Fabrication of SrTiO3 Layer on Pt Electrode for Label-Free Capacitive Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. SrTiO3 Perovskite Layer Deposition
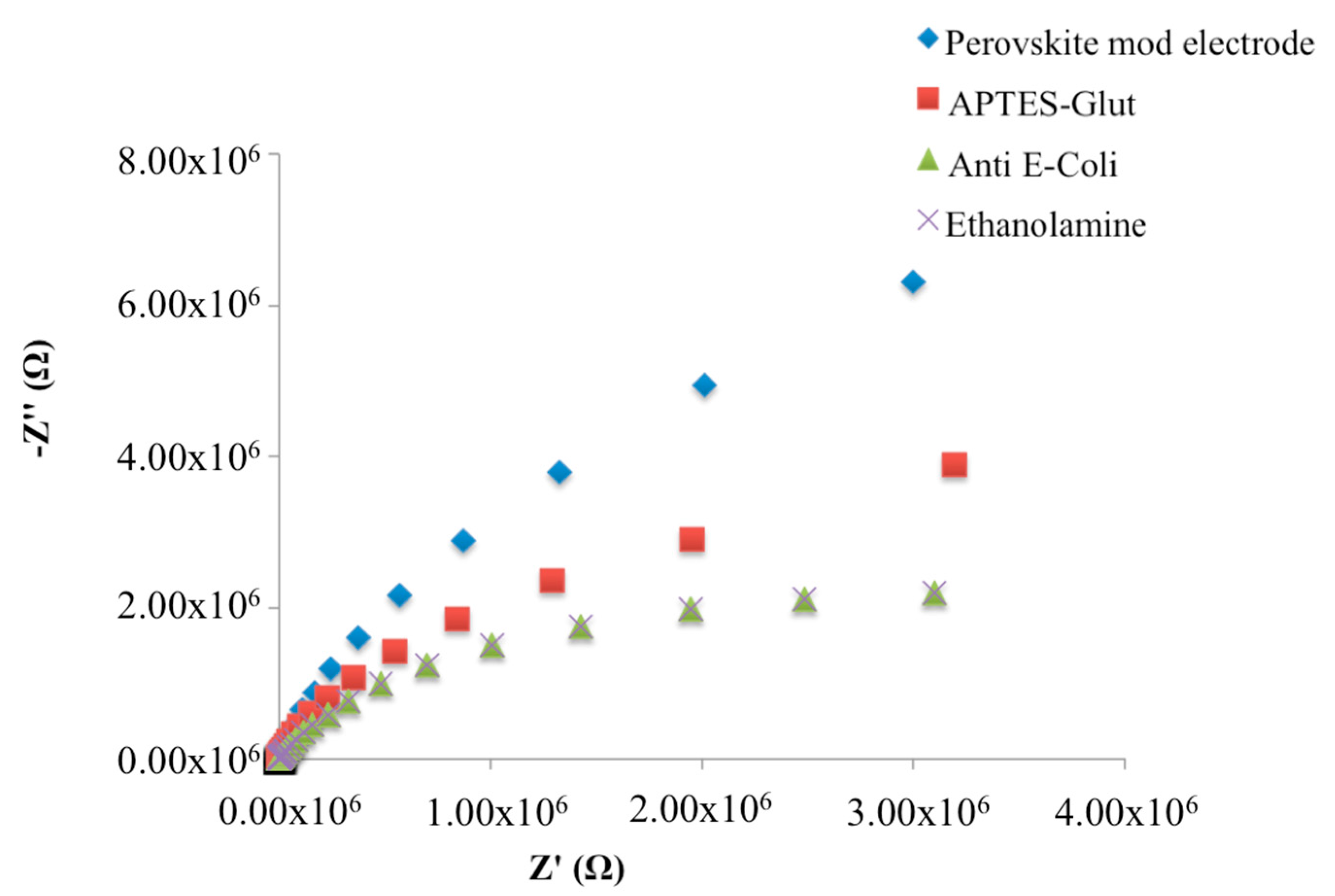
2.4. Immunosensor Manufacturing
2.5. Experimental Measurements
3. Results
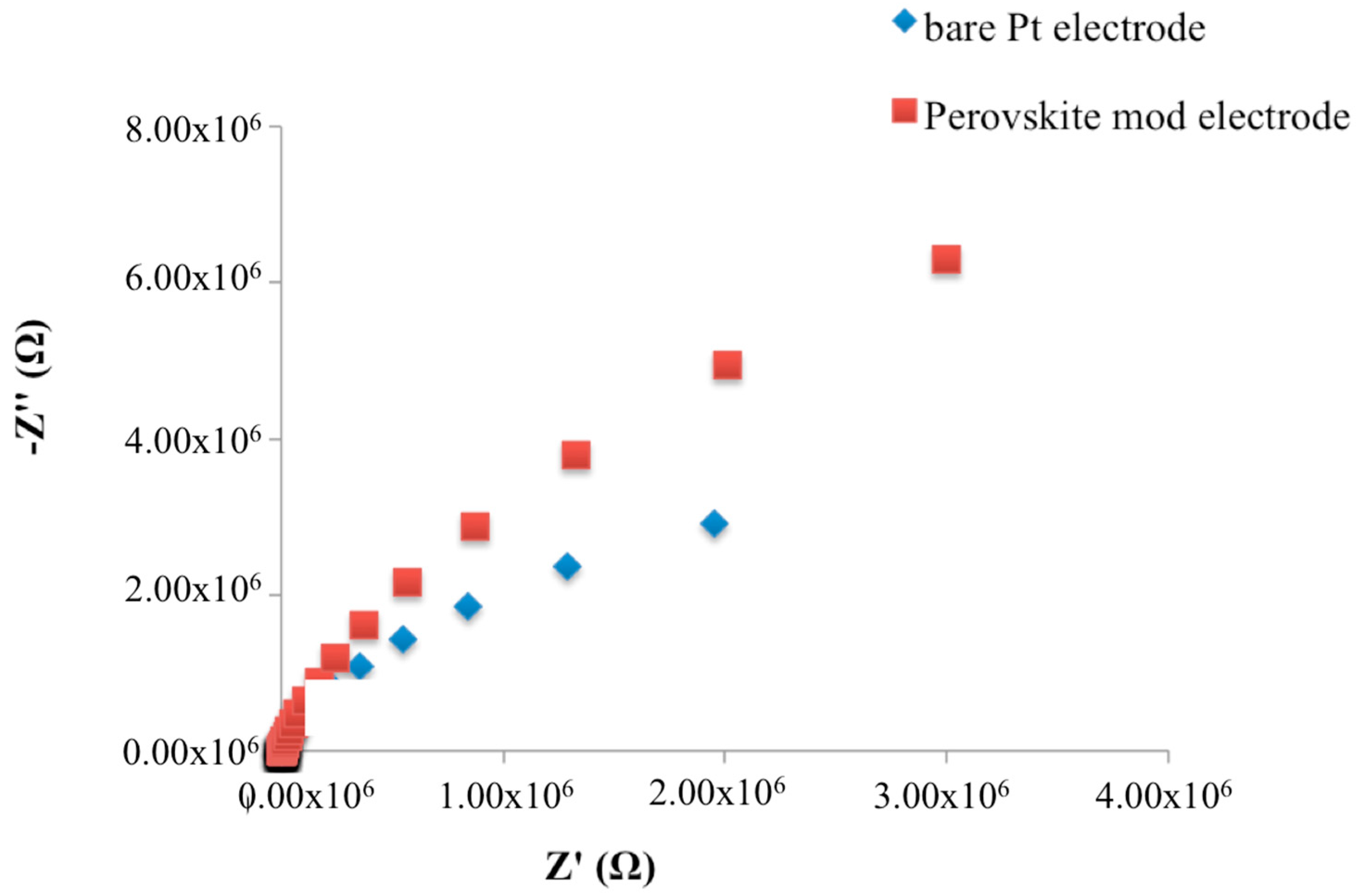
3.1. Characterization of the Electrode Modification Process
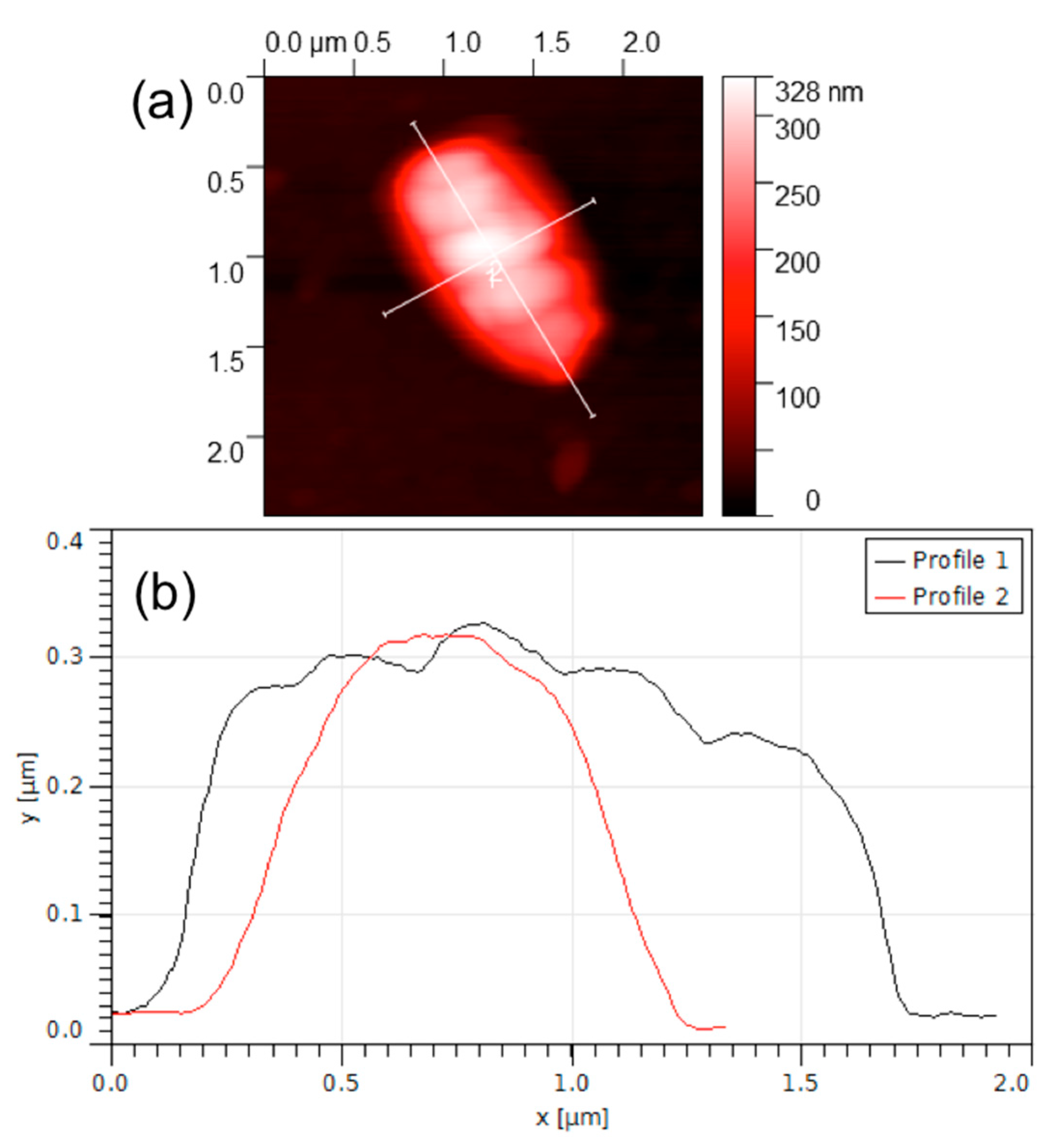
3.2. AFM Characterization
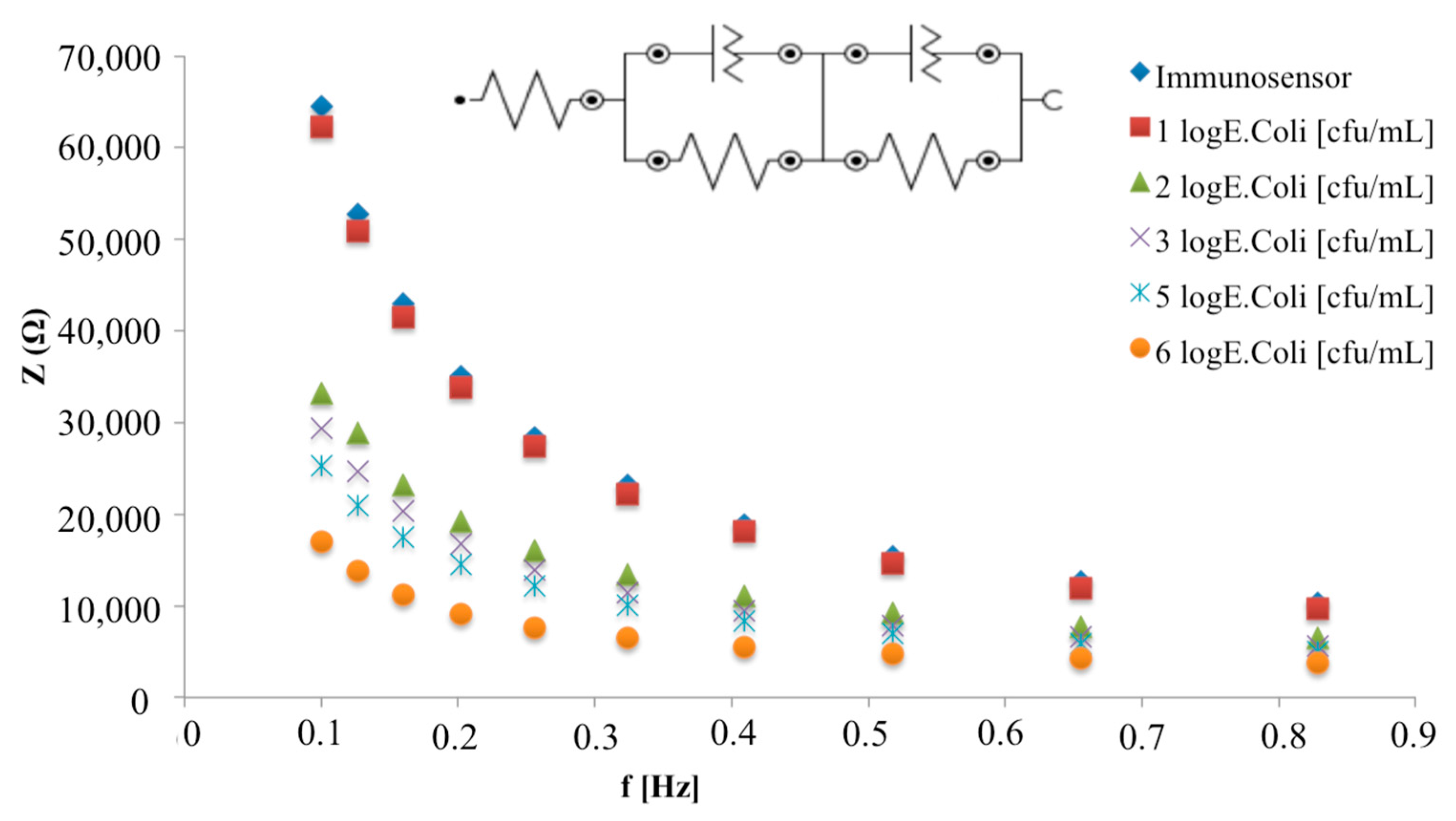
3.3. Analytical Parameters of an E. coli Capacitive Label-Free Immunosensor
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants—Trends and perspective. Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezginturk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2014, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Prodromidis, M.I. Impedimetric immunosensors—A review. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Turner, A.P.F.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectron. 2004, 19, 515–530. [Google Scholar] [CrossRef]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Saby, C.; Jaffrezic-Renault, N.; Martelet, C.; Colin, B.; Charles, M.H.; Delair, T.; Mandrand, B. Immobilization of antibodies onto a capacitance silicon-based transducer. Sens. Actuators B Chem. 1993, 16, 458–462. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Feng, M.; Min, L.; Yang, J.; Yu, S.; Zhang, Y.; Hu, X.; Yang, Z. Perovskite-type calcium titanate nanoparticles as novel matrix for designing sensitive electrochemical biosensing. Biosens. Bioelectron. 2017, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Y.; Tan, O.K.; Wei, Q.; Yao, M.W.; Tjin, S.C. Dielectric film for biosensor application. Sens. Actuators B Chem. 2006, 119, 78–83. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Kaabbuathong, N.; D’Epifanio, A.; Grilli, M.L.; Traversa, E.; Aono, H.; SadaOKA, Y. Nano-structured perovskite ocide electrodes for planar electrochemical sensors using tape casted YSZ layers. J. Eur. Ceram. Soc. 2004, 24, 1187–1190. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C.; Ferroni, M.; Sadaoka, Y.; Traversa, E. Screen—Printed perovskite-type thick films as gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 55, 99–110. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Chen, X.; Hu, J.; Chen, Z.; Feng, X.; Li, A. Nanoplated bismuth titanate sub-microspheres for protein immobilization and their corresponding direct electrochemistry and electrocatalysis. Biosens. Bioelectron. 2009, 24, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, H.; Chen, Z.; Li, A.; Feng, X.; Xi, B.; Hu, G. Sol–gel hydrothermal synthesis and enhanced biosensing properties of nanoplated lanthanum-substituted bismuth titanate microspheres. J. Mater. Chem. 2011, 21, 5352–5359. [Google Scholar] [CrossRef]
- Santos, A.; Davis, J.J.; Bueno, P.R. Fundamentals and Applications of Impedimetric an Redox Capacitive Biosensors. Anal. Bioanal. Tech. 2014. [Google Scholar] [CrossRef]
- Bernalte, E.; Marín-Sánchez, C.; Pinilla-Gil, E.; Brett, C.M.A. Characterisation of Screen-Printed Gold and Gold Nanoparticle-Modified Carbon Sensors by Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2013, 709, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Malvano, F.; Albanese, D.; Crescitelli, A.; Pilloton, R.; Esposito, E. Impedimetric Label-Free Immunosensor on Disposable Modified Screen-Printed Electrodes for Ochratoxin A. Biosensors 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- San Paulo, A.; Garcıa, R. High-Resolution Imaging of Antibodies by Tapping-Mode Atomic Force Microscopy: Attractive and Repulsive Tip-Sample Interaction Regimes. Biophys. J. 2000, 78, 1599–1605. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, H.; Cai, P.; Fein, J.B.; Chen, W. Atomic force microscopy measurements of bacterial adhesion and biofilm formation onto clay-sized particles. Sci. Rep. 2015, 5, 16857. [Google Scholar] [CrossRef] [PubMed]
- Duplan, V.; Frost, E.; Dubowski, J.J. A photoluminescence—Based quantum semiconductor biosensor for rapid in situ detection of Escherichia coli. Sens. Actuators B Chem. 2011, 160, 46–51. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Supaporn, D.; Kanatharana, P.; Wongkittisuksa, B.; Limbut, W.; Numnuam, A.; Limsakul, C.; Thavarungkul, P. Label—Free capacitive immunosensors for ultra-trace detection based on the increase of immobilized antibodies on silver nanoparticles. Anal. Chim. Acta 2011, 699, 232–241. [Google Scholar]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Matteo, M. A highly sensitive impedimetric label free immunosensor for Ochratoxin measurement in cocoa beans. Food Chem. 2016, 212, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Matteo, M. A new label—Free impedimetric aptasensor for gluten detection. Food Control 2017, 79, 200–206. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; De, A.; Chaudhuri, C.R.; Bandyopadhyay, K.B.; Sen, P. Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 Bacteria. Sens. Actuators B Chem. 2012, 171, 916–923. [Google Scholar] [CrossRef]
- Barreiros dos Santos, M.; Agusil, J.P.; Prieto-Simon, B.; SPorer, C.; Teixeire, V.; Samitier, J. Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ping, J.; Ye, Z.; Wu, J.; Ying, Y. Impedimetric immunosensor based on gold nanoparticles modified grapheme paper for label—Free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2013, 49, 492–498. [Google Scholar]
- Barreiros dos Santos, M.; Azevedo, S.; Agusil, J.P.; Prieto-Simon, B.; Sporer, C.; Torrents, E.; Juarez, A.; Teixeira, V.; Samitier, J. Lable-free ITO-based immunosensor for the detection of very low concentrations of pathogenic bacteria. Bioelectrochemistry 2015, 101, 146–152. [Google Scholar] [CrossRef] [PubMed]


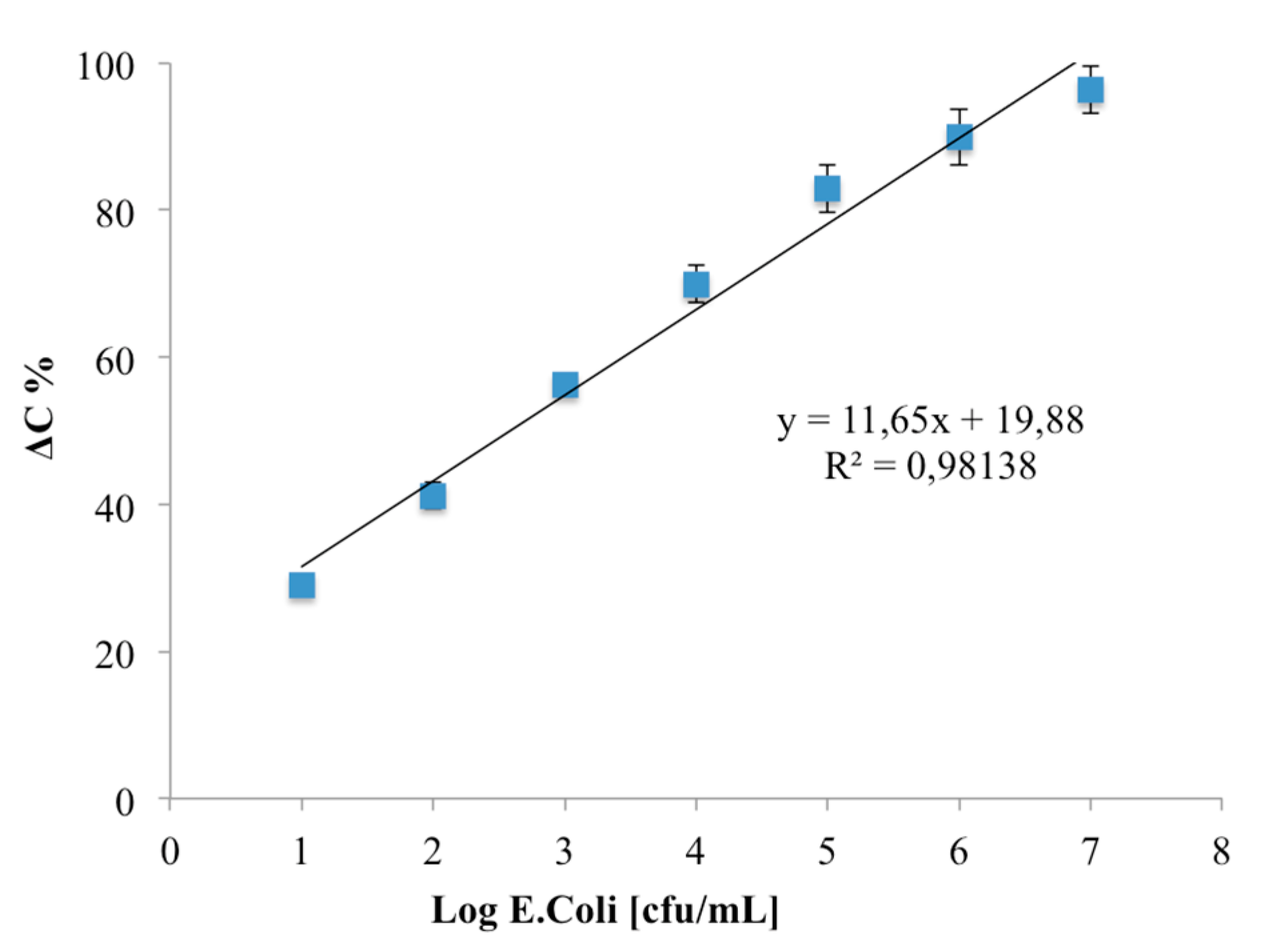
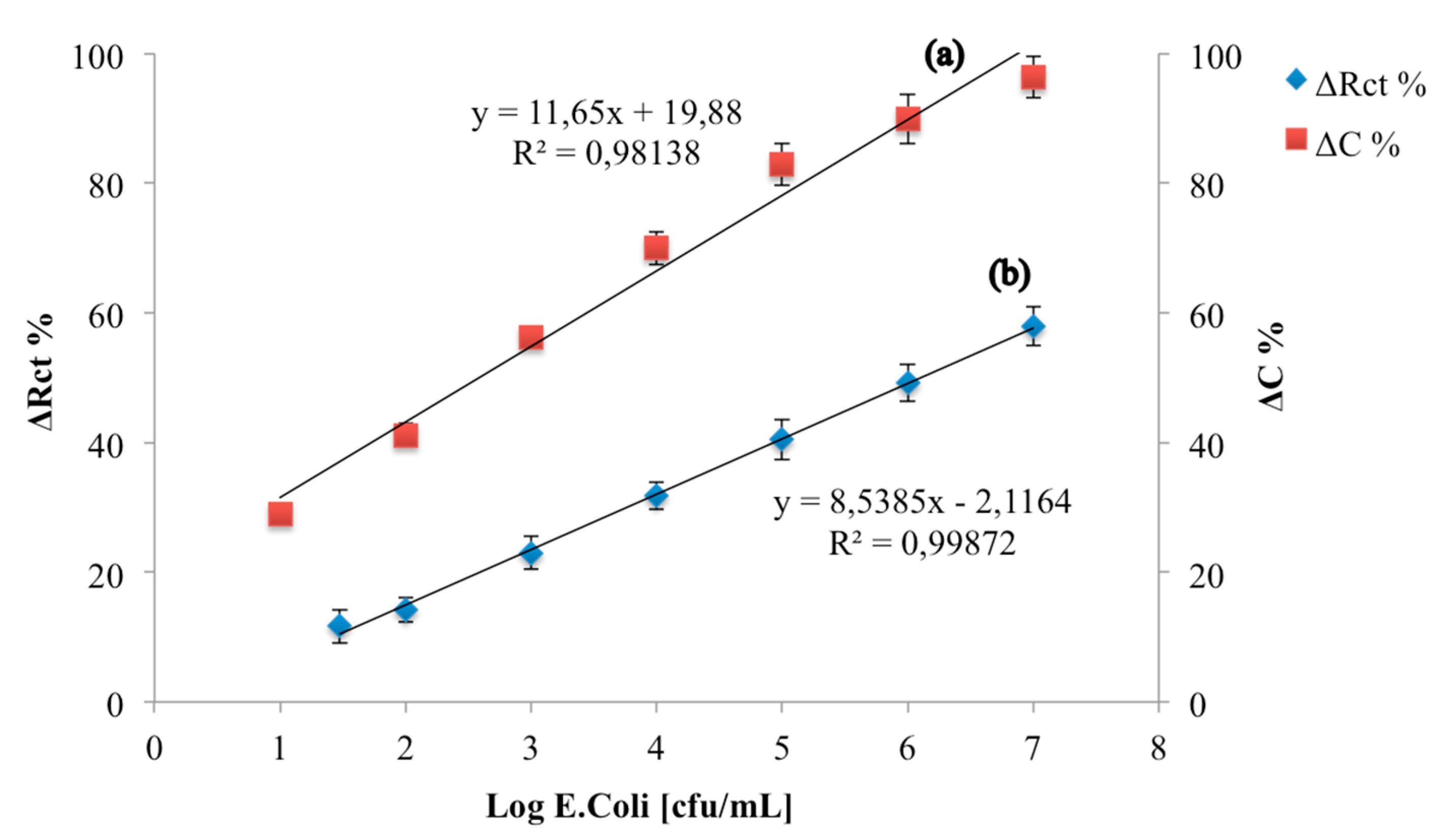
| Working Principle | Sensitivity (% Change) | Linear Range (log cfu/mL) | LOD (log cfu/mL) | RSD (%) |
|---|---|---|---|---|
| Capacitive | 11.65 | 1–7 | 1 | 3.1 |
| Resistive | 8.53 | 1.47–7 | 1.47 | 3.9 |
| Schematic Immunosensor Assembly | Working Principle | Linear Range (log cfu/mL) | LOD (cfu/mL) | References |
|---|---|---|---|---|
| Au + PANI + Glu + anti-E. coli | Resistive | 2–7 | 100 | [25] |
| Au + MHDA | Resistive | 1.47–8.48 | 2 | [26] |
| GOPE + AuNPs + Streptavidn + Biotin + anti-E. coli | Resistive | 2.17–8.17 | 150 | [27] |
| Epoxysilane-mod. ITO + PDMS + anti-E. coli | Resistive | 1–6 | 10 | [28] |
| Pt + SrTiO3 + APTES-Glu + anti-E. coli | Capacitive | 1–7 | 10 | This work |
| Au + Cys + Glu + anti-E. coli | Resistive | 1.47–1 | 30 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvano, F.; Maritato, L.; Carapella, G.; Orgiani, P.; Pilloton, R.; Di Matteo, M.; Albanese, D. Fabrication of SrTiO3 Layer on Pt Electrode for Label-Free Capacitive Biosensors. Biosensors 2018, 8, 26. https://doi.org/10.3390/bios8010026
Malvano F, Maritato L, Carapella G, Orgiani P, Pilloton R, Di Matteo M, Albanese D. Fabrication of SrTiO3 Layer on Pt Electrode for Label-Free Capacitive Biosensors. Biosensors. 2018; 8(1):26. https://doi.org/10.3390/bios8010026
Chicago/Turabian StyleMalvano, Francesca, Luigi Maritato, Giovanni Carapella, Pasquale Orgiani, Roberto Pilloton, Marisa Di Matteo, and Donatella Albanese. 2018. "Fabrication of SrTiO3 Layer on Pt Electrode for Label-Free Capacitive Biosensors" Biosensors 8, no. 1: 26. https://doi.org/10.3390/bios8010026







