alex Mason. 2017. "State-of-the-Art Methods for Skeletal Muscle Glycogen Analysis in Athletes—The Need for Novel Non-Invasive Techniques" Biosensors 7, no. 1: 11. https://doi.org/10.3390/bios7010011







