Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes
Abstract
:1. Introduction
2. SPR Methods
2.1. SPR Imaging in Multiplex: Multichannel SPR Biosensors
Enhancement of Sensitivity by Combining SPR with a Labeling or Capturing Method
2.2. EIS
2.2.1. Fabrication of Impedance Sensor
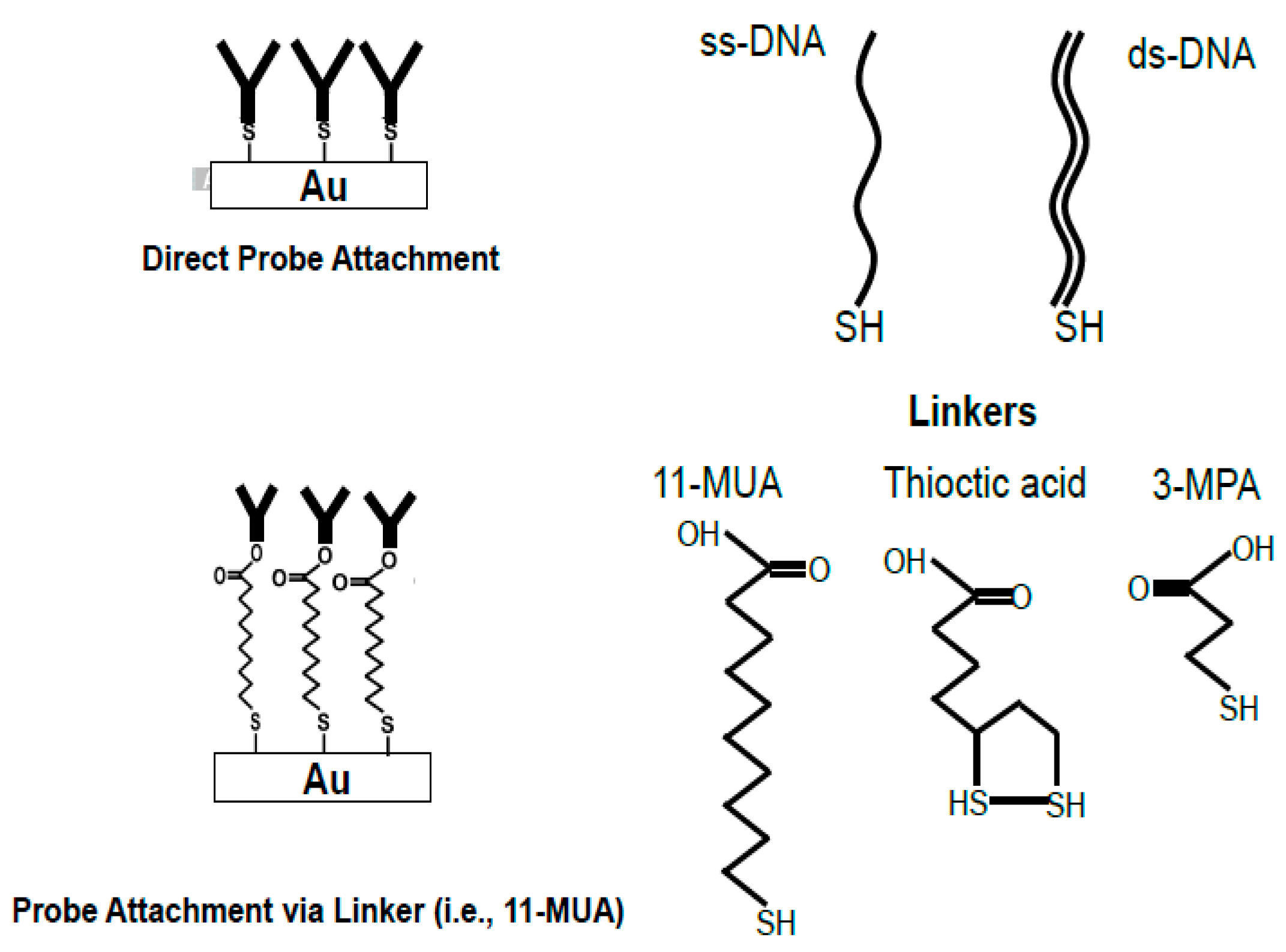
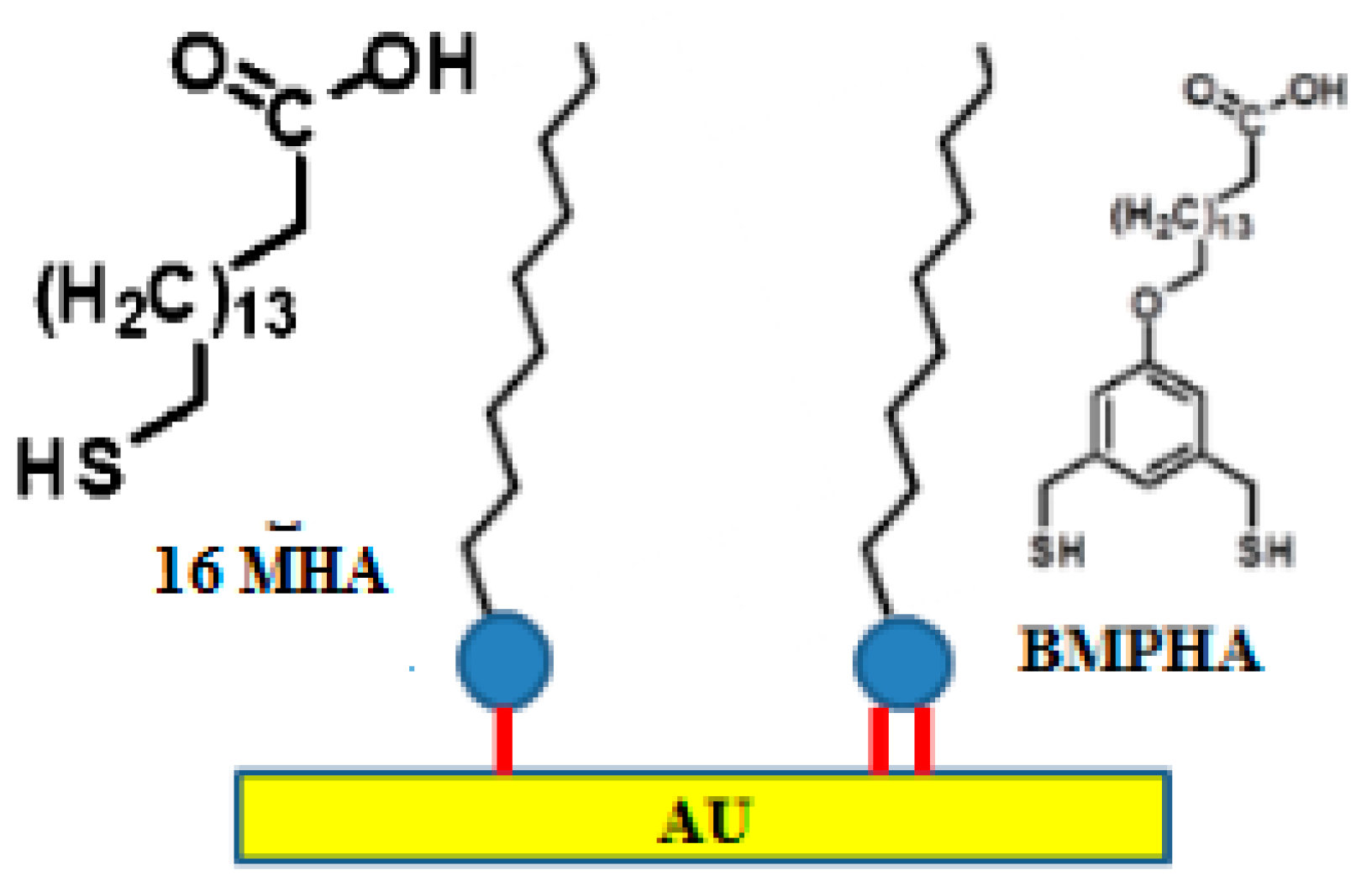
2.2.2. Linkage of Bio-Molecules onto Au Surface
2.2.3. Attaching Bio-Molecules
2.2.4. Technical Challenges for Impedance Biosensors
Susceptibility to Non-Specific Adsorption
Stability of Biomolecule Immobilization onto a Conductive Electrode Material
Complexity of Impedance Detection
2.3. Further Advancements and Present Perspectives
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Poltronieri, P.; De Blasi, M.D.; D’Urso, O.F. Detection of Listeria monocytogenes through Real Time PCR and biosensor methods. Plant Soil Environ. 2009, 9, 363–369. [Google Scholar]
- Poltronieri, P.; Cimaglia, F.; De Lorenzis, E.; Chiesa, M.; Mezzolla, V.; Reca, I.B. Protein chips for detection of Salmonella spp. from enrichment culture. Sensors 2016, 16, 574. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, F.; De Lorenzis, E.; Mezzolla, V.; Rossi, F.; Poltronieri, P. Detection of L. monocytogenes in enrichment cultures by immunoseparation and immunosensors. IEEE Sens. 2016, 16, 7045–7052. [Google Scholar] [CrossRef]
- Rodriguez-Lazaro, D.; Gonzalez-García, P.; Gattuso, A.; Gianfranceschi, M.V.; Hernandez, M. Reducing time in the analysis of Listeria monocytogenes in meat, dairy and vegetable products. Int. J. Food Microbiol. 2014, 184, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lauer, W.F.; Sidi, C.D.; Tourniaire, J.P. iQ-Check Salmonella II: Real-time polymerase chain reaction test kit. Performance tested method 010803. J. AOAC Int. 2009, 92, 1865–1870. [Google Scholar] [PubMed]
- Poltronieri, P.; Mezzolla, V.; Primiceri, E.; Maruccio, G. Biosensors for detection of food pathogens. Foods 2014, 3, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, P. Innovations in detection of deliberate or accidental contamination with biological agents in environment and foods. Challenges 2016, 7, 22. [Google Scholar] [CrossRef]
- D’Urso, O.F.; De Blasi, M.D.; Manera, M.G.; Latronico, M.F.; Rella, R.; Poltronieri, P. Listeria monocytogenes detection with surface plasmon resonance and protein arrays. IEEE Sens. 2008, 8, 458–461. [Google Scholar] [CrossRef]
- Menti, C.; Henriques, J.A.P.; Missell, F.P.; Roesch-Ely, M. Antibody-based magneto-elastic biosensors: Potential devices for detection of pathogens and associated toxins. Appl. Microbiol. Biotechnol. 2016, 100, 6149. [Google Scholar] [CrossRef] [PubMed]
- Rippa, M.; Castagna, R.; Pannico, M.; Musto, P.; Borriello, G.; Paradiso, R.; Galiero, G.; Bolletti Censi, S.; Zhou, J.; Zyss, J.; et al. Octupolar metastructures for a highly sensitive, rapid, and reproducible phage-based detection of bacterial pathogens by Surface-Enhanced Raman Scattering. ACS Sens. 2017, 2, 947. [Google Scholar] [CrossRef] [PubMed]
- Juan-Colás, J.; Johnson, S.; Krauss, T.F. Dual-mode Electro-Optical techniques for biosensing applications: A Review. Sensors 2017, 17, 2047. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R. Antibody-based sensors: Principles, problems and potential for detection of pathogens and associated toxins. Sensors 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Wang, A.; Slavik, M. Rapid, sensitive, and simultaneous detection of three foodborne pathogens using magnetic nanobead-based immunoseparation and quantum dot-based multiplex immunoassay. J. Food Prot. 2011, 74, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, Z.; Mao, Y.; Ji, Y.; Xu, H.; Xiong, Y.; Li, Y. Gold nanoparticle-based dynamic light scattering immunoassay for ultrasensitive detection of Listeria monocytogenes in lettuces. Biosens. Bioelectron. 2015, 66, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Gunda, N.S.; Jamal, I.; Mitra, K. Optical biosensors with an integrated Mach-Zender interferometer for detection of Listeria monocytogenes. Biomed. Microdevices 2014, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ohk, S.H.; Bhunia, A.K. Multiplex fiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol. 2013, 33, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Piro, B.; Reisberg, S. Recent advances in electrochemical immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Volpe, G.; Piermarini, S.; Delibato, E.; Palleschi, G. Electrochemical biosensors for rapid detection of foodborne Salmonella: A critical overview. Sensors 2017, 17, 1910. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáez-Sedeño, P.; Pingarrón, J.M. Electrochemical affinity biosensors in food safety. Chemosensors 2017, 5, 8. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Cimaglia, F.; Rosu, V.; Chiesa, M.; Poltronieri, P.; Aliverti, A.; Santino, A.; Sechi, L.A. Quantum dot nanoparticle-based lateral flow assay for rapid detection of Mycobacterium species using anti-FprA antibodies. Nanotechnol. Dev. 2012, 2, e5. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, Y.; Li, H.; Luo, L.; Xu, J.; Ye, C. Development of multiple cross displacement amplification label-based gold nanoparticles lateral flow biosensor for detection of Listeria monocytogenes. Int. J. Nanomed. 2017, 12, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.-H.; Ku, S. Current technical approaches for the early detection of foodborne pathogens: Challenges and opportunities. Int. J. Mol. Sci. 2017, 18, 2078. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzis, E.; Manera, M.G.; Cimaglia, F.; Montagna, G.; Chiesa, M.; Poltronieri, P.; Santino, A.; Rella, R. SPR based immunosensor for detection of Legionella pneumophila in water samples. Opt. Commun. 2013, 294, 420–426. [Google Scholar] [CrossRef]
- Nanduri, V.; Bhunia, A.K.; Tu, S.-I.; Paoli, G.C.; Brewster, J.D. SPR biosensor for the detection of L. monocytogenes using phage-displayed antibody. Biosens. Bioelectron. 2007, 23, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Miyachi, M.; Fujii, E.; Citterio, D.; Yamada, K.; Sato, Y.; Kurihara, K.; Kawaguchi, H.; Suzuki, K. SPR sensor signal amplification based on dye-doped polymer particles. Sci. Technol. Adv. Mater. 2006, 7, 150–155. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Zheng, S.; Liu, Y.; He, Z.; Luo, F. Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sens. Actuators B Chem. 2016, 230, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Monitoring of Escherichia coli O157:H7 in food samples using lectin based surface plasmon resonance biosensor. Food Chem. 2013, 136, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhan, S.; Huang, Z.; Hong, X. Review: Advances and applications of Surface Plasmon Resonance biosensing instrumentation. Instrum. Sci. Technol. 2013, 41, 574–607. [Google Scholar] [CrossRef]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical functionalization of plasmonic surface biosensors: A tutorial review on issues, strategies, and costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, J.; Jiang, M.; Chang, K.; Chen, R.; Ma, L.; Zhu, J.; Guo, Q.; Sun, H.; Hu, J. The development of a portable SPR bioanalyzer for sensitive detection of Escherichia coli O157:H7. Sensors 2016, 16, 1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tsuji, S.; Kitaoka, H.; Kobayashi, H.; Tamai, M.; Honjoh, K.-I.; Miyamoto, T. Simultaneous detection of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes at a very low level using Simultaneous Enrichment Broth and multichannel SPR biosensor. J. Food Sci. 2017, 82, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Marusov, G.; Sweatt, A.; Pietrosimone, K.; Benson, D.; Geary, S.J.; Silbart, L.K.; Challa, S.; Lagoy, J.; Lawrence, D.A.; Lynes, M.A. A microarray biosensor for multiplexed detection of microbes using grating-coupled surface plasmon resonance imaging. Environ. Sci. Technol. 2012, 46, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Chadtová Song, X.; Mrázek, J.; Lamačová, J.; Lynn, N.S.; Šedivák, P., Jr.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, C.S.; Chen, S.H.; Ye, R.; Wu, V.C. A piezoelectric immunosensor for specific capture and enrichment of viable pathogens by quartz crystal microbalance sensor, followed by detection with antibody-functionalized gold nanoparticles. Biosens. Bioelectron. 2012, 38, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, L.; Huang, Y.; Chen, L.; Zhang, G.; Shen, Z.; Zhang, J.; Xiao, Z.; Chen, T. Amplifying the signal of localized surface plasmon resonance sensing for the sensitive detection of Escherichia coli O157:H7. Sci. Rep. 2017, 7, 3288. [Google Scholar] [CrossRef] [PubMed]
- Rippa, M.; Castagna, R.; Tkachenko, V.; Zhou, J.; Petti, L. Engineered nanopatterned substrates for high-sensitive localized surface plasmon resonance: An assay on biomacromolecules. J. Mater. Chem. B 2017, 5, 5473–5478. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Electrochemical sensors. Anal. Chem. 2004, 76, 3285–3298. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Radhakrishnan, R.; Jahne, M.; Rogers, S.; Suni, I.I. Detection of Listeria monocytogenes by Electrochemical Impedance Spectroscopy. Electroanalysis 2013, 25, 2231–2237. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pali, M.; Lee, H.J.; Lee, T.R.; Suni, I.I. Impedance biosensor incorporating a Carboxylate-Terminated Bidentate Thiol for antibody immobilization. J. Electrochem. Soc. 2016, 163, B125–B130. [Google Scholar] [CrossRef]
- Bever, C.R.S.; Majkova, Z.; Radhakrishnan, R.; Suni, I.I.; McCoy, M.; Wang, Y.; Dechant, J.; Gee, S.; Hammock, B.D. Development and utilization of camelid VHH antibodies from Alpaca for 2,2′4,4′-tetrabrominated diphenyl ether detection. Anal. Chem. 2014, 86, 7875–7882. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA sensors and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Blankespoor, R.; Limoges, B.; Schöllhorn, B.; Syssa-Magalé, J.L.; Yazidi, D. Dense monolayers of metal-chelating ligands covalently attached to carbon electrodes electrochemically and their useful application in affinity binding of histidine-tagged proteins. Langmuir 2005, 21, 3362–3375. [Google Scholar] [CrossRef] [PubMed]
- Teh, H.F.; Gong, H.; Dong, X.-D.; Zeng, X.; Kuan Tan, A.L.; Yang, X.; Tan, S.N. Electrochemical biosensing of DNA with capture probe covalently immobilized onto glassy carbon surface. Anal. Chim. Acta 2005, 551, 23–29. [Google Scholar] [CrossRef]
- Ramesh, P.; Sampath, S. Electrochemical characterization of binderless, recompressed exfoliated graphite electrodes: Electron transfer kinetics and diffusion characteristics. Anal. Chem. 2003, 75, 6949–6957. [Google Scholar] [CrossRef] [PubMed]
- Maupas, H.; Soldatkin, A.P.; Martelet, C.; Jaffrezic-Renault, N.; Mandrand, B. Direct immunosensing using differential electrochemical measurements of impedimetric variations. J. Electroanal. Chem. 1997, 421, 165–171. [Google Scholar] [CrossRef]
- Rickert, J.; Göpel, W.; Beck, W.; Jung, G.; Heiduschka, P. A mixed self assembled monolayer for an impedimetric immunosensors. Biosens. Bioelectron. 1996, 11, 757–768. [Google Scholar] [CrossRef]
- Steel, A.B.; Levicky, R.L.; Herne, T.M.; Tarlov, M.J. Immobilization of nucleic acids at solid surfaces: Effect of oligonucleotide length on layer assembly. Biophys. J. 2000, 79, 975–981. [Google Scholar] [CrossRef]
- Patel, N.; Davies, M.C.; Hartshorne, M.; Heaton, R.J.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Immobilization of protein molecules onto homogeneous and mixed carboxylate-terminated self assembled monolayers. Langmuir 1997, 13, 6485–6490. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Bange, A.; Halsall, H.B.; Heineman, W.R. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, D.R.; Gobi, V.K.; Miura, N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens. Actuators B 2007, 121, 158–177. [Google Scholar] [CrossRef]
- Gaus, K.; Hall, E.A. Surface Plasmon resonance sensor for heparin measurements in blood plasma. Biosens. Bioelectron. 1998, 13, 1307–1315. [Google Scholar] [CrossRef]
- Andersson, L.I.; Hardenborg, E.; Sandberg-Stall, M.; Moller, K.; Henriksson, J.; Bramsby-Sjostrom, I.; Olsson, L.I.; Abdel-Rahim, M. Development of a molecularly imprinted polymer based solid-phase extraction of local anaesthetics from human plasma. Anal. Chim. Acta 2004, 526, 147–154. [Google Scholar] [CrossRef]
- Singh, R.; Suni, I.I. Minimizing non-specific adsorption in protein biosensors that utilize electrochemical impedance spectroscopy. J. Electrochem. Soc. 2010, 157, J334–J337. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Srimsobat, L.; Jamison, A.C.; Lee, T.R. Stability: A key issue for self-assembled monolayers on gold as thin film coatings and nanoparticle protectants. Colloid Surf. A 2011, 390, 1–19. [Google Scholar] [CrossRef]
- Srisombat, L.; Zhang, S.; Lee, T.R. Thermal stability of Mono-, Bis-, and Tris-chelating alkanethiol films assembled on gold nanoparticles and evaporated flat gold. Langmuir 2010, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Wang, X.; Williams, K.; Levicky, R. Thermostable DNA immobilization and temperature effects on surface hybridization. Langmuir 2012, 28, 8446–8455. [Google Scholar] [CrossRef] [PubMed]
- Chinwangso, P.; Jamison, A.C.; Randall Lee, T. Multidendate adsorbates for self assembled monolayer films. Acc. Chem. Res. 2011, 44, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jamison, A.C.; Yuan, Y.; Li, C.-H.; Rittikulsittichai, S.; Rusakova, I.; Randall Lee, T. Robust carboxylic acid terminated organic thin films and nanoparticle protectants generated from bidendate alkanethiols. Langmuir 2013, 29, 10432–10439. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M.A.; Oliveira-Brett, A.M.; Serrano, S.H.P. An EIS study of DNA-modified electrodes. Electrochim. Acta 1999, 44, 4233–4239. [Google Scholar] [CrossRef]
- Davis, F.; Nabok, A.V.; Higson, S.P. Species differentiation by DNA-modified carbon electrodes using AC impedimetric approach. Biosens. Bioelectron. 2005, 20, 1531–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Peck, J.R.; van der Weide, D.W.; Hamers, R.J. Direct electrical detection of hybridization at DNA-modified silicon surface. Biosens. Bioelectron. 2004, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Butler, J.E.; Russell, J.N.; Hamers, R.J. Direct electrical detection of antibody-antigen binding on diamond and silicon substrates using electrical impedance spectroscopy. Analyst 2007, 132, 296–306. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.S.; Zhang, Y.; Hesketh, P.J.; Maclay, G.J.; Gendel, S.M.; Stetter, J.R. Impedance based sensing of the of the specific binding reaction between Staphylococcus enterotoxin B and its antibody on an ultrathin Pt film. Biosens. Bioelectron. 1995, 10, 675–682. [Google Scholar] [CrossRef]
- Pak, S.C.; Penrose, W.; Hesketh, P.J. An ultrathin platinum film sensor to measure biomolecular binding. Biosens. Bioelectron. 2001, 16, 371–379. [Google Scholar] [CrossRef]
- Mantzila, A.G.; Prodromidis, M.I. Performance of impedimetric biosensors based on anodically formed Ti/TiO2 electrodes. Electroanalysis 2005, 20, 1878–1885. [Google Scholar] [CrossRef]
- Mantzila, A.G.; Prodromidis, M.I. Development and study of anodic Ti/TiO2 electrodes and their potential use as impedimetric immunosensors. Electrochim. Acta 2006, 51, 3537–3542. [Google Scholar] [CrossRef]
- Ruan, C.M.; Yang, L.; Li, Y.B. Immunobiosensor chips for detection of Escherichia coli O157:H57 using electrochemical impedance spectroscopy. Anal. Chem. 2002, 74, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Corry, B.; Janelle, U.; Crawley, C. Probing direct binding affinity in electrochemical antibody-based sensors. Anal. Chim. Acta 2003, 496, 103–116. [Google Scholar] [CrossRef]
- Huang, Y.; Suni, I.I. Degenerate Si as an electrode material for electrochemical biosensors. J. Electrochem. Soc. 2008, 155, J350–J354. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Suni, I.I. Antibody regeneration on degenerate Si electrodes for calibration and reuse of impedance biosensors. Sens. Bio-Sens. 2016, 7, 20–24. [Google Scholar] [CrossRef]
- Morgan, H.; Green, N.G. (Eds.) AC Electrokinetics: Colloids and Nanoparticles; Research Studies Press: Baldock, UK, 2003. [Google Scholar]
- Wang, D.; Sigurdson, M.; Meinhart, C.D. Experimental analysis of particle and fluid motion in AC electrokinetics. Exp. Fluids 2005, 38, 1–10. [Google Scholar] [CrossRef]
- Ahualli, S.; Jimenez, M.L.; Carrique, F.; Delgado, A.V. AC electrokinetics of concentrated suspensions of soft particles. Langmuir 2009, 25, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Biased AC electro-osmosis for on-chip bioparticle processing. IEEE Trans. Nanotechnol. 2006, 5, 84–89. [Google Scholar] [CrossRef]
- Wu, J. Interactions of electrical fields with fluids: Laboratory-on-a-chip applications. IET Nanobiotechnol. 2008, 2, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, A.; Ramos, A.; Gonzalez, A.; Green, N.G.; Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: Scaling laws. J. Phys. D Appl. Phys. 2003, 36, 2584. [Google Scholar] [CrossRef]
- Lian, M.; Islam, N.; Wu, J. AC electrothermal manipulation of conductive fluids and particles for lab-chip applications. IET Nanobiotechnol. 2007, 1, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, K.; Wadhwa, A.; Eda, S.; Li, S.; Wu, J. Development of an AC electrokinetics-based immunoassay system for on-site serodiagnosis of infectious diseases. Sens. Actuators A 2011, 171, 406–413. [Google Scholar] [CrossRef]
- Jarocka, U.; Wąsowicz, M.; Radecka, H.; Malinowski, T.; Michalczuk, L.; Radecki, J. Impedimetric immunosensor for detection of plum pox virus in plant extracts. Electroanalysis 2011, 23, 2197. [Google Scholar] [CrossRef]
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. Immunosensor based on antibody binding fragments attached to gold nanoparticles for detection of avian influenza virus H5N1. Sensors 2014, 14, 15714–15728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, S.; Polonschii, C.; Gheorghiu, M.; Bratu, D.; Dobre, A.; Gheorghiu, E. Assessment of pathogenic bacteria using periodic actuation. Lab Chip 2013, 13, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Primiceri, E.; Chiriacò, M.S.; De Feo, F.; Santovito, E.; Fusco, V.; Maruccio, G. A multipurpose biochip for food pathogen detection. Anal. Methods 2016, 88, 3055–3060. [Google Scholar] [CrossRef]
- Bouguelia, S.; Roupioz, Y.; Slimani, S.; Mondani, L.; Casabona, M.G.; Durmort, C.; Vernet, T.; Calemczuk, R.; Livache, T. On-chip microbial culture for the specific detection of very low levels of bacteria. Lab Chip 2013, 13, 4024–4032. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Polonschii, C.; Gheorghiu, M.; Bratu, D.; Gheorghiu, E. Biosensing based on Magneto-Optical Surface Plasmon Resonance. Methods Mol. Biol. 2017, 1571, 73–88. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, R.; Poltronieri, P. Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes. Biosensors 2017, 7, 63. https://doi.org/10.3390/bios7040063
Radhakrishnan R, Poltronieri P. Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes. Biosensors. 2017; 7(4):63. https://doi.org/10.3390/bios7040063
Chicago/Turabian StyleRadhakrishnan, Rajeswaran, and Palmiro Poltronieri. 2017. "Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes" Biosensors 7, no. 4: 63. https://doi.org/10.3390/bios7040063





