Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3
Abstract
:1. Introduction
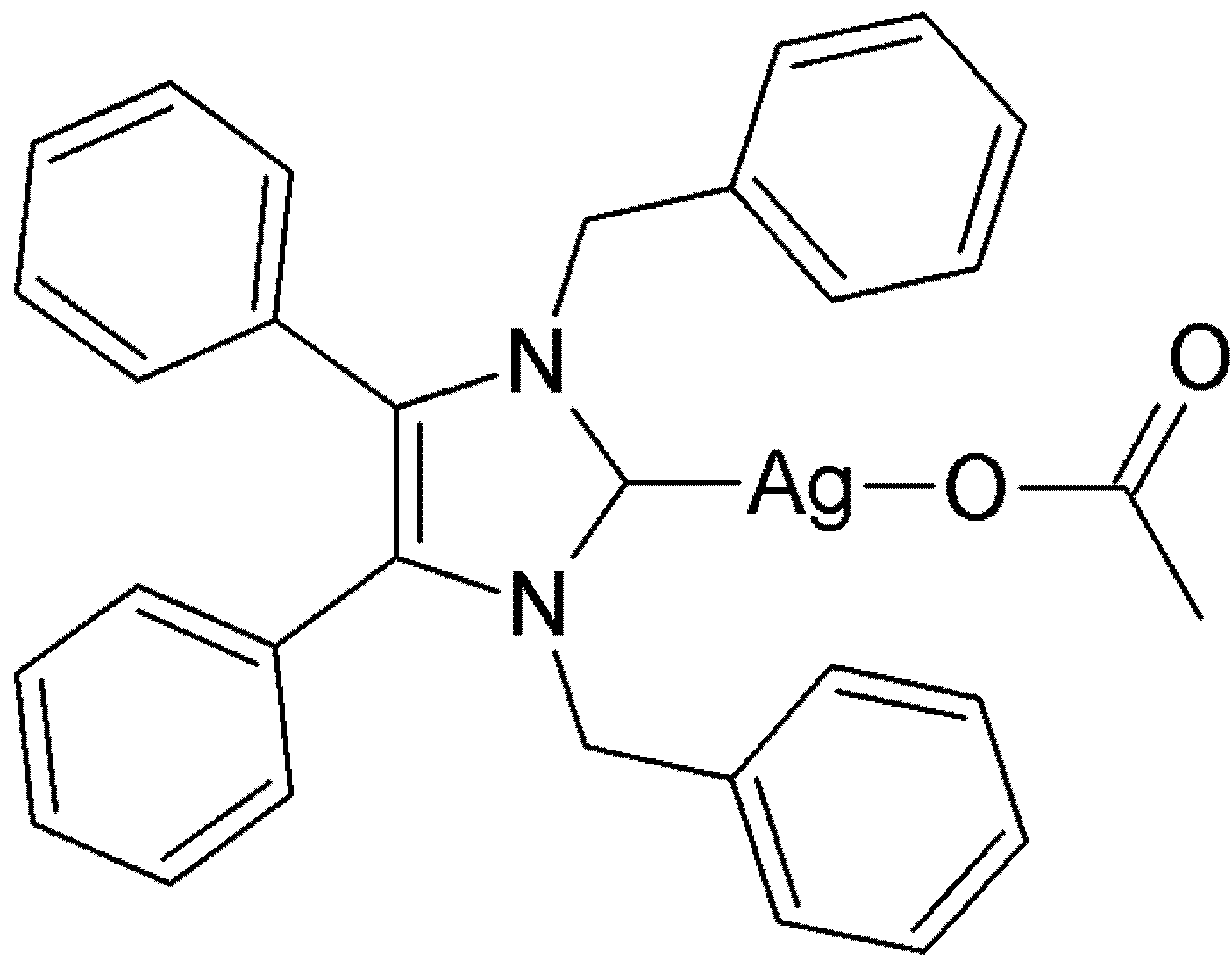
2. Results and Discussion
| Bacterial Strain | MIC [μg/mL] |
|---|---|
| Mycobacterium bovis BCG Pasteur | 20 |
| Mycobacterium smegmatis | 5 |
| Salmonella typhimurium | 12.5 |
| Staphylococcus aureus (MSSA) | 12.5 |
| Staphylococcus aureus (MRSA) | 12.5 |
| Pseudomonas aeruginosa | 3.13 |
| Escherichia coli | 6.25 |
3. Experimental
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Sutherland, R.; Rolinson, G.N. Characteristics of methicillin-resistant staphylococci. J. Bacteriol. 1964, 87, 887–899. [Google Scholar]
- Bartlett, J.G. Methicillin-resistant Staphylococcus aureus infections. Top. HIV Med. 2008, 16, 151–155. [Google Scholar]
- Lienhardt, C.; Raviglione, M.; Spigelman, M.; Hafner, R.; Jaramillo, E.; Hoelscher, M.; Zumla, A.; Gheuens, J. New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J. Infect. Dis. 2012, 205 (Suppl. 2), S241–S249. [Google Scholar]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef]
- Panzner, M.J.; Deeraksa, A.; Smith, A.; Wright, B.D.; Hindi, K.M.; Kascatan-Nebioglu, A.; Torres, A.G.; Judy, B.M.; Hovis, C.E.; Hilliard, J.K.; et al. Synthesis and in vitro Efficacy Studies of Silver Carbene Complexes on Biosafety Level 3 Bacteria. Eur. J. Inorg. Chem. 2009, 2009, 1739–1745. [Google Scholar]
- Patil, S.; Tacke, M. NHC-Silver(I) Acetates as Bioorganometallic Anticancer and Antibacterial Drugs. In Insights into Coordination, Bioinorganic and Applied Inorganic Chemistry; Melník, M., Segľa, P., Tatarko, M., Eds.; Press of Slovak University of Technology: Bratislava, Slovakia, 2011; pp. 555–566. [Google Scholar]
- Patil, S.; Deally, A.; Gleeson, B.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Novel Benzyl-Substituted N-Heterocyclic Carbene-Silver Acetate Complexes: Synthesis, Cytotoxicity and Antibacterial Studies. Metallomics 2011, 3, 74–88. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharkey, M.A.; O'Gara, J.P.; Gordon, S.V.; Hackenberg, F.; Healy, C.; Paradisi, F.; Patil, S.; Schaible, B.; Tacke, M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics 2012, 1, 25-28. https://doi.org/10.3390/antibiotics1010025
Sharkey MA, O'Gara JP, Gordon SV, Hackenberg F, Healy C, Paradisi F, Patil S, Schaible B, Tacke M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics. 2012; 1(1):25-28. https://doi.org/10.3390/antibiotics1010025
Chicago/Turabian StyleSharkey, Michael A., James P. O'Gara, Stephen V. Gordon, Frauke Hackenberg, Claire Healy, Francesca Paradisi, Siddappa Patil, Bettina Schaible, and Matthias Tacke. 2012. "Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3" Antibiotics 1, no. 1: 25-28. https://doi.org/10.3390/antibiotics1010025




