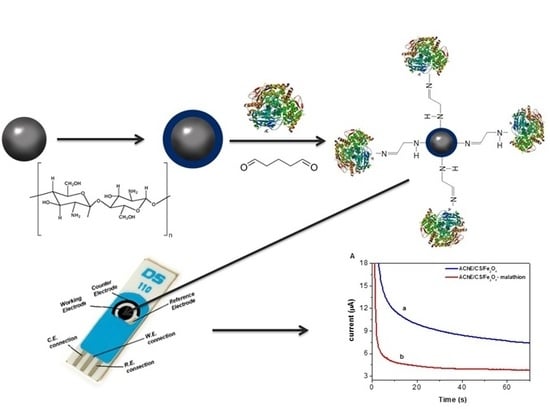
Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
2.2. Synthesis of Nanoparticles
2.3. Functionalization of the Nanoparticles with Chitosan (CS/Fe3O4)
2.4. AChE Functionalization of the Chitosan-Modified Fe3O4 Particles (AChE/CS/Fe3O4)
2.5. Optimization of Experimental Conditions
2.6. Detection of Malathion in Tomato Sauce and Pond Water
3. Results and Discussion
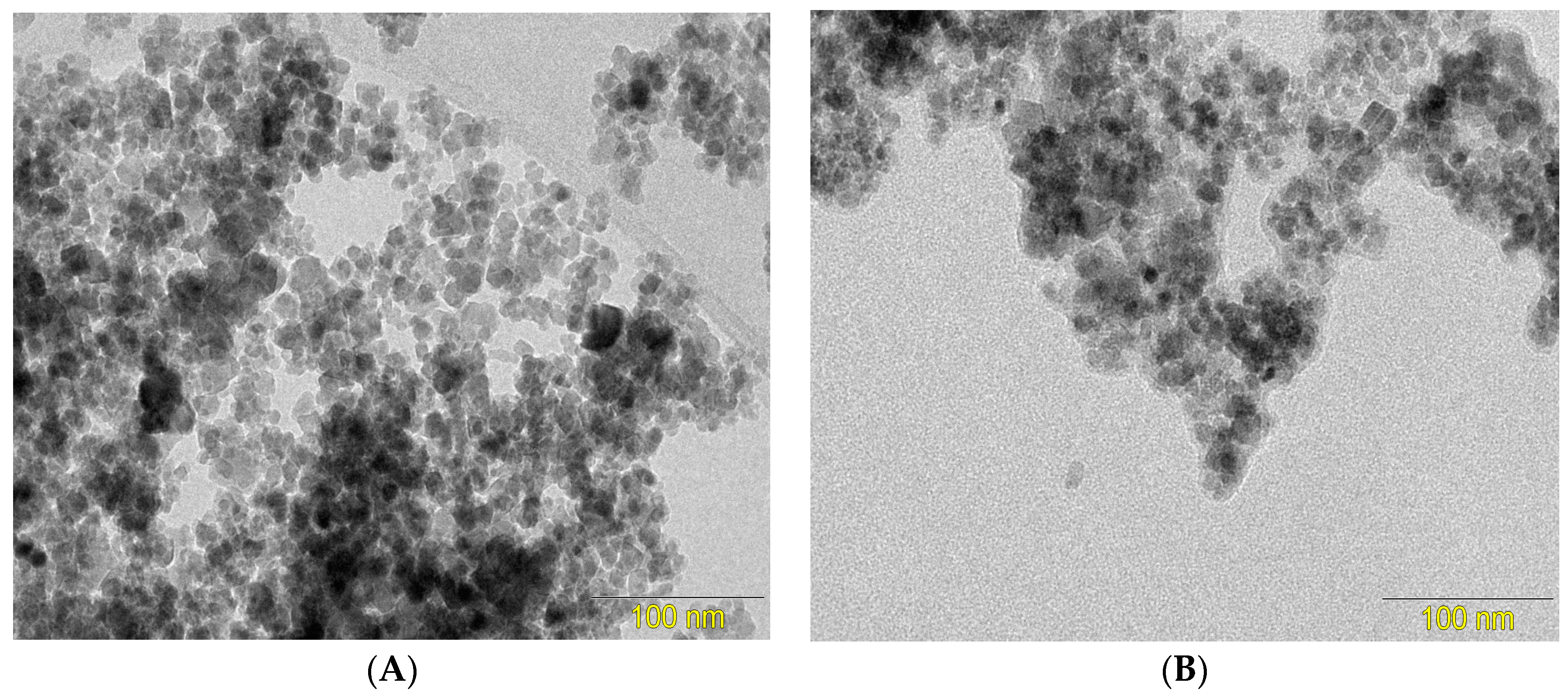
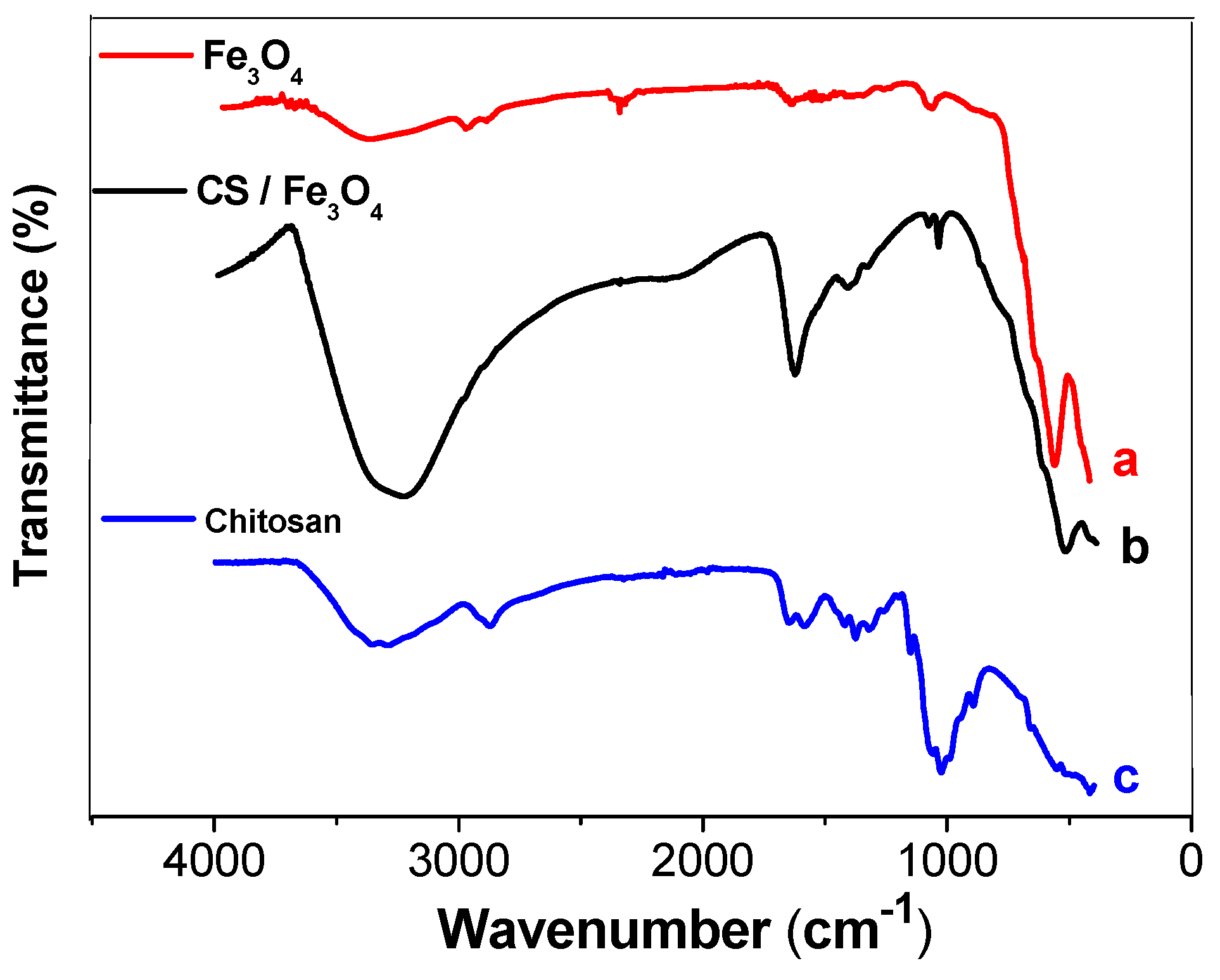
3.1. Characterization of the Fe3O4 and CS/Fe3O4 Magnetic Nanoparticles
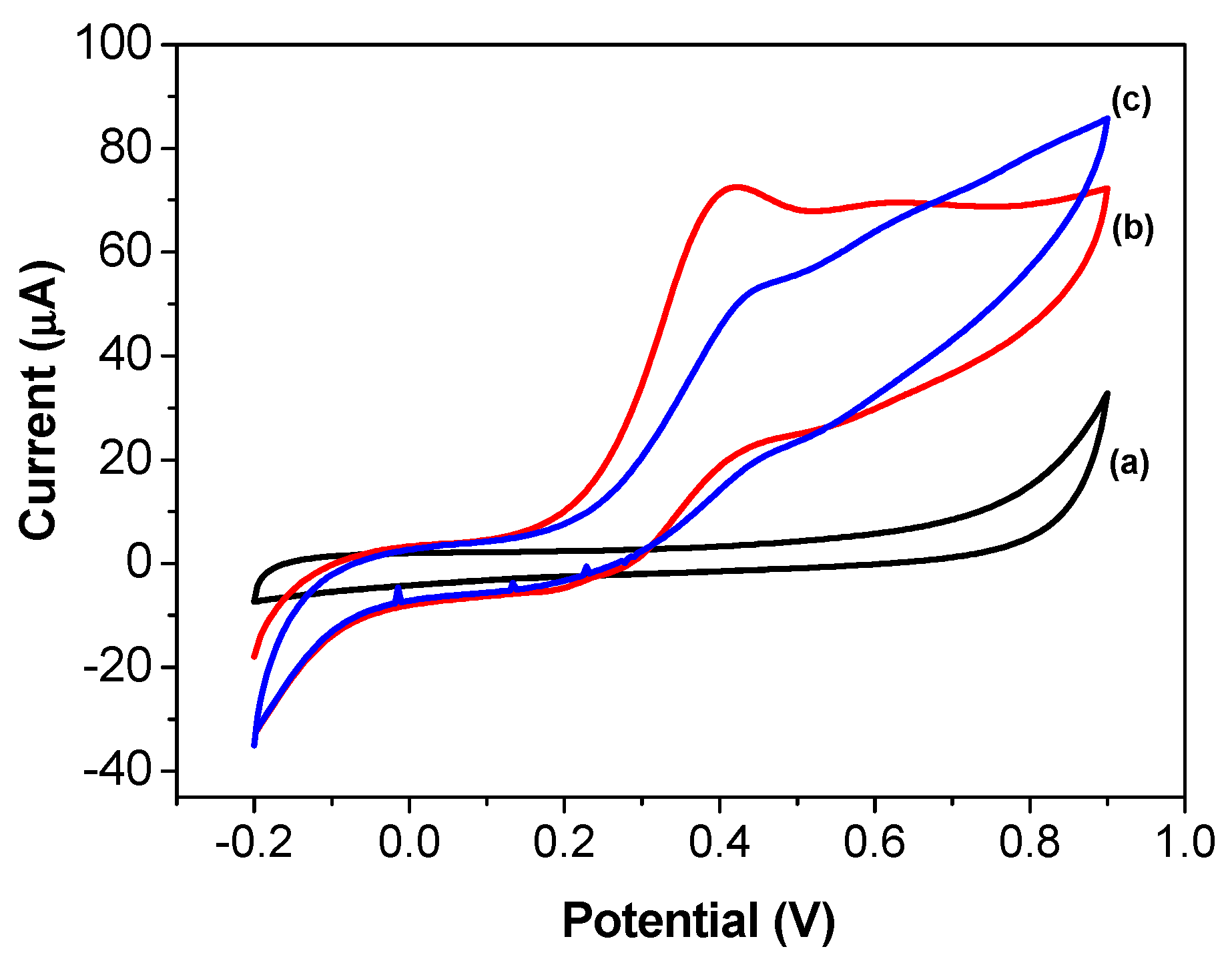
3.2. Cyclic Voltammetry Evaluation of Thiocholine Oxidation on the Screen-Printed Carbon Electrode Modified with AChE/CS/Fe3O4
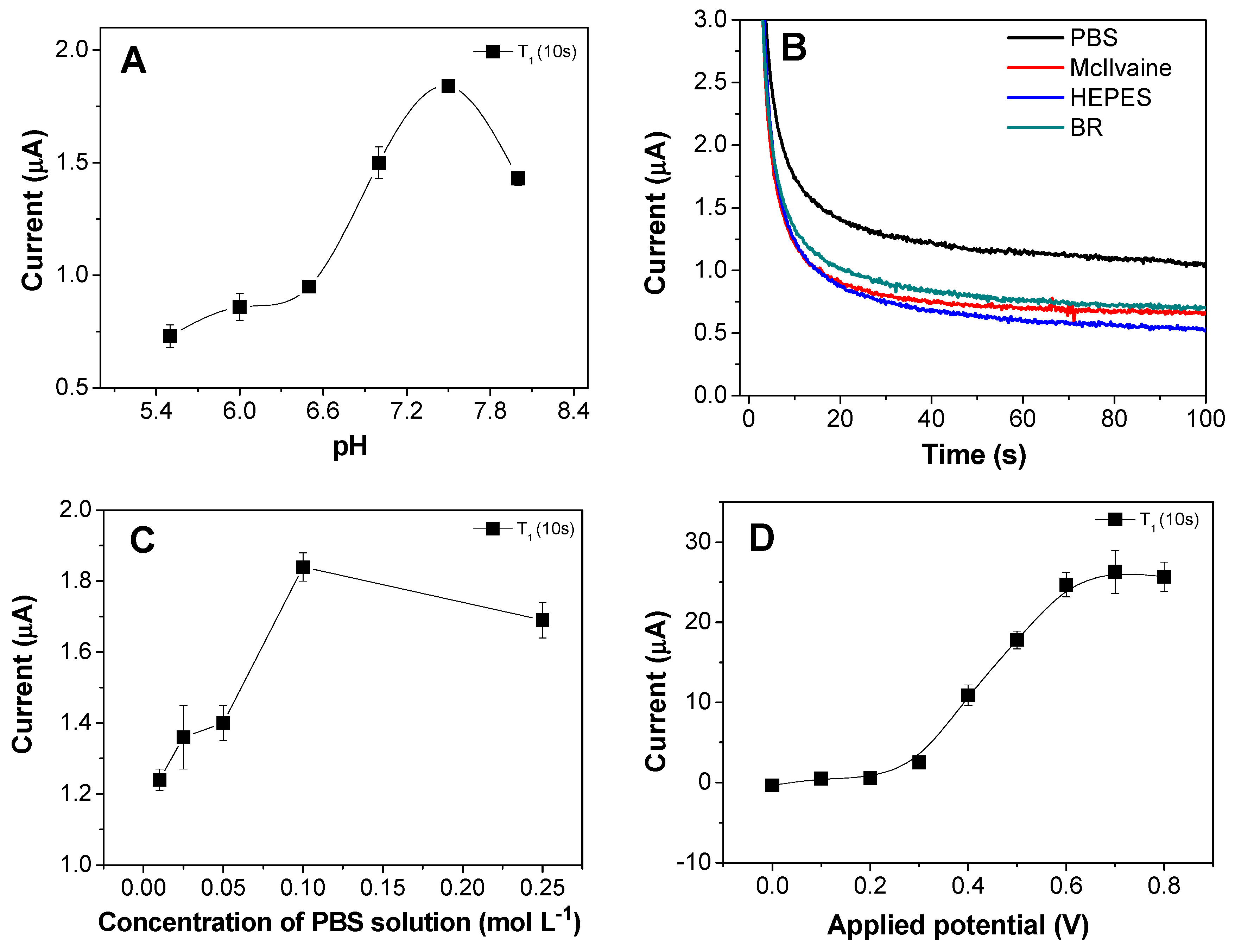
3.3. Optimization of Experimental Conditions
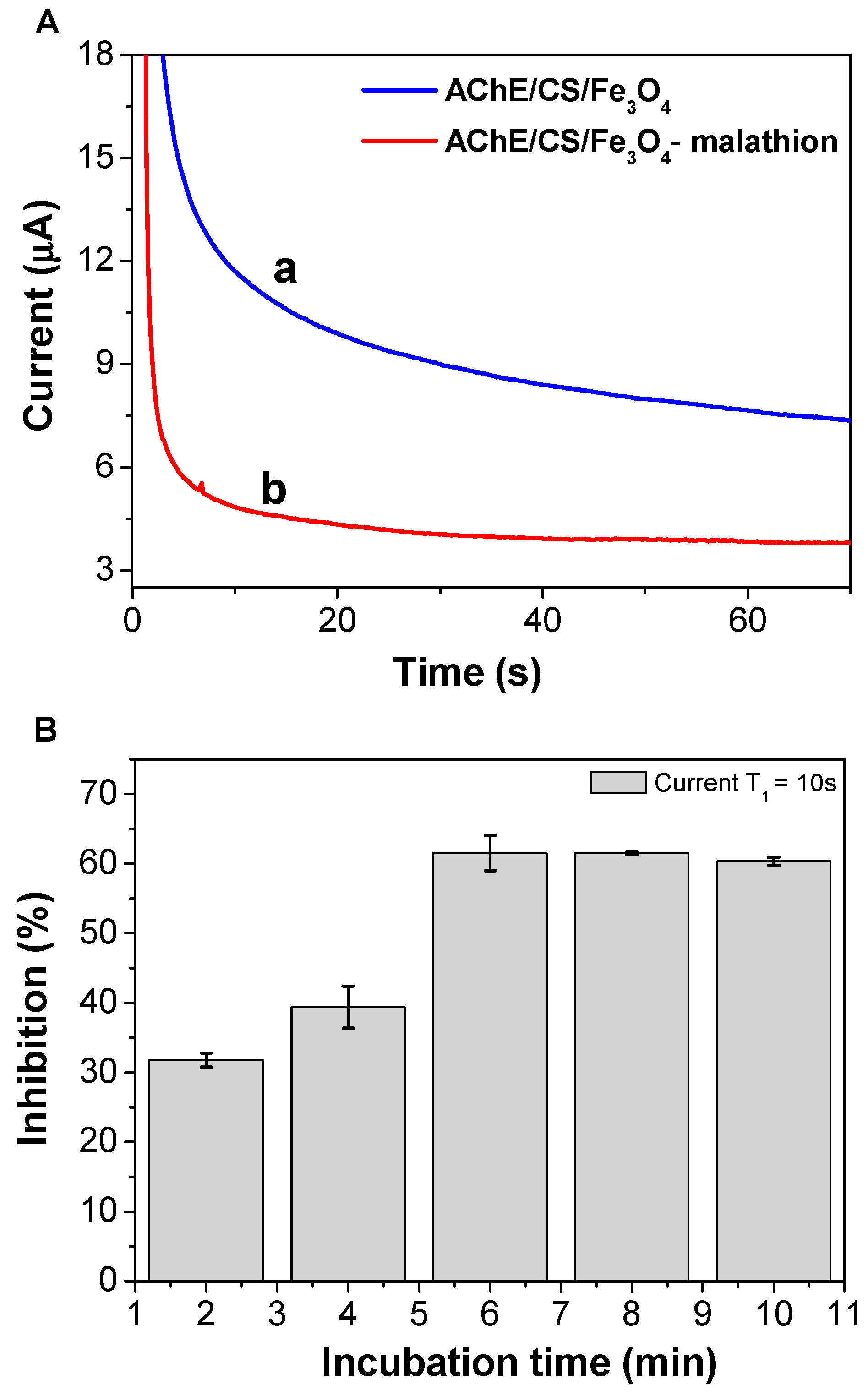
3.4. Temporal Behavior of Sensor Inhibition by Malathion
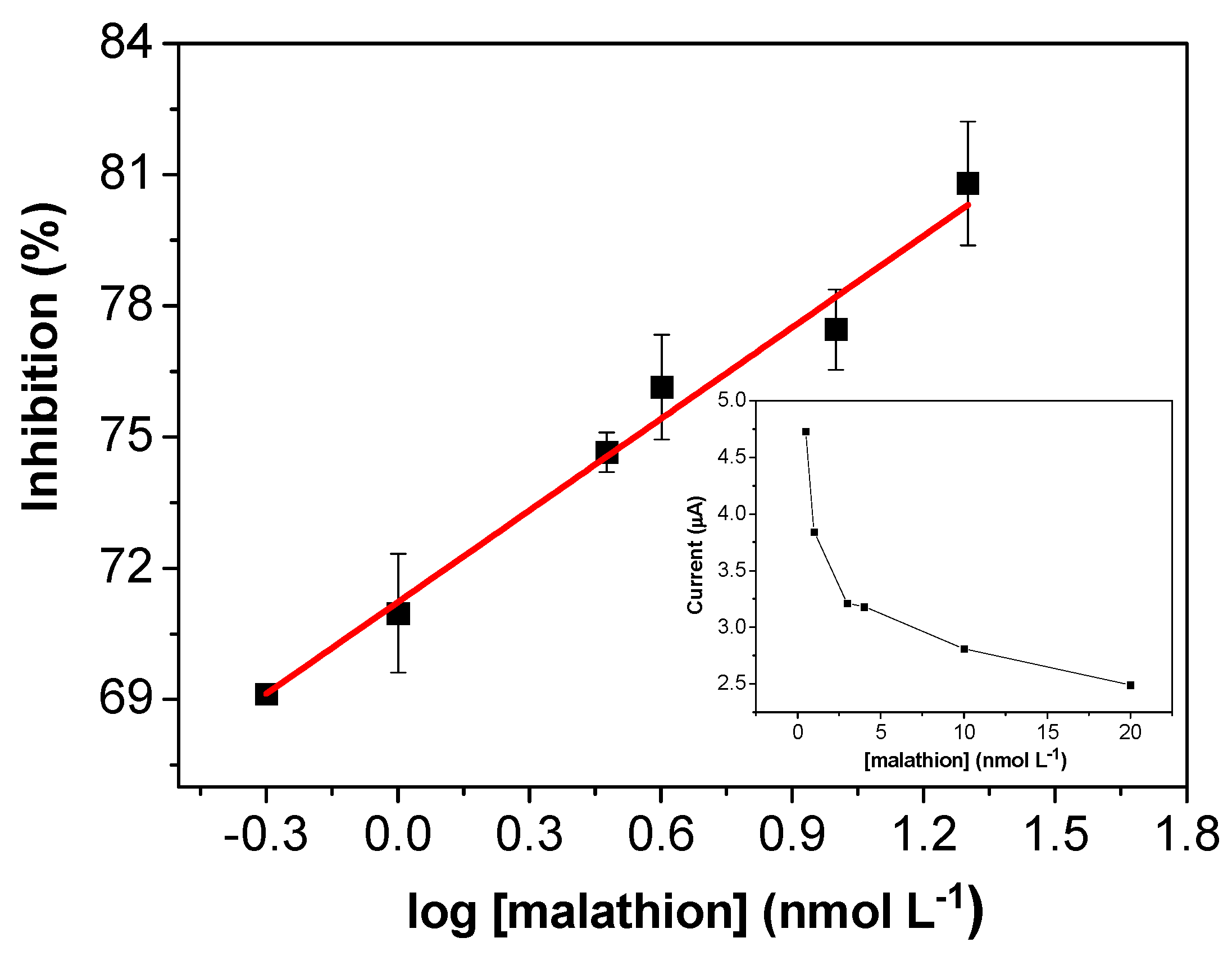
3.5. Analytical Performance of the AChE/CS/Fe3O4-Modified Screen-Printed Carbon Electrode for Determination of Malathion Pesticide
3.6. Determination of Malathion in Tomato Sauce and Pond Water Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, J. A novel acetylcholinesterase biosensor based on ionic liquids-AuNPs-porous carbon composite matrix for detection of organophosphate pesticides. Sens. Actuators B Chem. 2015, 211, 290–296. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Wu, M.; Wang, X.; Hou, T.; Li, F. Electrochemical biosensing strategy for highly sensitive pesticide assay based on mercury ion-mediated DNA conformational switch coupled with signal amplification by hybridization chain reaction. Sens. Actuators B Chem. 2016, 236, 597–604. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.K.; Kim, K.H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Cakir, Z.; Emet, M.; Serinken, M.; Karcioglu, O.; Kandis, H.; Uzkeser, M. Acute abdomen associated with organophosphate poisoning. J. Emerg. Med. 2011, 41, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Pundir, C.S. An amperometric biosensor based on acetylcholinesterase immobilized onto iron oxide nanoparticles/multi-walled carbon nanotubes modified gold electrode for measurement of organophosphorus insecticides. Anal. Chim. Acta 2011, 701, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.A.; Okonkwo, J.O. Talanta Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Prabhakar, N. Current scenario in organophosphates detection using electrochemical biosensors. Trends Anal. Chem. 2017. [Google Scholar] [CrossRef]
- Wu, S.; Huang, F.; Lan, X.; Wang, X.; Wang, J.; Meng, C. Electrochemically reduced graphene oxide and Nafion nanocomposite for ultralow potential detection of organophosphate pesticide. Sens. Actuators B Chem. 2013, 177, 724–729. [Google Scholar] [CrossRef]
- Arduini, F.; Ricci, F.; Tuta, C.S.; Moscone, D.; Amine, A.; Palleschi, G. Detection of carbamic and organophosphorous pesticides in water samples using a cholinesterase biosensor based on Prussian Blue-modified screen-printed electrode. Anal. Chim. Acta 2006, 580, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Su, H.; Qu, X.; Ju, P.; Cui, L.; Ai, S. Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide-gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides. Sens. Actuators B Chem. 2011, 160, 1255–1261. [Google Scholar] [CrossRef]
- Jeyapragasam, T.; Saraswathi, R. Electrochemical biosensing of carbofuran based on acetylcholinesterase immobilized onto iron oxide-chitosan nanocomposite. Sens. Actuators B Chem. 2014, 191, 681–687. [Google Scholar] [CrossRef]
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2006, 396, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Alkire, R.C.; Kolb, D.M.; Kibler, L.A.; Lipkowski, J. Chemically Modified Electrodes. In Advances in Electrochemical Science and Engineering; WILEY-VCH: Weinheim, Germany, 2009; Volume 11, pp. 1–56. ISBN 9783527680436. [Google Scholar]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, E.; Hadjmohammadi, M.R.; Kiekens, F.; De Wael, K. Mixed Hemi/Ad-Micelle Sodium Dodecyl Sulfate-Coated Magnetic Iron Oxide Nanoparticles for the Efficient Removal and Trace Determination of Rhodamine-B and Rhodamine-6G. Anal. Chem. 2015, 87, 7894–7901. [Google Scholar] [CrossRef] [PubMed]
- Chaichi, M.J.; Ehsani, M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol-H2O2-gold nanoparticle chemiluminescence detection system. Sens. Actuators B Chem. 2016, 223, 713–722. [Google Scholar] [CrossRef]
- Castilho, M.D.S.; Laube, T.; Yamanaka, H.; Alegret, S.; Pividori, M.I. Magneto Immunoassays for Plasmodium falciparum Histidine-Rich. Anal. Chem. 2011, 83, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sánchez, M.; Mayorga-Martinez, C.C.; Watanabe, T.; Ivandini, T.A.; Honda, Y.; Pino, F.; Nakata, A.; Fujishima, A.; Einaga, Y.; Merkoçi, A. Microfluidic platform for environmental contaminants sensing and degradation based on boron-doped diamond electrodes. Biosens. Bioelectron. 2016, 75, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, A.; Kopel, P.; Cegan, A.; Pohanka, M. Construction of an Acetylcholinesterase Sensor Based on Synthesized Paramagnetic Nanoparticles, a Simple Tool for Neurotoxic Compounds Assay. Sensors 2017, 17, 676. [Google Scholar] [CrossRef] [PubMed]
- Ribani, M.; Bottoli, C.B.G.; Collins, C.H.; Jardim, I.C.S.F.; Melo, L.F.C. Validação em métodos cromatográficos e eletroforéticos. Quim. Nova 2004, 27, 771–780. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Amrollahi, M.A.; Makizadeh, S. Decoration of Fe3O4 magnetic nanoparticles on graphene oxide nanosheets. RSC Adv. 2015, 5, 105499–105506. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. A technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Stoytcheva, M. Electrochemical evaluation of the kinetic parameters of a heterogeneous enzyme reaction in presence of metal ions. Electroanalysis 2002, 14, 923–927. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, M. Development of acetylcholinesterase biosensor based on platinum-carbon aerogels composite for determination of organophosphorus pesticides. Food Control 2014, 36, 49–54. [Google Scholar] [CrossRef]
- Shi, M.; Xu, J.; Zhang, S.; Liu, B.; Kong, J. A mediator-free screen-printed amperometric biosensor for screening of organophosphorus pesticides with flow-injection analysis (FIA) system. Talanta 2006, 68, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Guidone, S.; Amine, A.; Palleschi, G.; Moscone, D. Acetylcholinesterase biosensor based on self-assembled monolayer-modified gold-screen printed electrodes for organophosphorus insecticide detection. Sens. Actuators B Chem. 2013, 179, 201–208. [Google Scholar] [CrossRef]
- Dou, J.; Fan, F.; Ding, A.; Cheng, L.; Sekar, R.; Wang, H.; Li, S. A screen-printed, amperometric biosensor for the determination of organophosphorus pesticides in water samples. J. Environ. Sci. 2012, 24, 956–962. [Google Scholar] [CrossRef]
- Domínguez-renedo, O.; Alonso-lomillo, M.A.; Recio-cebrián, P.; Arcos-martínez, M.J. Screen-printed acetylcholinesterase-based biosensors for inhibitive determination of permethrin. Sci. Total Environ. 2012, 426, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Catalina, D.; Carvajal, S.; Peñuela, G. Effect of chlorpyrifos on the inhibition of the enzyme acetylcholinesterase by cross-linking in water-supply samples and milk from dairy cattle. Talanta 2013, 111, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ben, N.; Bakas, I.; Istamboulié, G.; Ait-ichou, I.; Ait-addi, E.; Rouillon, R.; Noguer, T. Sol-gel immobilization of acetylcholinesterase for the determination of organophosphate pesticides in olive oil with biosensors. Food Control 2013, 30, 657–661. [Google Scholar] [CrossRef]
- Suprun, E.; Evtugyn, G.; Budnikov, H.; Ricci, F.; Moscone, D.; Palleschi, G. Acetylcholinesterase sensor based on screen-printed carbon electrode modified with prussian blue. Anal. Bioanal. Chem. 2005, 383, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Lee, Y.; Lin, T.; Shih, H.; Chang, F.; Lin, H.P. Development of an amperometric micro-biodetector for pesticide monitoring and detection. J. Taiwan Inst. Chem. Eng. 2009, 40, 113–122. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Fu, J.; Guo, Y.; Sun, X.; Yang, Q.; Wang, X. Electrochemical acetylcholinesterase biosensor based on multi-walled carbon nanotubes/dicyclohexyl phthalate modified screen-printed electrode for detection of chlorpyrifos. J. Electroanal. Chem. 2017, 801, 185–191. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Pundir, C.S. Immobilization of rat brain acetylcholinesterase on porous gold-nanoparticle-CaCO3 hybrid material modified Au electrode for detection of organophosphorous insecticides. Int. J. Biol. Macromol. 2011, 49, 923–929. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Noguer, T.; Marty, J.L.; Istamboulie, G. Ultra-sensitive biosensor based on genetically engineered acetylcholinesterase immobilized in poly (vinyl alcohol)/Fe-Ni alloy nanocomposite for phosmet detection in olive oil. Food Chem. 2016, 203, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yu, Y.; Fan, K.; Ji, F.; Wu, J.; Ying, Y. A nano-silver enzyme electrode for organophosphorus pesticide detection. Anal. Bioanal. Chem. 2016, 408, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
| AChE Based Biosensor | Pesticide | Technique | Linear Response Range (nmol L−1) | LOD (nmol L−1) | Ref. |
|---|---|---|---|---|---|
| AChE/Fe3O4–CH/GCE | Carbofuran | SWV/CV | 5.0–90 | 3.6 | [12] |
| MPs-AChE | Carbofuran | SWV | 39–625 | 20 | [20] |
| AChE-AuNPs-CaCO3 | Malathion/C | EIS/Amperometry | 0.1–100 | 0.1 | [35] |
| PVA-AWP/Fe-Ni/NP AChE | Phosmet-oxon | CV/Amperometry | 0.1–5 | 0.1 | [36] |
| AChE–AuNPs/MPS/Au | Carbamate | CV/Amperometry | 3–2 × 103 | 1.0 | [37] |
| Nano-silver/AChE/chitosan | Paraoxon | CV/Amperometry | 0–3.63 × 102 | 14.5 | [38] |
| AChE/CS/Fe3O4 | Malathion | CV/Amperometry | 0.5–20 | 0.3 | This work |
| Samples | Malathion Added (mol L−1) | Malathion Expected (mol L−1) | Malathion Found (mol L−1) | Recovery (%) |
|---|---|---|---|---|
| Tomato sauce | 0 | 0 | - | - |
| 2.5 × 10−7 | 2.5 × 10−7 | 2.7 × 10−7 | 108.0 (±1.95) a | |
| 7.5 × 10−7 | 7.5 × 10−7 | 7.4 × 10−7 | 98.7 (±2.65) a | |
| 1.2 × 10−6 | 1.2 × 10−6 | 1.2 × 10−6 | 100.0 (±2.66) a | |
| Pond water | 0 | 0 | - | - |
| 2.5 × 10−7 | 2.5 × 10−7 | 2.4 × 10−7 | 96.0 (±3.40) a | |
| 7.5 × 10−7 | 7.5 × 10−7 | 7.5 × 10−7 | 100.0 (±1.01) a | |
| 1.2 × 10−6 | 1.2 × 10−6 | 1.3 × 10−6 | 108.3 (±0.87) a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, N.F.M.; Neto, S.Y.; Luz, R.D.C.S.; Damos, F.S.; Yamanaka, H. Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles. Biosensors 2018, 8, 16. https://doi.org/10.3390/bios8010016
Rodrigues NFM, Neto SY, Luz RDCS, Damos FS, Yamanaka H. Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles. Biosensors. 2018; 8(1):16. https://doi.org/10.3390/bios8010016
Chicago/Turabian StyleRodrigues, Núbia Fernanda Marinho, Sakae Yotsumoto Neto, Rita De Cássia Silva Luz, Flávio Santos Damos, and Hideko Yamanaka. 2018. "Ultrasensitive Determination of Malathion Using Acetylcholinesterase Immobilized on Chitosan-Functionalized Magnetic Iron Nanoparticles" Biosensors 8, no. 1: 16. https://doi.org/10.3390/bios8010016