Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements
Abstract
:1. Introduction
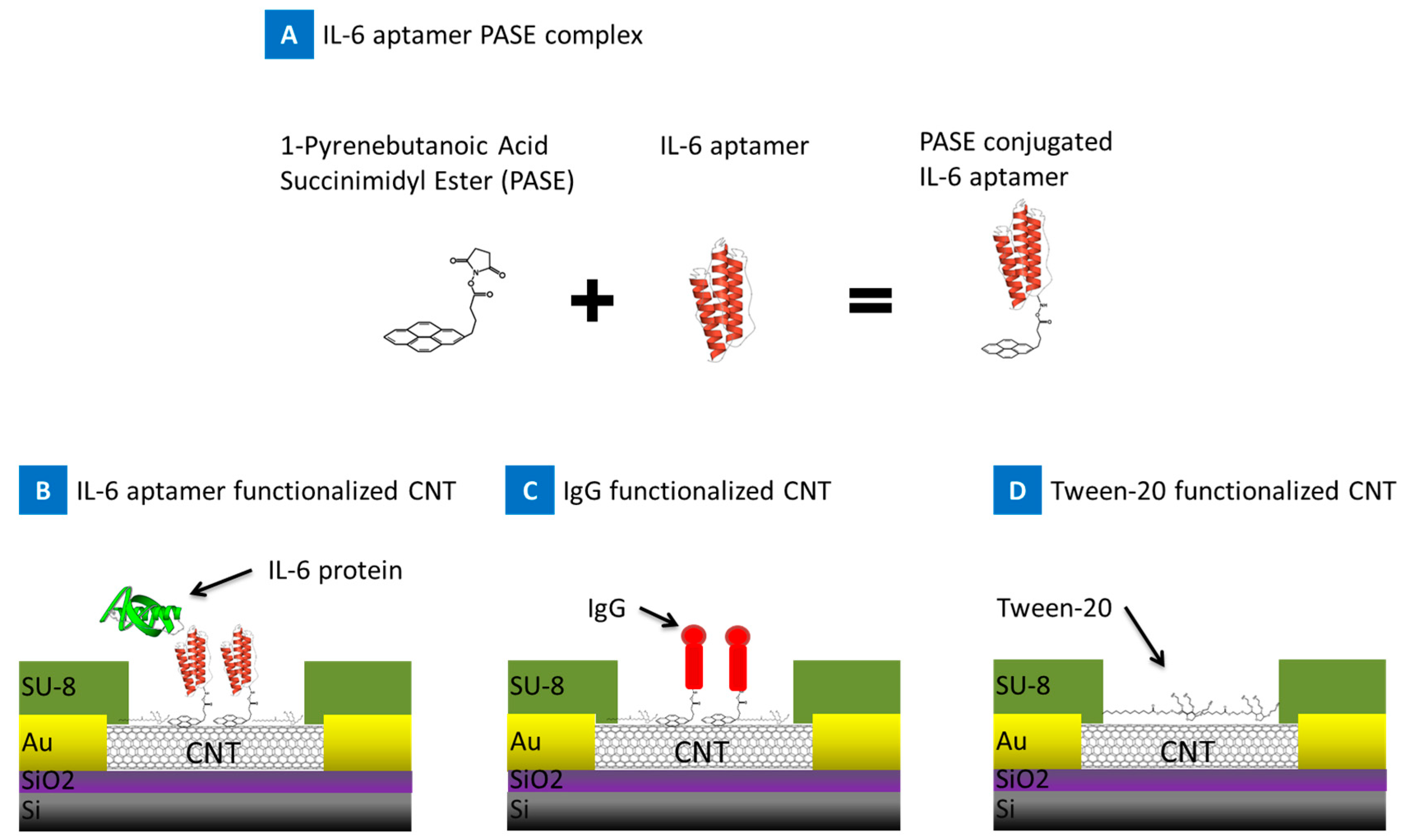
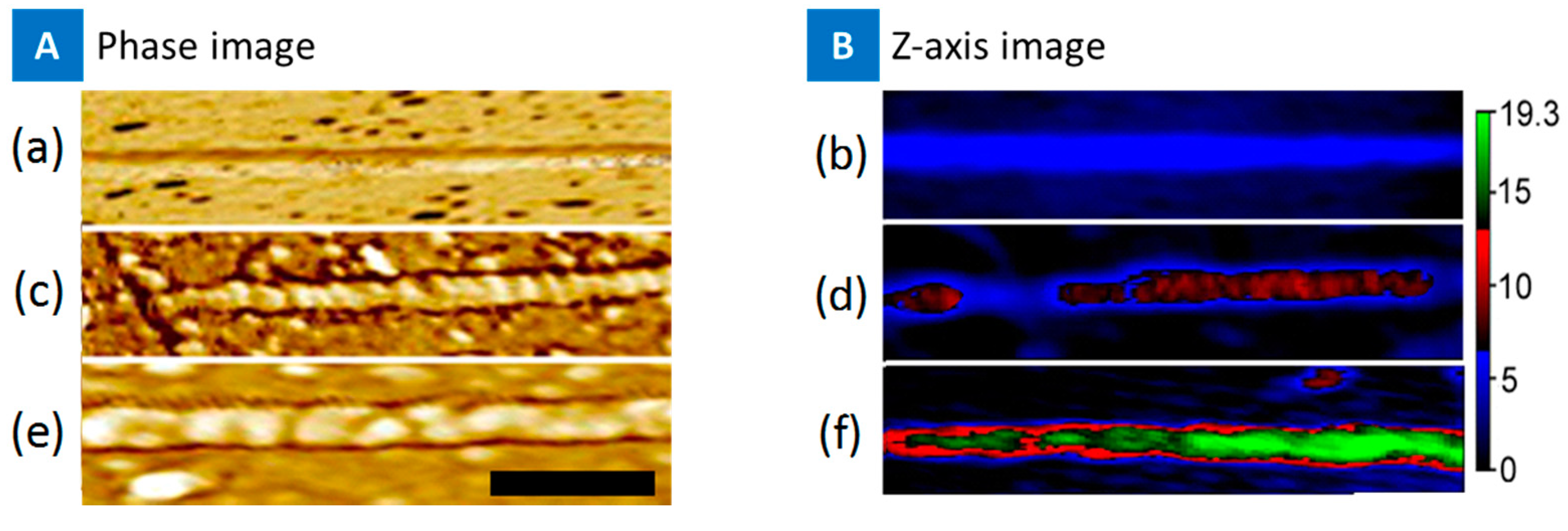
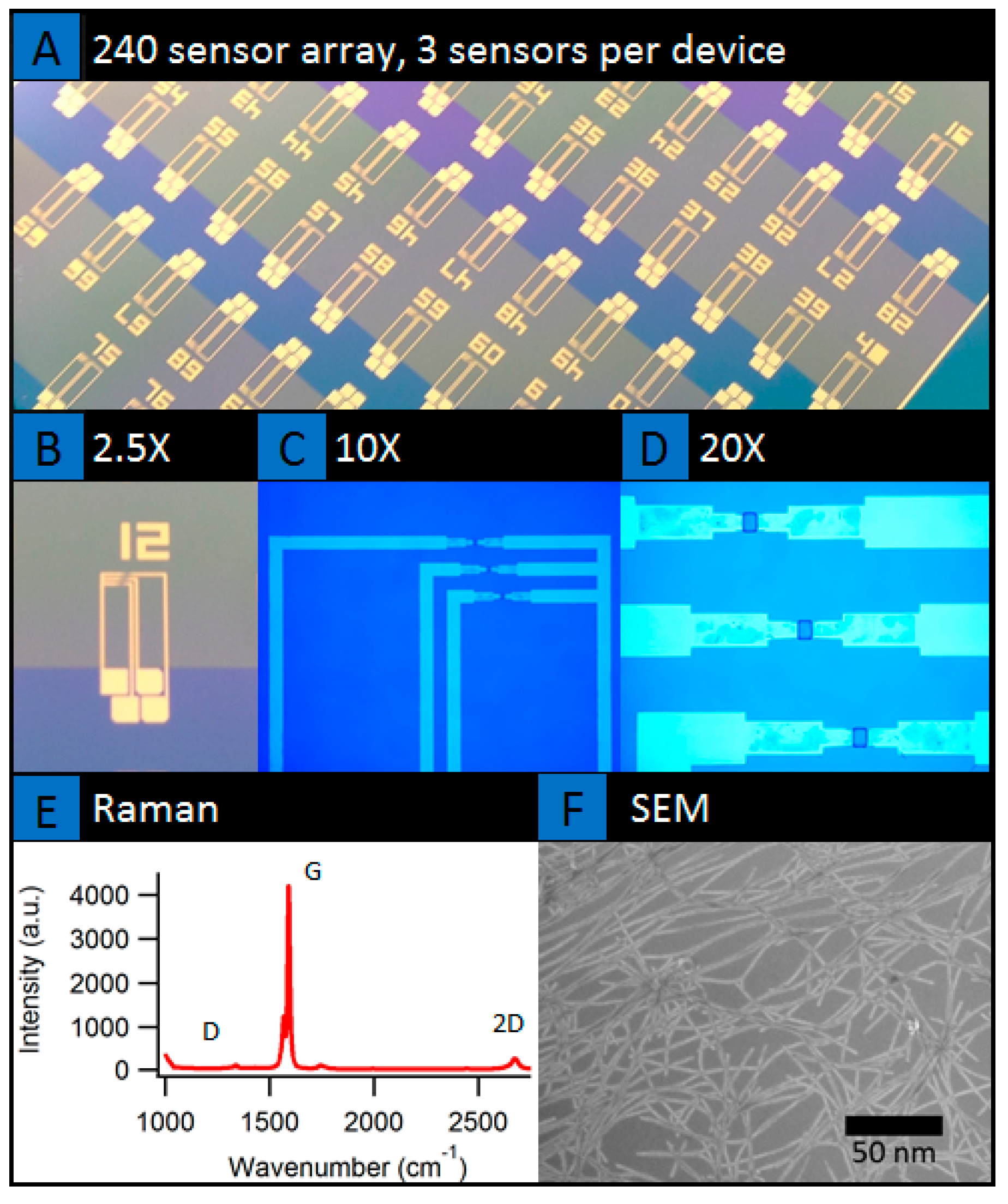
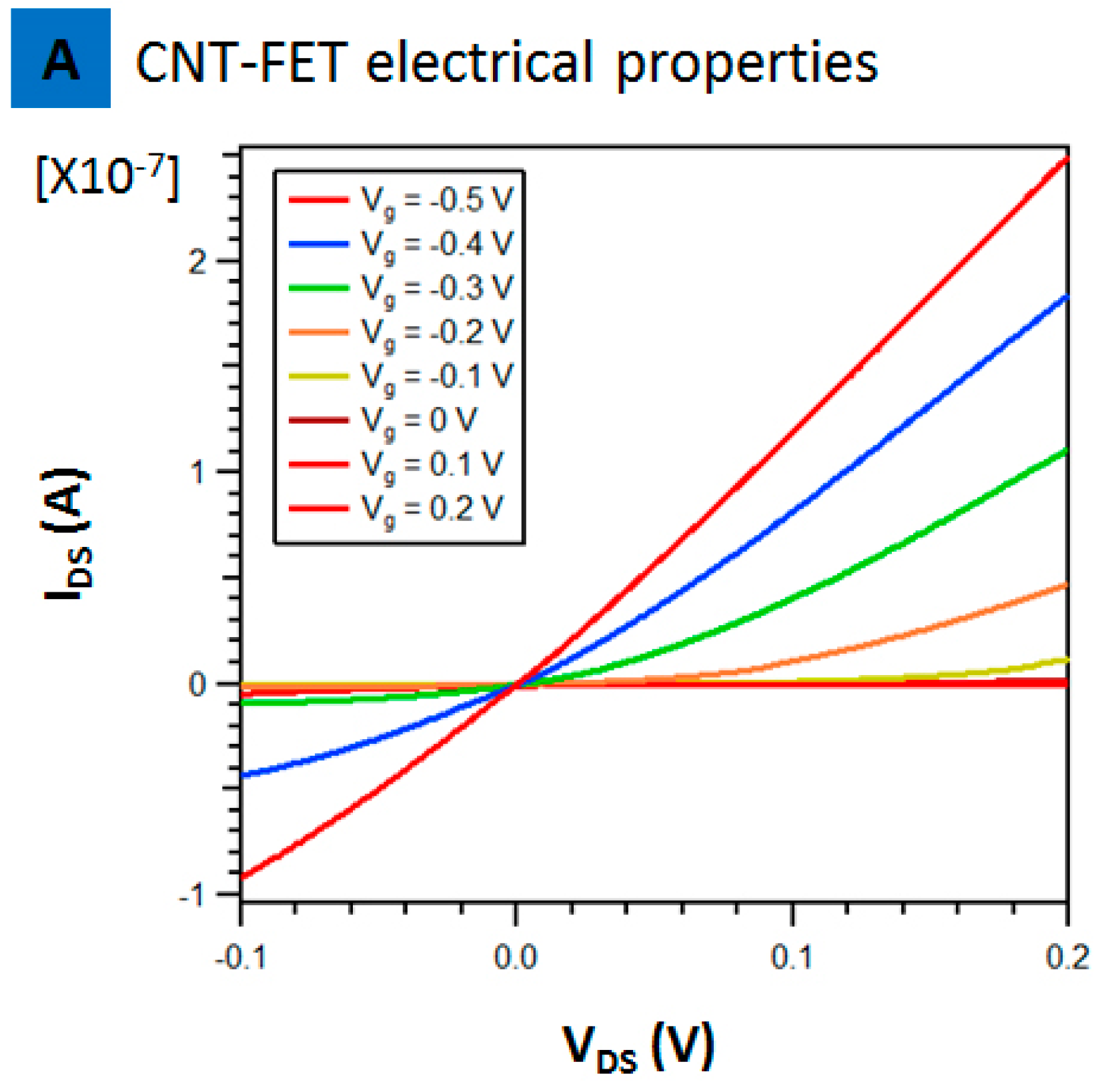
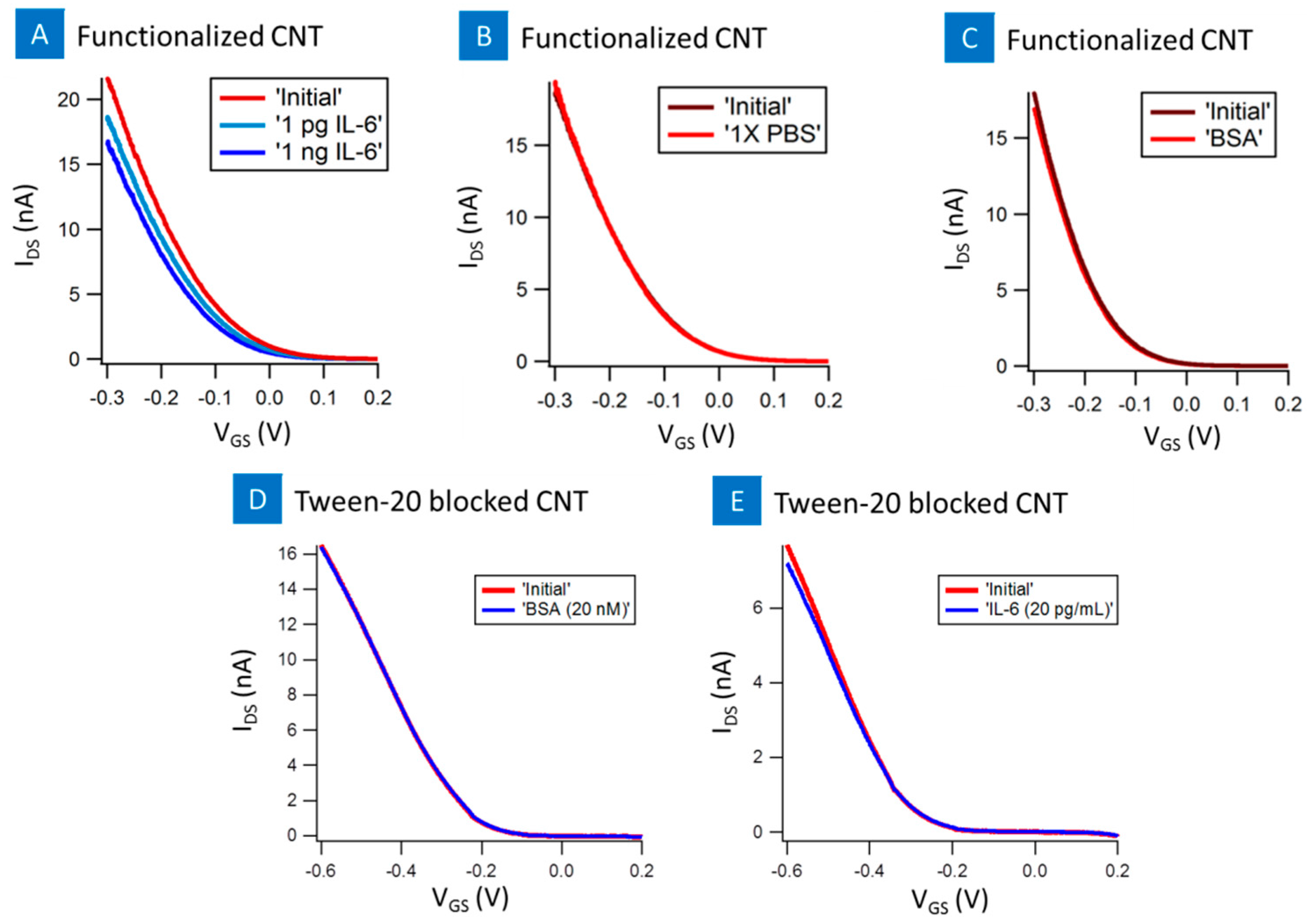
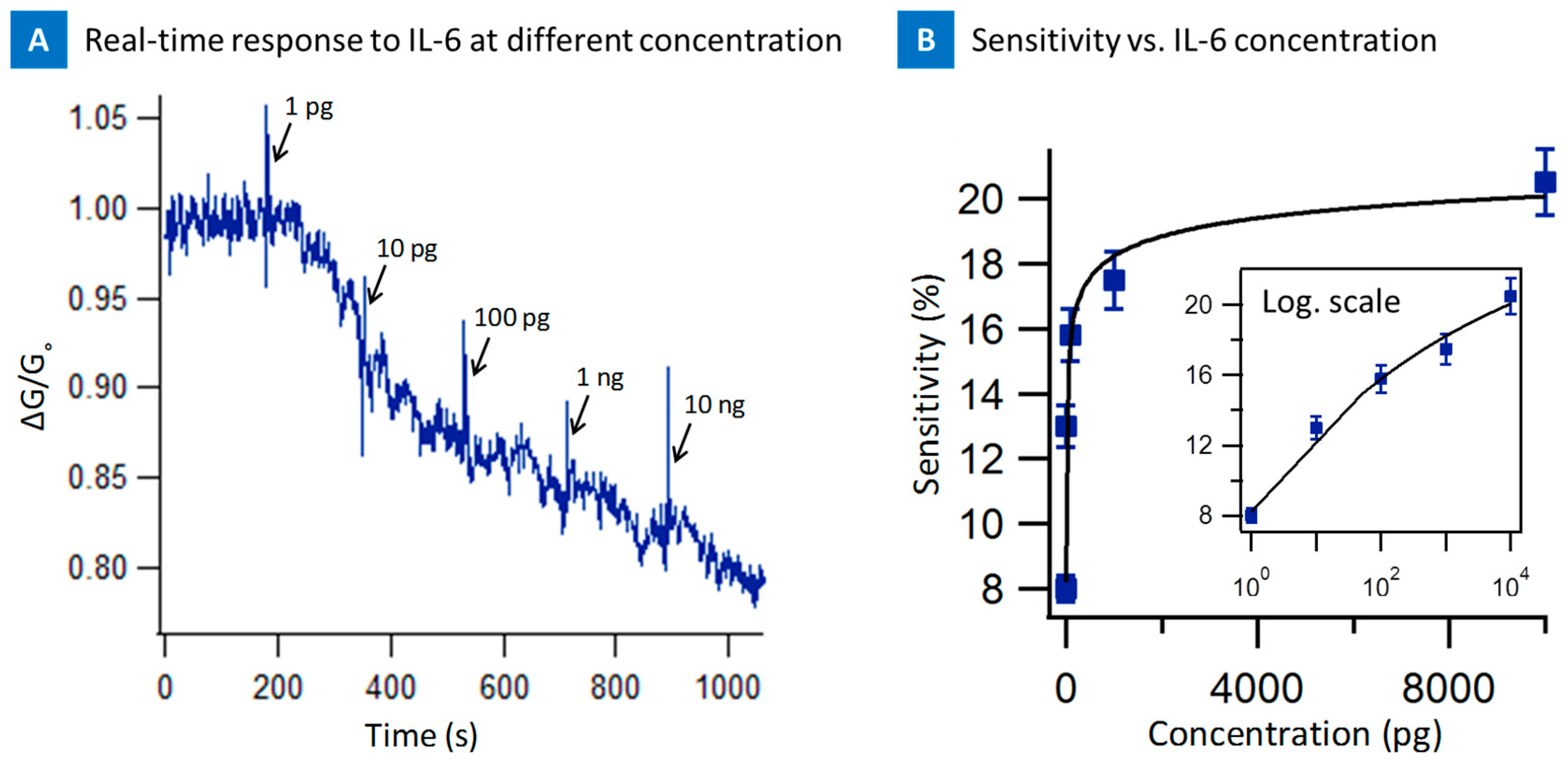
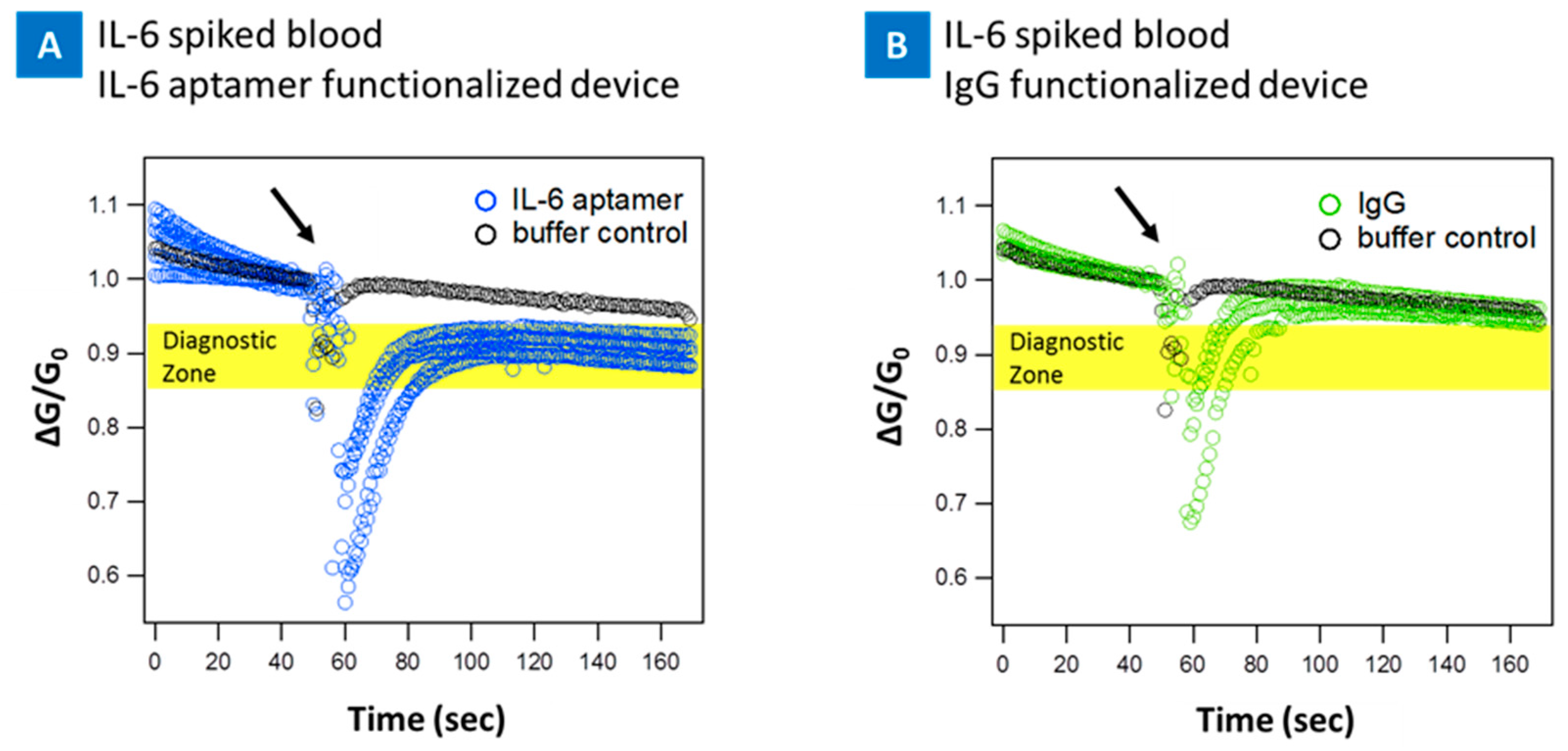
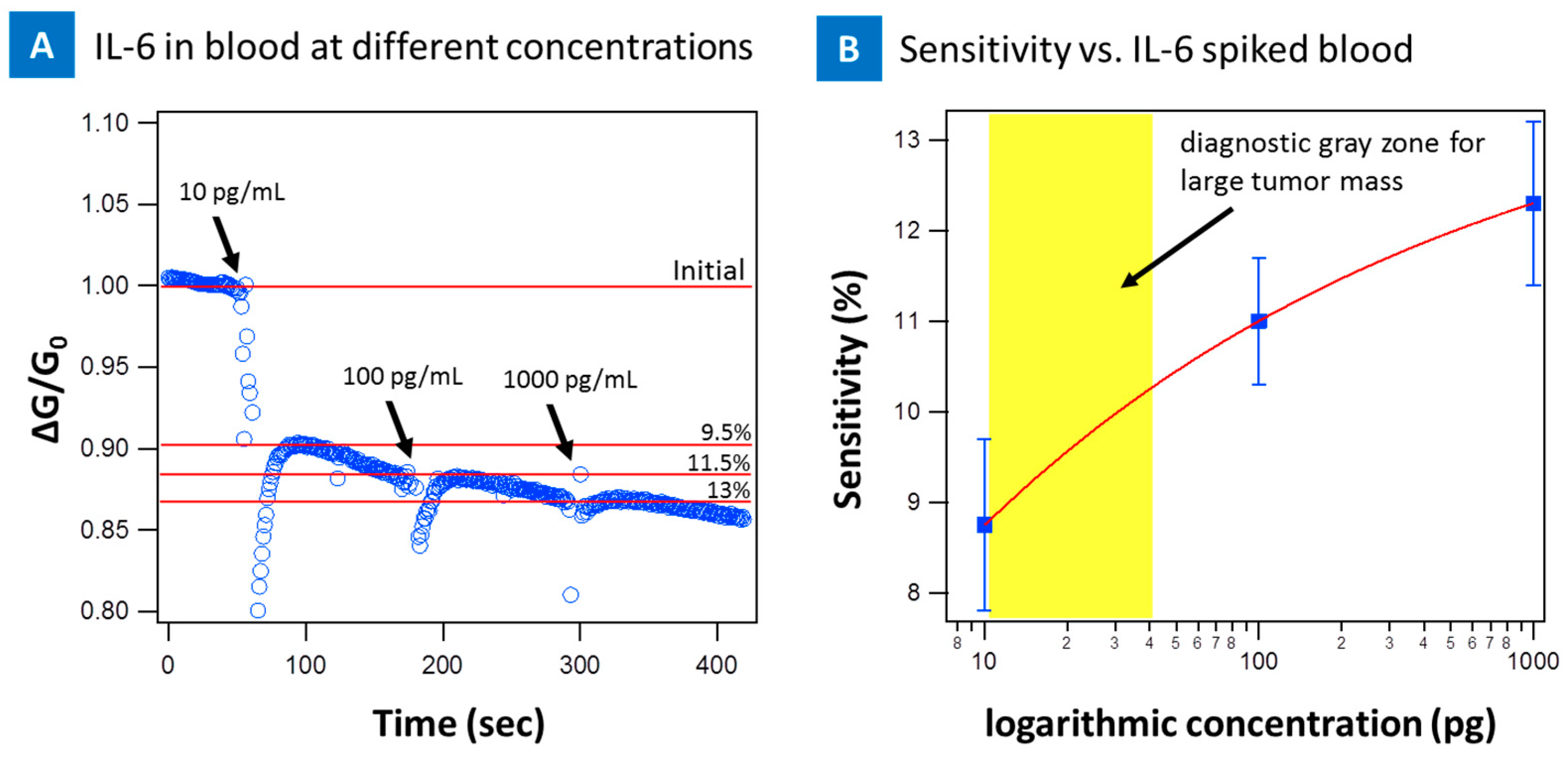
2. Results
3. Discussion and Conclusions
4. Materials and Methods
4.1. Device Fabrication
4.2. Device Functionalization
4.3. Blood Sample Preparation
4.4. Testing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.G.; Wang, D.W.; Dai, H.J. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Star, A.; Gabriel, J.C.P.; Bradley, K.; Gruner, G. Electronic detection of specific protein binding using nanotube fet devices. Nano Lett. 2003, 3, 459–463. [Google Scholar] [CrossRef]
- Khosravi, F.; King, B.; Panchapakesan, B.; Rai, S.; Kloecker, G.; Wickstrom, E. Nanotube devices for digital profiling of cancer biomarkers and circulating tumor cells. IEEE Int. Conf. Nano 2013, 107–112. [Google Scholar]
- Wang, J.; Liu, G.D.; Jan, M.R. Ultrasensitive electrical biosensing of proteins and DNA: Carbon-nanotube derived amplification of the recognition and transduction events. J. Am. Chem. Soc. 2004, 126, 3010–3011. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.T.; Tseng, Y.C.; Ormategui, N.; Loinaz, I.; Eritja, R.; Bokor, J. Label-free DNA biosensors based on functionalized carbon nanotube field effect transistors. Nano Lett. 2009, 9, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Lau, C.M.; Lohani, A.; Mhaisalkar, S.G.; Kasim, J.; Shen, Z.X.; Ho, X.N.; Rogers, J.A.; Li, L.J. Electrical detection of femtomolar DNA via gold-nanoparticle enhancement in carbon-nanotube-network field-effect transistors. Adv. Mater. 2008, 20, 2389. [Google Scholar] [CrossRef]
- Dastagir, T.; Forzani, E.S.; Zhang, R.; Amlani, I.; Nagahara, L.A.; Tsui, R.; Tao, N. Electrical detection of hepatitis c virus rna on single wall carbon nanotube-field effect transistors. Analyst 2007, 132, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Cho, N.S. Diagnosis of human hepatitis b virus in non-treated blood by the bovine igg DNA-linked carbon nanotube biosensor. J. Clin. Virol. 2009, 44, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Wickstrom, E.; Panchapakesan, B. Nanotube-antibody biosensor arrays for the detection of circulating breast cancer cells. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- King, B.C.; Clark, M.; Burkhead, T.; Sethu, P.; Rai, S.; Kloecker, G.; Panchapakesan, B. Electrical detection of specific versus non-specific binding events in breast cancer cells. Biosens. Nanomed. V 2012, 8460. [Google Scholar] [CrossRef]
- Khosravi, F.; Trainor, P.; Rai, S.N.; Kloecker, G.; Wickstrom, E.; Panchapakesan, B. Label-free capture of breast cancer cells spiked in buffy coats using carbon nanotube antibody micro-arrays. Nanotechnology 2016, 27, 13LT02. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Trainor, P.J.; Lambert, C.; Kloecker, G.; Wickstrom, E.; Rai, S.N.; Panchapakesan, B. Static micro-array isolation, dynamic time series classification, capture and enumeration of spiked breast cancer cells in blood: The nanotube–ctc chip. Nanotechnology 2016, 27, 44LT03. [Google Scholar] [CrossRef] [PubMed]
- Minot, E.D.; Janssens, A.M.; Heller, I.; Heering, H.A.; Dekker, C.; Lemay, S.G. Carbon nanotube biosensors: The critical role of the reference electrode. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Heller, I.; Janssens, A.M.; Mannik, J.; Minot, E.D.; Lemay, S.G.; Dekker, C. Identifying the mechanism of biosensing with carbon nanotube transistors. Nano Lett. 2008, 8, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Lee, B.Y.; Hong, S.; Sim, S.J. Ultrasensitive carbon nanotube-based biosensors using antibody-binding fragments. Anal. Biochem. 2008, 381, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, S.; Salimi, A.; Teymourian, H.; Hallaj, R. Label-free electrochemical ige aptasensor based on covalent attachment of aptamer onto multiwalled carbon nanotubes/ionic liquid/chitosan nanocomposite modified electrode. Biosens. Bioelectron. 2013, 43, 218–225. [Google Scholar] [CrossRef] [PubMed]
- So, H.-M.; Won, K.; Kim, Y.H.; Kim, B.-K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.-O. Single-walled carbon nanotube biosensors using aptamers as molecular recognition elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance vegf aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Munge, B.S.; Krause, C.E.; Malhotra, R.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Electrochemical immunosensors for interleukin-6. Comparison of carbon nanotube forest and gold nanoparticle platforms. Electrochem. Commun. 2009, 11, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker il-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, M. Electrochemical sensor utilizing ferrocene loaded porous polyelectrolyte nanoparticles as label for the detection of protein biomarker il-6. Sens. Actuators B Chem. 2011, 158, 361–365. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine-40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The role of il-6 and stat3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: Implications for survival. Cancer Immunol. Immunother. 2006, 55, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mo, H.-Y.; Xiong, G.; Zhang, L.; He, J.; Huang, Z.-F.; Liu, Z.-W.; Chen, Q.-Y.; Du, Z.-M.; Zheng, L.-M. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J. Biol. Chem. 2012, 287, 35484–35495. [Google Scholar] [CrossRef] [PubMed]
- Anestakis, D.; Petanidis, S.; Kalyvas, S.; Nday, C.M.; Tsave, O.; Kioseoglou, E.; Salifoglou, A. Mechanisms and αpplications of ιnterleukins in cancer immunotherapy. Int. J. Mol. Sci. 2015, 16, 1691–1710. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Yao, Q.; Liu, Y.; Du, S.; Liu, A.; Guo, Z.; Sun, A.; Ruan, J.; Chen, L.; Ye, C. Il-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int. J. Oncol. 2012, 40, 1171–1179. [Google Scholar] [PubMed]
- Gasche, J.A.; Hoffmann, J.; Boland, C.R.; Goel, A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int. J. Cancer 2011, 129, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Ni, Z.; Dyer, K.; Tweardy, D.J.; Gao, A.C. Interleukin-6 induces prostate cancer cell growth accompanied by activation of Stat3 signaling pathway. Prostate 2000, 42, 239–242. [Google Scholar] [CrossRef]
- Chung, T.D.; Yu, J.J.; Spiotto, M.T.; Bartkowski, M.; Simons, J.W. Characterization of the role of il-6 in the progression of prostate cancer. Prostate 1999, 38, 199–207. [Google Scholar] [CrossRef]
- Zhang, G.; Adachi, I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999, 19, 1427–1432. [Google Scholar] [PubMed]
- Blay, J.-Y.; Negrier, S.; Combaret, V.; Attali, S.; Goillot, E.; Merrouche, Y.; Mercatello, A.; Ravault, A.; Tourani, J.-M.; Moskovtchenko, J.-F. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992, 52, 3317–3322. [Google Scholar] [PubMed]
- Chung, Y.C.; Chang, Y.F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J. Surg. Oncol. 2003, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.-W.; Liu, L.-J.; Huang, J. Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int. J. Oncol. 2014, 45, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Kanakura, Y.; Aozasa, K.; Johkoh, T.; Nakamura, M.; Nakano, S.; Nakano, N.; Ikeda, Y.; Sasaki, T.; Nishioka, K. Humanized anti–interleukin-6 receptor antibody treatment of multicentric castleman disease. Blood 2005, 106, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Keystone, E.; Tony, H.; Cantagrel, A.; Van Vollenhoven, R.; Sanchez, A.; Alecock, E.; Lee, J.; Kremer, J. Il-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008, 67, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Maini, R.N. Interleukin-6: A new therapeutic target. Arthritis Res. Ther. 2006, 8, S5. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E. Interleukin-6 and new strategies for the treatment of cancer, hyperproliferative diseases and paraneoplastic syndromes. Expert Opin. Ther. Targets 2005, 9, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Nemeth, J.A.; Trikha, M. Cnto 328, a monoclonal antibody to il-6, inhibits human tumor-induced cachexia in nude mice. Int. J. Cancer 2004, 111, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Chia, J.S.; Chang, Y.F.; Chiang, C.P. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and chinese medicinal herbs on patients with oral lichen planus. J. Oral Pathol. Med. 2002, 31, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Madhok, R.; Crilly, A.; Watson, J.; Capell, H.A. Serum interleukin 6 levels in rheumatoid arthritis: Correlations with clinical and laboratory indices of disease activity. Ann. Rheum. Dis. 1993, 52, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, N.; Terao, K.; Mima, T.; Nakahara, H.; Takagi, N.; Kakehi, T. Mechanisms and pathologic significances in increase in serum interleukin-6 (il-6) and soluble il-6 receptor after administration of an anti–il-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and castleman disease. Blood 2008, 112, 3959–3964. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.; Keating, M.J.; Reuben, J.M.; O'Brien, S.; Lee, B.-N.; Lerner, S.; Kurzrock, R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome. Blood 2001, 97, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Debye, P. Dielectric properties of pure liquids. Chem. Rev. 1936, 19, 171–182. [Google Scholar] [CrossRef]
- King, B.; Panchapakesan, B. Vacuum filtration based formation of liquid crystal films of semiconducting carbon nanotubes and high performance transistor devices. Nanotechnology 2014, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; King, B.C.; Loomis, J.; Campo, E.M.; Hegseth, J.; Cohn, R.W.; Terentjev, E.; Panchapakesan, B. Nanotube liquid crystal elastomers: Photomechanical response and flexible energy conversion of layered polymer composites. Nanotechnology 2014, 25, 355501. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Arichi, N.; Tokugawa, S.; Yoshioka, I.; Namba, Y.; Kishikawa, H.; Takahara, S.; Ichikawa, Y. Hepatocyte growth factor and interleukin-6 in combination with prostate volume are possible prostate cancer tumor markers in patients with gray-zone psa levels. Prostate Cancer Prostatic Dis. 2008, 11, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chang, Y.F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J. Surg. Oncol. 2003, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Brichory, F.M.; Misek, D.E.; Yim, A.M.; Krause, M.C.; Giordano, T.J.; Beer, D.G.; Hanash, S.M. An immune response manifested by the common occurrence of annexins i and ii autoantibodies and high circulating levels of il-6 in lung cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 9824–9829. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravi, F.; Loeian, S.M.; Panchapakesan, B. Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements. Biosensors 2017, 7, 17. https://doi.org/10.3390/bios7020017
Khosravi F, Loeian SM, Panchapakesan B. Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements. Biosensors. 2017; 7(2):17. https://doi.org/10.3390/bios7020017
Chicago/Turabian StyleKhosravi, Farhad, Seyed Masoud Loeian, and Balaji Panchapakesan. 2017. "Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements" Biosensors 7, no. 2: 17. https://doi.org/10.3390/bios7020017





