Activity of Sulfa Drugs and Their Combinations against Stationary Phase B. burgdorferi In Vitro
Abstract
:1. Introduction
2. Results and Discussion
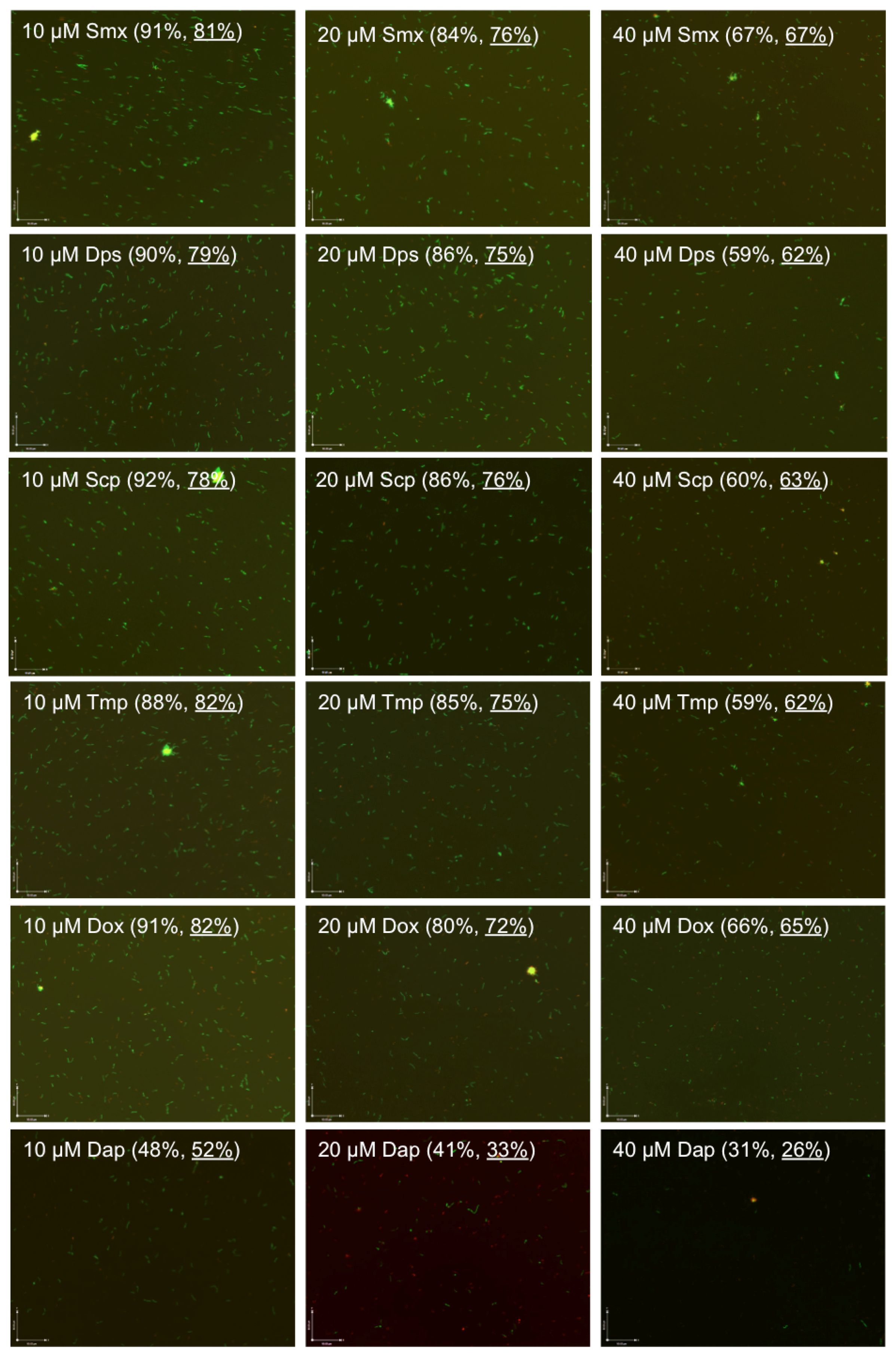
2.1. Comparison of the Relative Anti-Persister Activity of Commonly Used Sulfa Drugs Sulfamethoxazole, Dapsone, Sulfachlorpyridazine, and Trimethoprim for Their Activity against Stationary Phase B. burgdorferi Culture
2.2. Comparison of the Relative Anti-Persister Activity of Sulfamethoxazole, Dapsone, Sulfachlorpyridazine, and Trimethoprim in Drug Combinations at Respective Serum Drug Concentrations
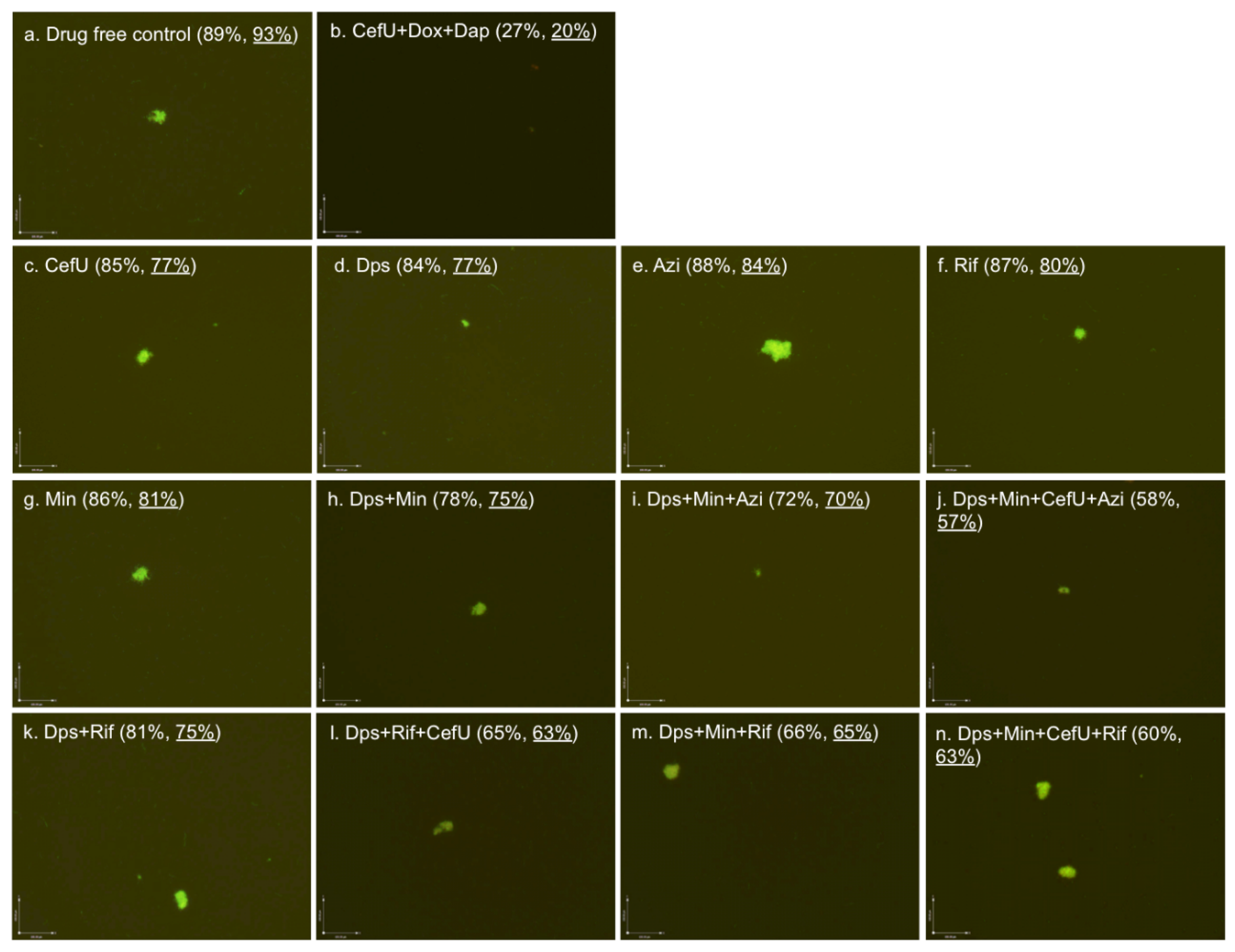
2.3. Effect of Dapsone Drug Combinations with Clinically Used Drugs on Stationary Phase B. burgdorferi Culture
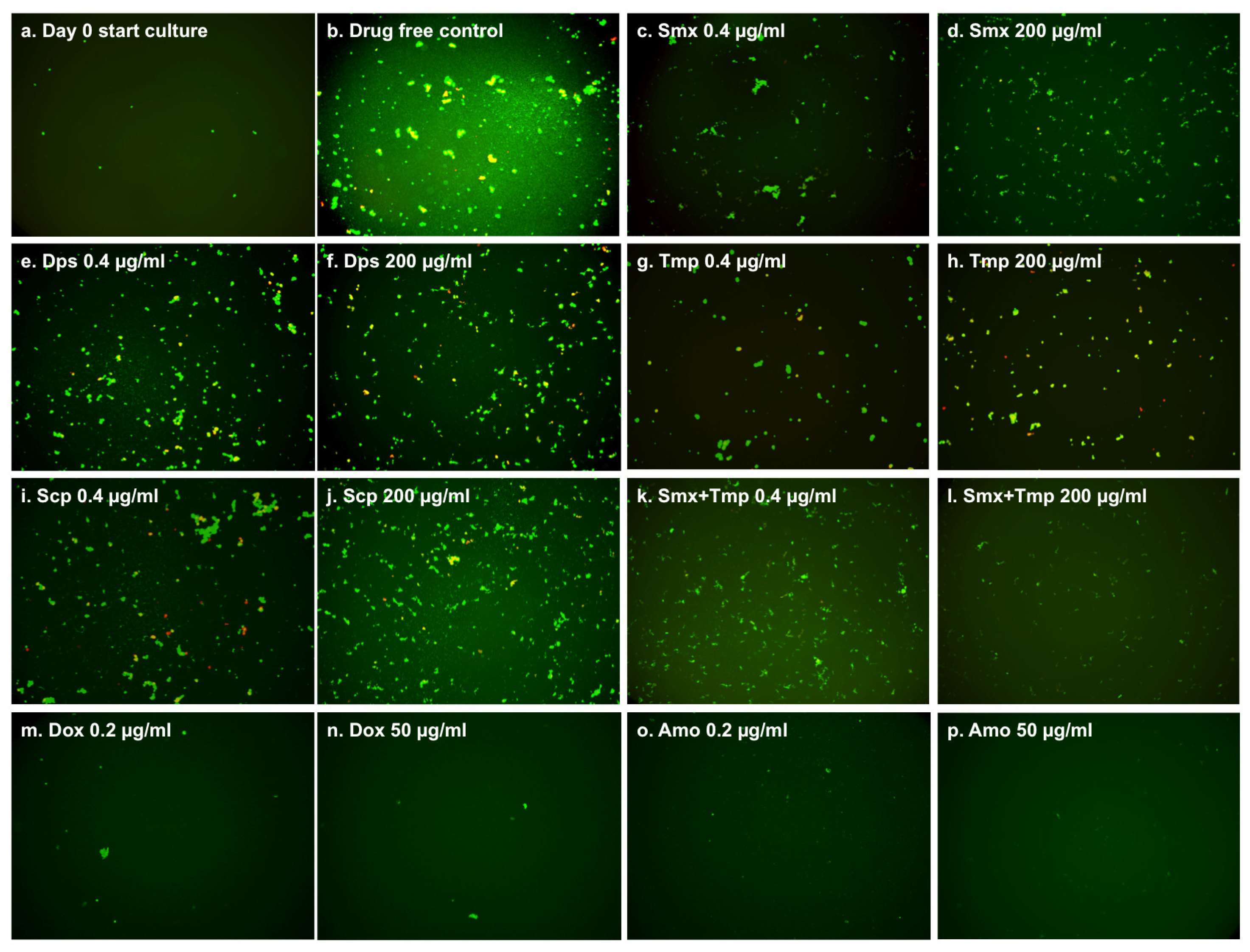
2.4. Effect of Sulfamethoxazole, Dapsone, Sulfachlorpyridazine, and Trimethoprim on Growing B. burgdorferi
3. Experimental Section
3.1. Strain, Media and Culture Techniques
3.2. Drugs
3.3. Microscopy
3.4. Evaluation of Drugs and Drug Combinations for Their Activities against B. burgdorferi Stationary Phase Cultures
3.5. Minimum Inhibitory Concentration (MIC) Determination
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- CDC. Lyme Disease. Available online: http://www.cdc.gov/lyme/ (accessed on 13 September 2015).
- Radolf, J.D.; Caimano, M.J.; Stevenson, B.; Hu, L.T. Of ticks, mice and men: Understanding the dual-host lifestyle of lyme disease spirochaetes. Nat. Rev. Microbiol. 2012, 10, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the infectious diseases society of america. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- CDC. Post-Treatment Lyme Disease Syndrome. Available online: http://www.cdc.gov/lyme/postLDS/index.html (accessed on 18 September 2016).
- Steere, A.C.; Gross, D.; Meyer, A.L.; Huber, B.T. Autoimmune mechanisms in antibiotic treatment-resistant lyme arthritis. J. Autoimmun. 2001, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, L.K.; Gonzalez, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine lyme borreliosis therapy. J. Clin. Investig. 2012, 122, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.J.; Neitzel, D.; Reed, K.D.; Belongia, E.A. Coinfections acquired from ixodes ticks. Clin. Microbiol. Rev. 2006, 19, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, E.; Imai, D.; Feng, S.; Barthold, S.W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE 2014, 9, e86907. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar] [PubMed]
- Marques, A.; Telford, S.R., 3rd; Turk, S.P.; Chung, E.; Williams, C.; Dardick, K.; Krause, P.J.; Brandeburg, C.; Crowder, C.D.; Carolan, H.E.; et al. Xenodiagnosis to detect Borrelia burgdorferi infection: A first-in-human study. Clin. Infect. Dis. 2014, 58, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microb. Infect. 2014. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the causative agent of lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 2015, 59, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Caskey, J.R.; Embers, M.E. Persister development by Borrelia burgdorferi populations in vitro. Antimicrob. Agents Chemother. 2015, 59, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Persister mechanisms in borrelia burgdorferi: Implications for improved intervention. Emerg. Microbes Infect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Sullivan, D.; Zhang, Y. Identification of additional anti-persister activity against Borrelia burgdorferi from an FDA drug library. Antibiotics 2015. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.; Freeman, P. The use of dapsone as a novel “persister” drug in the treatment of chronic lyme disease/post treatment lyme disease syndrome. J. Clin. Exp. Dermatol. Res. 2016. [Google Scholar] [CrossRef]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Zhang, Y. Eradication of biofilm-like microcolony structures of Borrelia burgdorferi by daunomycin and daptomycin but not mitomycin C in combination with doxycycline and cefuroxime. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Purser, J.E.; Norris, S.J. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2000, 97, 13865–13870. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Ctrl | Smx | Smx + Tmp | Dps | Dps + Tmp | Scp | Scp + Tmp | Tmp |
|---|---|---|---|---|---|---|---|---|
| Ctrl | 95% | 84% b | 86% | 83% | 85% | 84% b | 86% b | 84% |
| Dox | 85% | 76% | 76% | 77% | 76% | 76% | 78% | 76% |
| CefU | 83% | 77% | 76% | 77% | 76% | 74% | 76% | 75% |
| Cip | 82% | 78% | 77% | 77% | 77% | 76% | 77% | 77% |
| CefU + Dox | 78% | 76% | 77% | 77% | 77% | 76% | 77% | 77% |
| CefU + Cip | 78% | 76% | 76% | 75% | 76% | 77% | 76% | 74% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Zhang, S.; Shi, W.; Zhang, Y. Activity of Sulfa Drugs and Their Combinations against Stationary Phase B. burgdorferi In Vitro. Antibiotics 2017, 6, 10. https://doi.org/10.3390/antibiotics6010010
Feng J, Zhang S, Shi W, Zhang Y. Activity of Sulfa Drugs and Their Combinations against Stationary Phase B. burgdorferi In Vitro. Antibiotics. 2017; 6(1):10. https://doi.org/10.3390/antibiotics6010010
Chicago/Turabian StyleFeng, Jie, Shuo Zhang, Wanliang Shi, and Ying Zhang. 2017. "Activity of Sulfa Drugs and Their Combinations against Stationary Phase B. burgdorferi In Vitro" Antibiotics 6, no. 1: 10. https://doi.org/10.3390/antibiotics6010010






