1. Introduction
Piezoresistive microcantilevers (MCLs) were firstly proposed by Tortonese et al. in 1993 to minimize the entire system of an atomic force microscope, as well as to simplify the operation of the microscope [
1]. Since then, piezoresistive MCLs have been developed and are widely used as pressure sensors [
2], hygrometers [
3], and accelerometers [
4]. In biomedical sensing applications, Gunter et al., in 2003, detected aerosol-based and solution-based vaccinia virus by using embedded piezoresistive MCL sensors [
5]. Wee et al., in 2005, reported a self-sensing, piezoresistive MCL sensor for the electrical detection of prostate cancer and cardiac disease markers [
6]. Meanwhile, an MCL with an internal piezoresistive component has been utilized for an in situ, label-free, highly specific, and rapid DNA detection assay developed by Mukhopadhyay et al. in 2005 [
7].
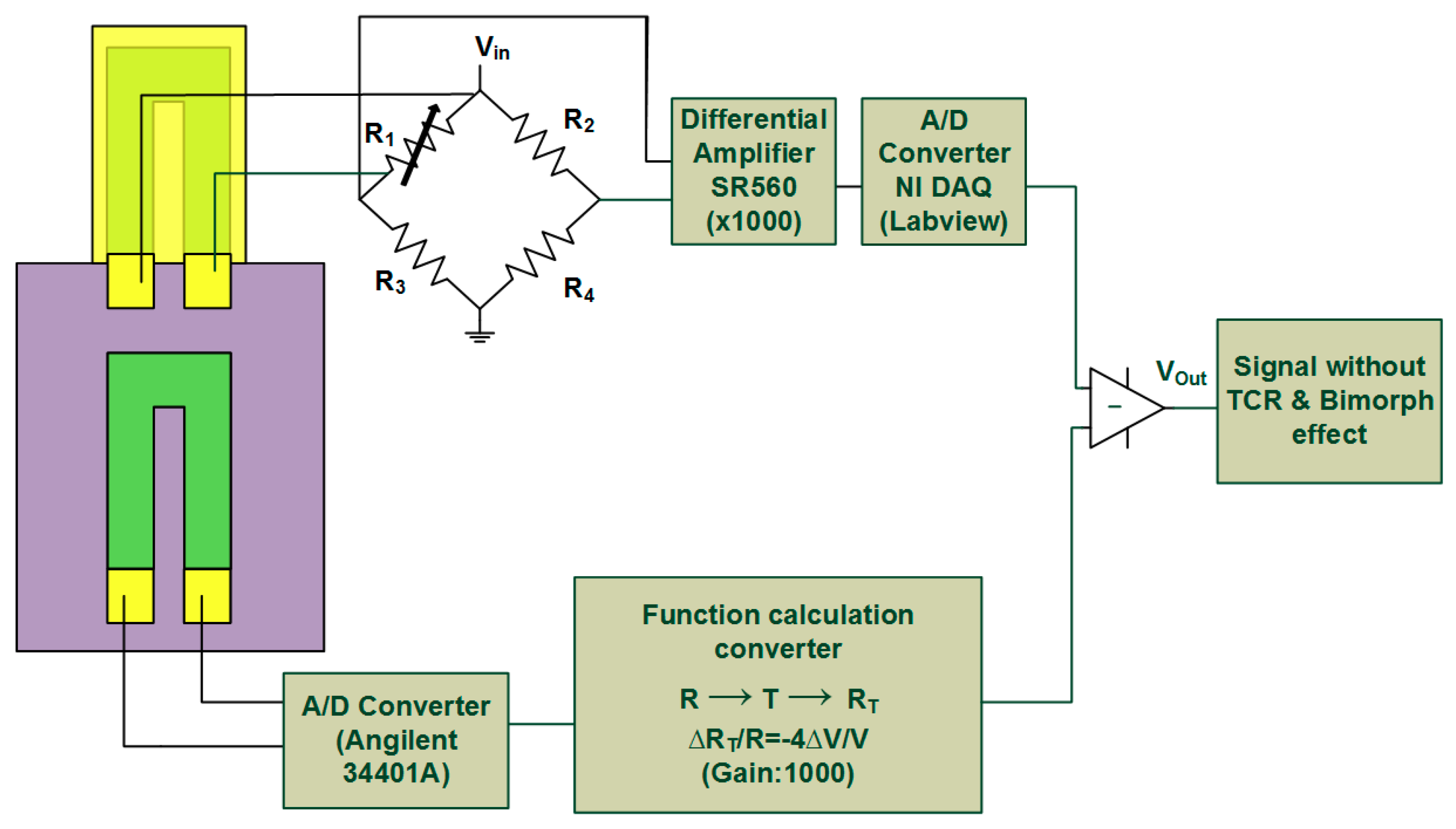
Conventionally, among these studies, the change in resistance is measured as an electrical signal by placing the piezoresistor in a Wheatstone bridge configuration. One sensing and one reference free-standing MCL beam with embedded piezoresistors as well as another two fixed resistors forming a Wheatstone bridge are used for eliminating environment disturbance. The sensing MCL is deposited with a sensitized film for detecting target analytes. Because of the unbalanced surface stress caused by the selective binding on the sensing MCL, the sensors are able to translate the bio-recognition events into nanomechanical deflection, leading to a resistance change proportional to the analyte concentration. Even so, the heterogeneous surface material properties for both beams contribute to the sensing and reference MCLs, which have independent deflection responses. The unexpected and disparate responses to most biological solvents or buffer fluids can affect the readout of real signals resulting in a problem of irreproducible results. In response to this issue, Yen et al. developed a single, free-standing, piezoresistive MCL sensor for detecting a biomolecule (C-reactive protein) with an improved precision [
8]. However, the thermal-induced signal, including the temperature coefficient of resistance (TCR) and material bimorph effect, becomes a major issue in this configuration.
Although several groups proposed different methods [
3,
9,
10,
11] to reduce thermal effects on piezoresistive MCL sensors, most of them are based on double beam configurations and not applicable for bio/chemical detections. Thaysen used similar materials to make the thermal expansion coefficient of the two MCLs the same [
12], but this method required an additional fabrication process, resulting in higher cost and lower yields. Differences in micromachining processes also made its design ineffective. Johansson et al. used SU-8 photoresist which is less sensitive to heat than the structure MCLs [
11]. Although the signal of thermal effect can be reduced, the resistors are less robust because they are made of gold. The SU-8 cantilevers with integrated metallic piezoresistive readout are suitable for detection of small surface stress changes as long as the temperature is reasonably well controlled by the temperature control system. However, an additional temperature control system can only control the ambient temperature, but the temperature of the specimen that is injected into the sensor cannot be changed immediately because of the bimorph effect. These limitations will greatly reduce the practicality of piezoresistive MCL-based sensors.
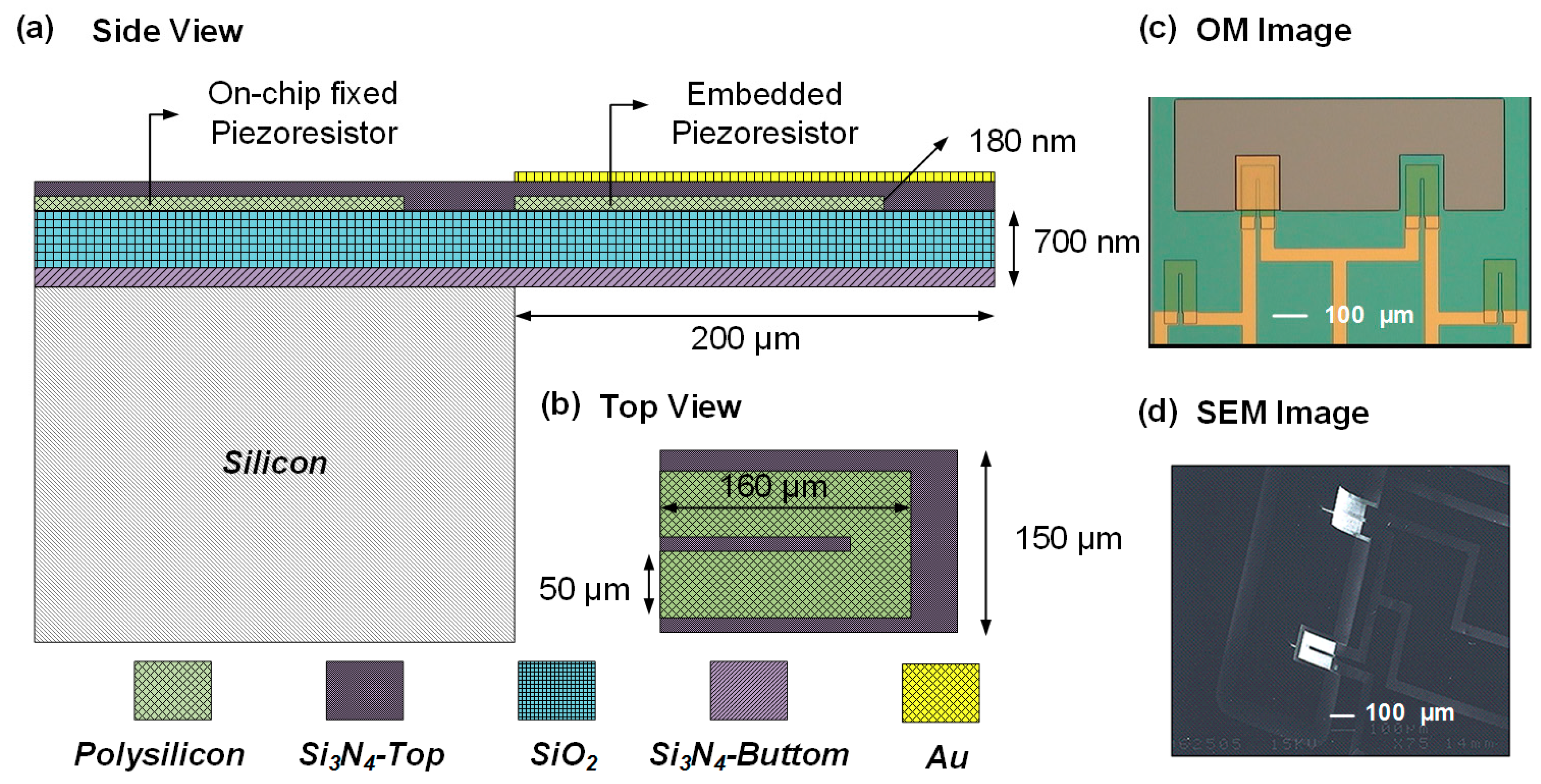
In this study, we propose a thermal self-elimination method for a biosensor based on a single, free-standing, piezoresistive MCL configuration. This strategy includes employing a fixed piezoresistor as an on-chip temperature sensor and an embedded piezoresistor in the MCL as a self-sensing temperature transducer. Through temperature gradient measurement, the relationship between these two resistances and the temperature is expressed as two quadratic functions. With the measurement of the temperature and through the interchange of these functions, we can eliminate the TCR and bimorph effect on the sensor. This method has an excellent temperature compensation ability in an environment with large temperature variations. Furthermore, in the absence of any thermostat device, the method was examined by using a piezoresistive MCL-based biosensor for C-reactive protein detections. The sensing capability of the biosensor and the feasibility of the system configuration were successfully verified.
3. Results and Discussion
3.1. On-Chip Piezoresistive Thermometer
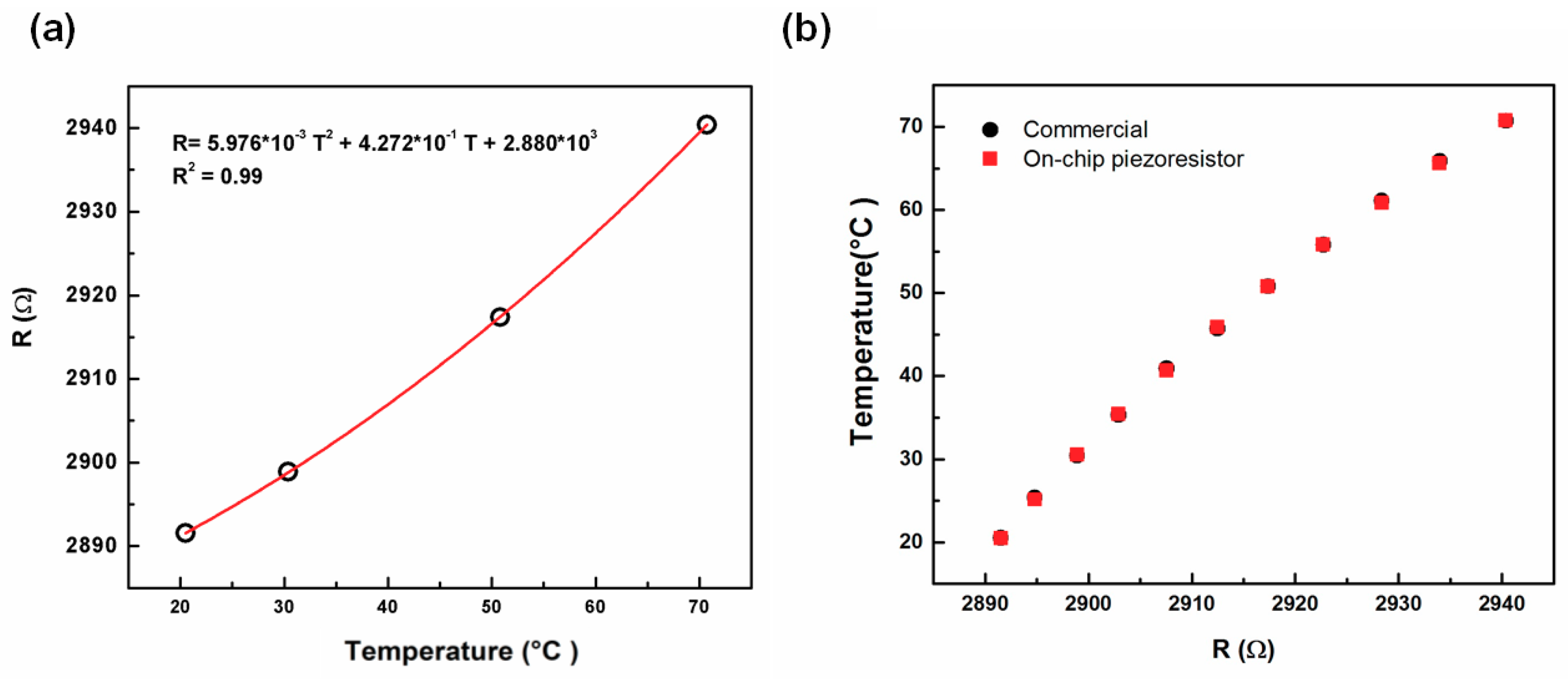
The relationship between the relative changes in the resistivity of TCR and temperature of the embedded fixed piezoresistor from 20 °C to 70 °C is shown in
Figure 5a. This relationship can be fitted to a quadratic equation. From the results, the on-chip fixed piezoresistor can be utilized as a thermometer to determine real-time temperature in the microchannel through a mathematical transformation. The result was compared with the temperature measured by a commercial thermometer shown in
Figure 5b. The difference of the measured temperature between the two devices was 0.2% which confirmed the reliability of the on-chip fixed piezoresistor as a thermometer.
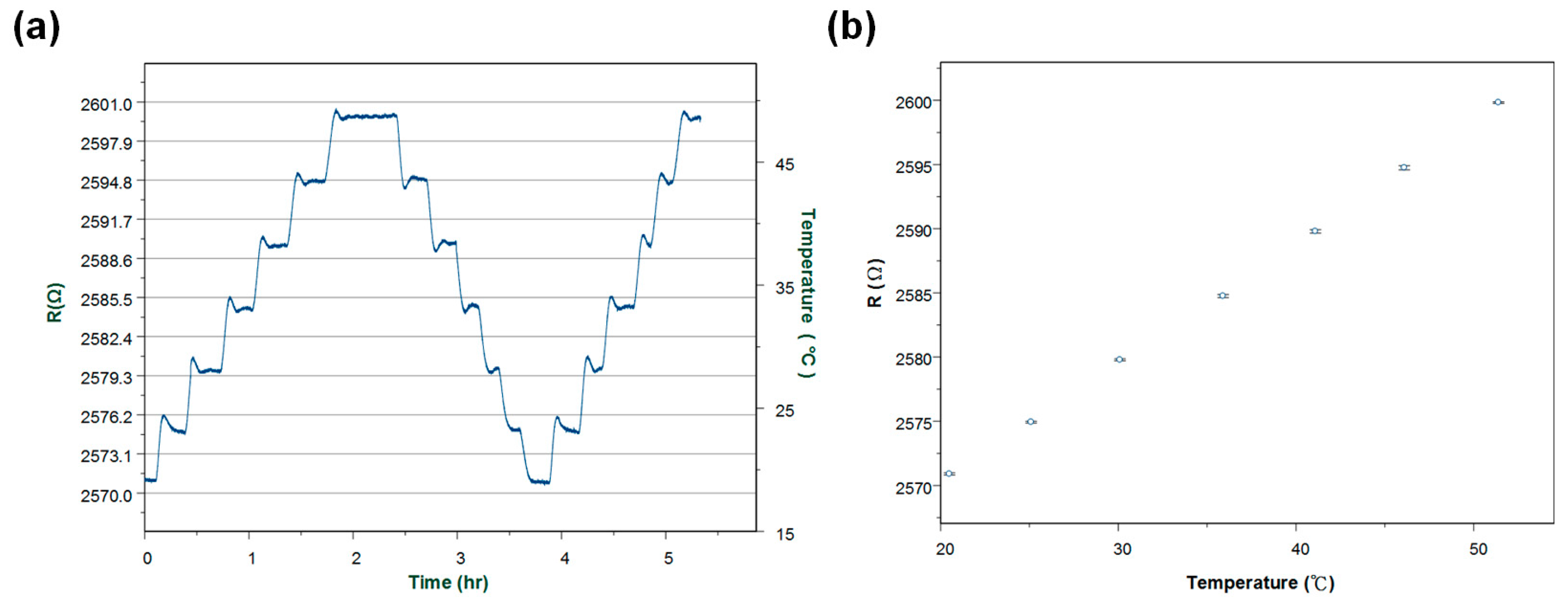
Moreover, the hysteresis for temperature measurements was studied. Through at least three experiments which lasted for 5 h (
Figure 6a), it was found that resistance change was about ±1 ohm with respect to a temperature variation of ±0.2 °C in doing up and down sweeps of the temperature. During various measurements, the coefficient of variation was 0.04% as shown in
Figure 6b.
3.2. Thermal Effects Elimination
By using the same calibration method as in the previous section, we can obtain two quadratic functions in the same form using Functions (1) and (2), which are
When measuring the resistance change in ROC of the on-chip piezoresistor, the real temperature can be obtained from (4), followed by substituting the temperature into (5) and getting temperature-induced resistance change RMCL of the freestanding piezoresistive MCL. This two-step mathematical transformation was automatically performed by our LabVIEW software. The resulting value of the resistance change was output as the compensation voltage through the Wheatstone bridge and the amplifier circuit. The output signal was finally obtained by subtracting the compensation voltage from the induced voltage of sensing piezoresistive MCL.
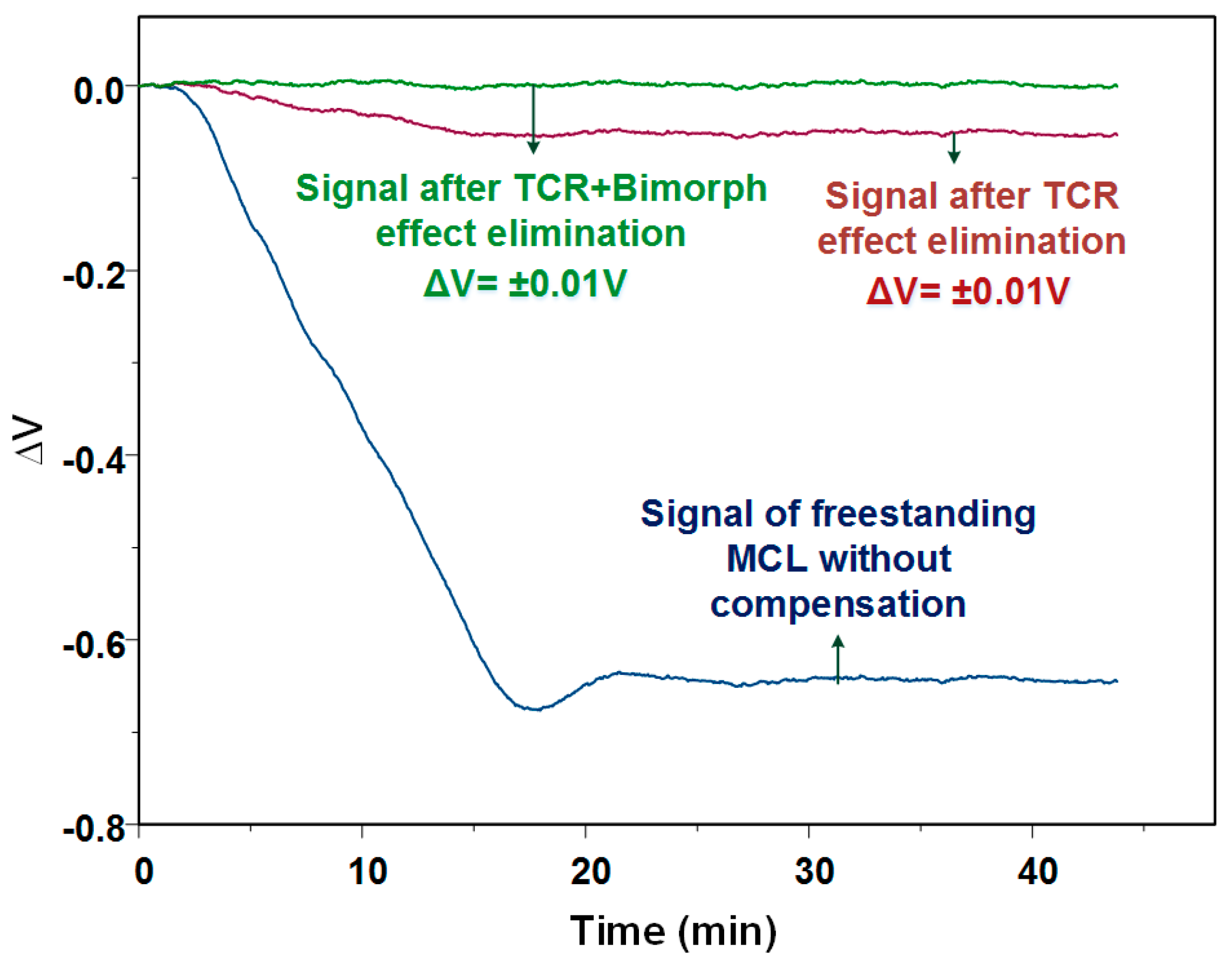
Figure 7 shows the output signal before and after thermal elimination, when the temperature increased form 13.6 °C to 40.7 °C. The input voltage for the Wheatstone bridge was 0.3 V. It was found that the voltage change caused by the total thermal effect was about 23.6 μV/°C, while the bimorph effect produced a change of 2 μV/°C. It revealed that the output signal induced by TCR was about ten times larger than that induced by the bimorph effect. The final output signal also showed that the effective elimination of thermal effects by applying this method. If recording the signal after thermal effect elimination for analysis, it can be found that the output voltage variation was 0.3 μV/°C, which was almost two orders of magnitude less than it was before processing the thermal elimination method.
We propose several reasons to explain the existing signal noise. First, since the temperature control system used in this experiment had ±0.2 °C of control error, the temperature calibration error resulted in a small error in the temperature calibration curve. The use of a more accurate temperature control device for measurement can improve accuracy. Another approach is to take the measured temperature error into the correction of the quadratic function in order to obtain more precise parameters a–f. Second, the noise came from an electric circuit and the reading error induced by the electrical instrument. Using an amplifying with low-pass filtering circuit for the electrical readout may decrease spurious signals.
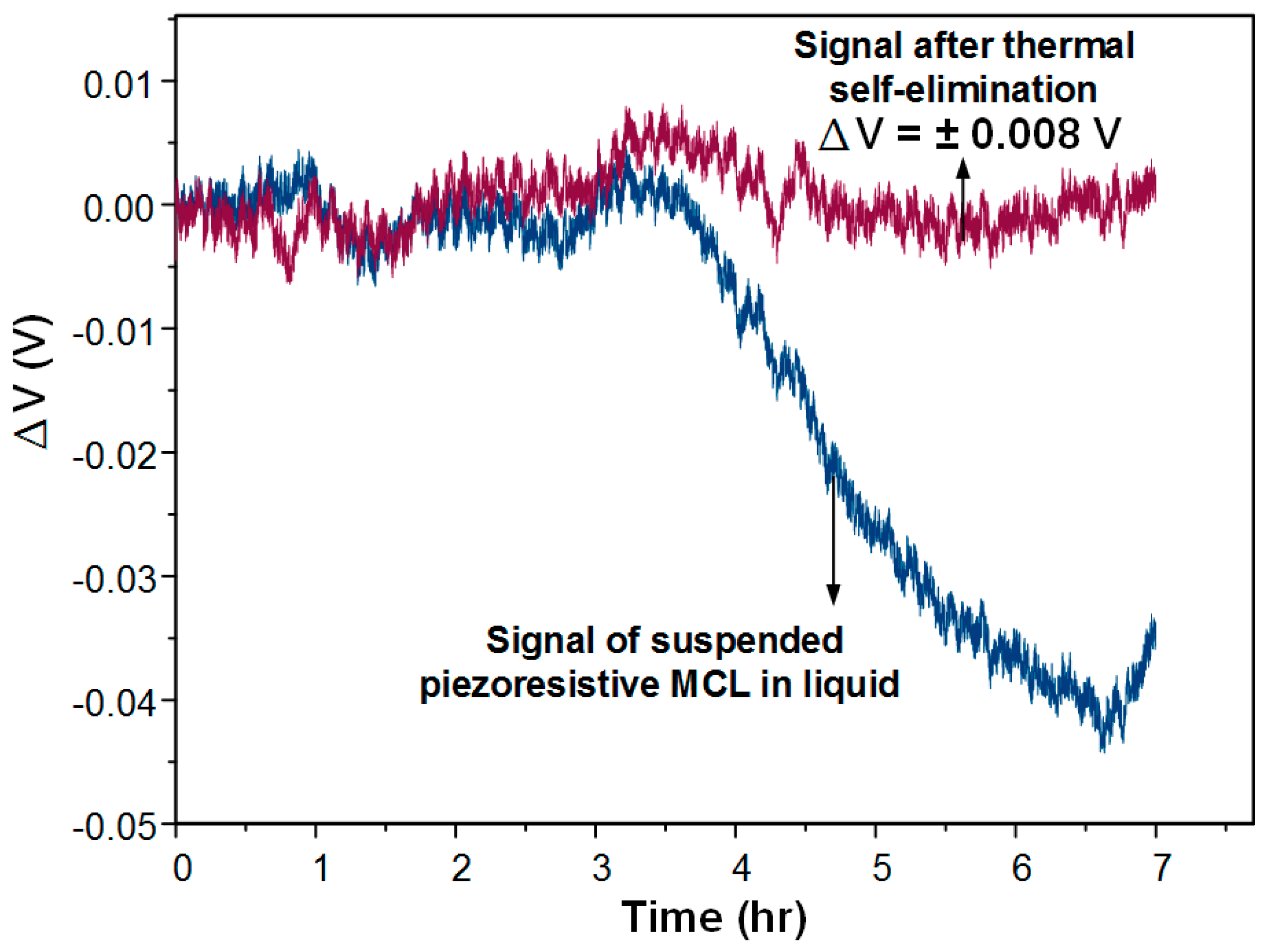
The piezoresistive MCL sensor was further tested in an aqueous environment for a long time (7 h). The solution injected was PBS buffer and the flow rate was controlled at 0.6 mL/h. The result shows that the real-time compensation method still worked in the fluid, as shown is
Figure 8. We found that the signal change from the freestanding MCL was 0.04 V at 3 h induced from 1.8 °C of room temperature change. The signal after compensation was maintained at around 0 V; however, it was not affected by the temperature-induced signal drift. The noise of the signal can result from flow field oscillations such as small bubbles on the interface of the fluid and surface of the MCL. This was a real response signal of the sensing MCL having nothing to do with the temperature effect.
3.3. C-Reactive Protein Detection with Real-Time Thermal Effects Elimination
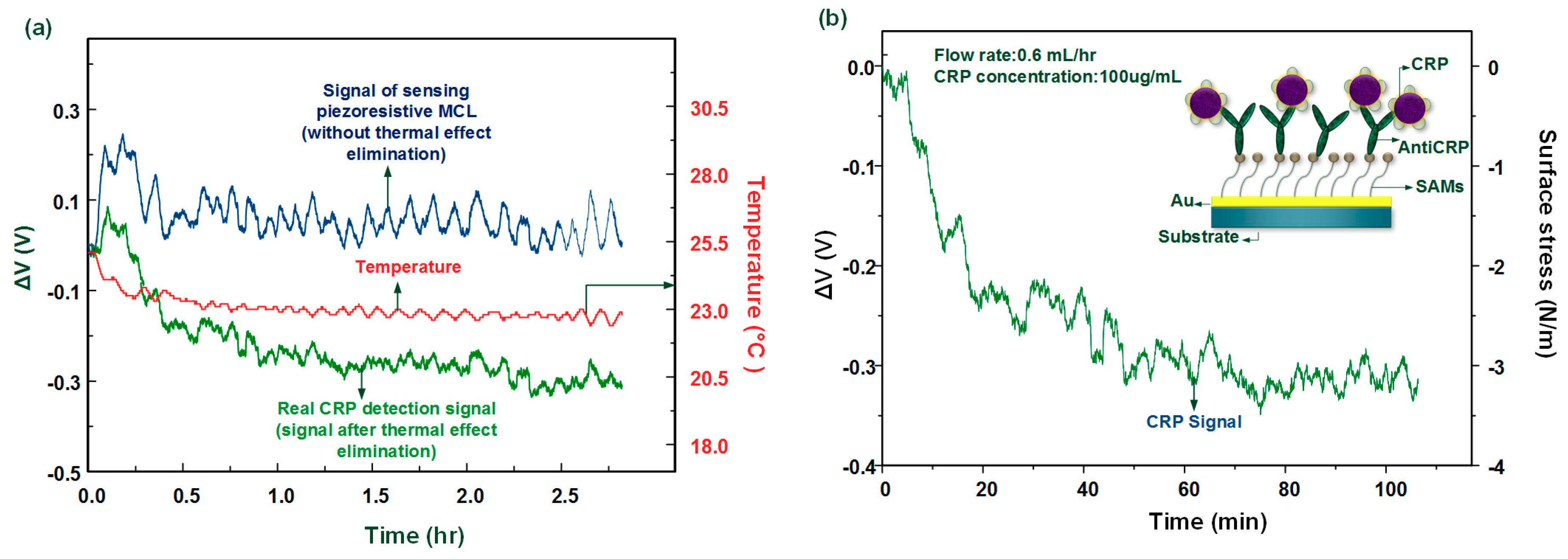
In protein detection, 100 μg/mL of the CRP solution (pH 7.2) was injected into the MCL sensing chip through the microchannel. The whole measurement was processed at room temperature without any temperature control system. The result shows that the proposed method successfully improves the piezoresistive MCL sensor for biomolecule detection as shown in
Figure 9a. The original signal without thermal effect elimination varied around 0 V due to the thermal induced signal coupled with the biochemical-induced signal. Such signals cannot provide any information for the existence of CRP. After applying the real-time thermal self-compensation method, the real tendency of the signal of CRP captured by anti-CRP was expressed.
Moreover, from
Figure 9a, we found that the system can successfully measure the biological sensing signal as well as the temperature signal. We found that the ambient temperature decreased by about 2 °C, which caused the corresponding TCR and bimorph effect, leading to a false readout signal. The voltage change signal of the antigen–antibody interaction can be converted into a value for the change of the surface stress [
13] as shown in
Figure 9b. In CRP detection, the signal indicated that the MCL deflects upward and the surface stress change was about −3.1 N/m (compressive surface stress). This biologically induced surface stress change was consistent when compared with our previous work [
8], which used a large external temperature control device to maintain the environment temperature. Therefore, this method was shown to successfully remove the thermal effects when using a freestanding MCL-based sensor for biomolecular detection. The whole system was miniaturized onto a chip consisting of sensing and fixed MCLs combined with the computing software, which was used to develop the multi-functional MCL array.
To improve performance of this method, we propose several ideas. First, in the current design, the position of the on-chip fixed piezoresistor is 100 μm from the suspended sensing MCL leading to a temperature difference between the two devices in the liquid environment. We suggest that the two devices be placed side by side to decrease the differences in temperature-sensing. Second, the whole process in the experiment is necessary to avoid bubble generation in the microchannel, since bubbles can greatly disrupt the flow field in the reaction area, resulting in measurement interruption and signal fluctuation. Moreover, before the injection of each sample, the tubing should be cleaned in order to avoid contamination with residue from the previous sample.