Potentially Important Therapeutic Interactions between Antibiotics, and a Specially Engineered Emulsion Drug Vehicle Containing Krill-Oil-Based Phospholipids and Omega-3 Fatty Acids
Abstract
:1. Introduction
2. Bloodstream Infections in the ICU: SIRS, Sepsis, and Endotoxin—A Lethal Combination
2.1. Systemic Inflammatory Response Syndrome (SIRS)
- (1)
- a temperature of >38 °C or <36 °C;
- (2)
- a heart rate of >90/minute;
- (3)
- a respiratory rate of >20/minute (or PaCO2 < 32 mmHg);
- (4)
- a white blood cell count of >12,000/mm3 or <4000/mm3 (or 10% immature bands).
2.2. Sepsis and Endotoxin
3. New Therapeutic Combinations: Antibiotics + BLIs + Phospholipids + Omega-3 Fatty Acids
3.1. Polyfunctional Ingredients
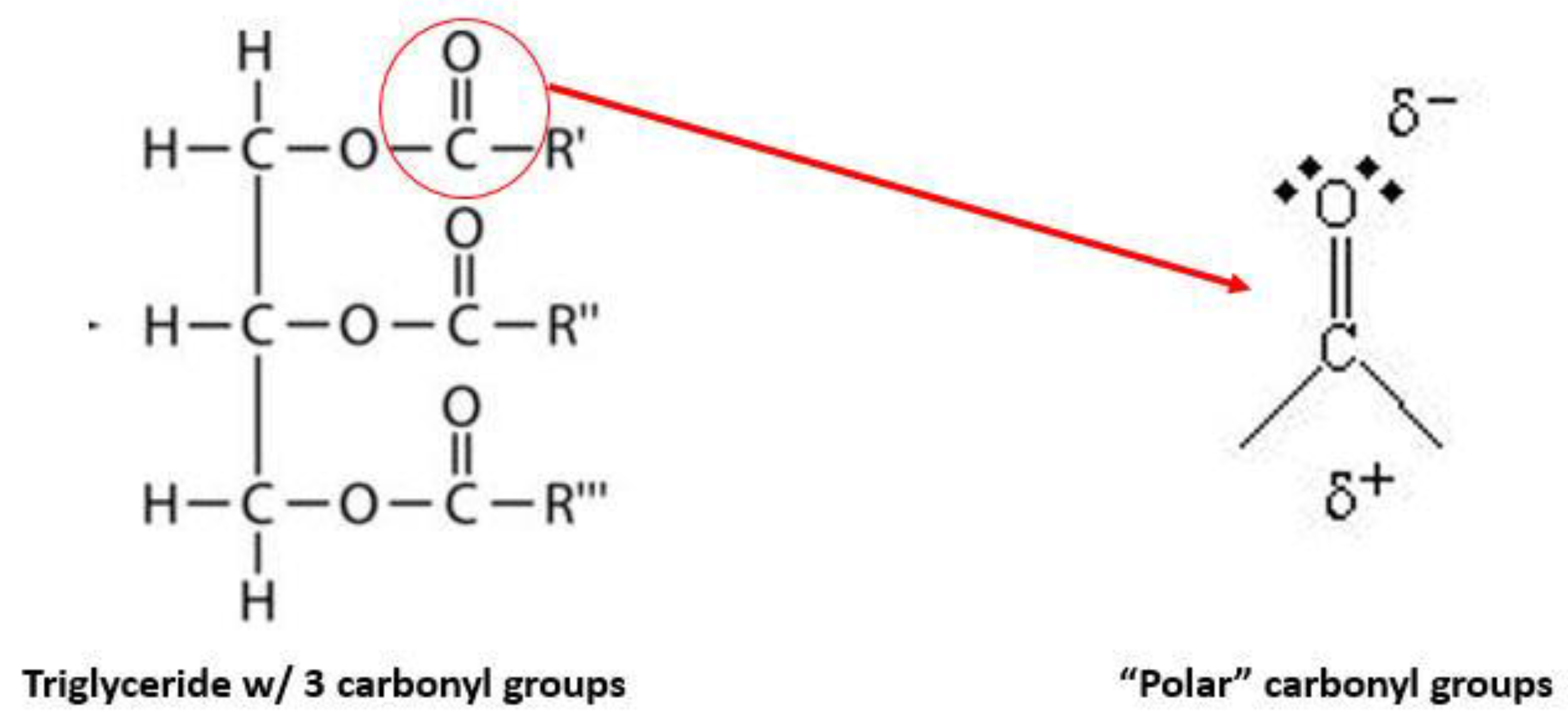
3.1.1. Krill Oil Phospholipids
3.1.2. Fish Oil Triglycerides Enriched in Omega-3 Fatty Acids
3.1.3. Medium Chain Triglyceride Oil
3.1.4. Sodium Oleate
3.1.5. Alpha Tocopherol
4. Conclusions
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| API | active pharmaceutical ingredient |
| PK/PD | pharmacokinetic/pharmacodynamics |
| ICU | intensive care unit |
| SIRS | systemic inflammatory response syndrome |
| CRP | C-reactive protein |
| PGs | prostaglandins |
| TXAs | thromboxanes |
| LKT | leukotrienes |
| FDA | Food and Drug Administration |
| NSAID | nonsteroidal anti-inflammatory drug |
| LA | linoleic acid |
| ARA | arachidonic acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| KO | krill oil |
| PL | phospholipids |
| EP | European Pharmacopoeia |
| MCT | medium chain triglycerides |
| LCT | long chain triglycerides |
References
- Allergan; AstraZeneca; Cipla; DSM Sinochem Pharmaceuticals. Industry Roadmap for Progress on Combating Antimicrobial Resistance; F. Hoffman-La Roche Ltd.: Basel, Switzerland; GSK; Johnson & Johnson; Merck & Co., Inc.: Kenilworth, NJ, USA; Novartis: Basel, Switzerland; Pfizer: New York, NY, USA; Sanofi: Paris, France; Shionogi & Co., Ltd.: Osaka, Japan; Wockhardt: Mumbai, India, 2016. [Google Scholar]
- Talkington, K.; Shore, C.; Kothari, P. A Scientific Roadmap for Antibiotic Discovery; The Pew Charitable Trust: Philadelphia, PA, USA, 2016. [Google Scholar]
- Bush, K.; Page, M.G.P. What we may expect from novel antibacterial agents in the pipeline with respect to resistance and pharmacodynamic principles. J. Pharmacokinet. Pharmacodyn. 2017, 44, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.L.; Papp-Wallace, K.M.; Hujer, A.M.; Domitrovic, T.N.; Hujer, K.M.; Hurless, K.N.; Tuohy, M.; Hall, G.; Bonomo, R.A. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: Resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. A resurgence of β-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int. J. Antimicrob. Agents 2015, 46, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Nicoli, D.F. Analytical methods for determining the size (distribution) in parenteral dispersions. In Non-Biological Complex Drugs; Crommelin, D.J.A., de Vlieger, J.S.B., Eds.; Springer: Cham, Switzerland, 2015; pp. 193–259. [Google Scholar]
- Calder, P.J. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Clin. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874.
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995, 273, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Delodder, F.; Liaudet, L.; Tozzi, P.; Schlaepfer, J.; Chiolero, R.L.; Tappy, L. Three short perioperative infusions of n-3 PUFAs reduce the systemic inflammation induced by cardiopulmonary bypass surgery: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Hacquebard, M.; Portois, L.; Dupont, I.E.; Deckelbaum, R.J.; Malaisse, W.J. Rapid cellular enrichment of eicosapentaenoate after single intravenous injection of a novel medium-chain triacylglycerol:fish oil emulsion in humans. Am. J. Clin. Nutr. 2010, 91, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Dellenger, R.P.; Tomayko, J.F.; Angus, D.C.; Opal, S.; Cupo, M.A.; McDermott, S.; Ducher, A.; Calandra, T.; Cohen, J.; Lipid Infusion and Patient Outcomes in Sepsis (LIPOS) Investigators. Efficacy and safety of a phospholipid emulsion (GR270773) in Gram-negative severe sepsis: Results of a phase II multicenter, randomized, placebo-controlled, dose defining trial. Crit. Care Med. 2009, 37, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Clementi, A.; Brocca, A.; Ronco, C. Endotoxin effects on cardiac and renal functions and cardiorenal syndromes. Blood Purif. 2017, 44, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Klem, D.J.; Foster, D.; Schorr, C.A.; Kazempour, K.; Walker, P.M.; Dellinger, R.P. The EUPHRATES trial (Evaluating the Use of Polymyxin B. Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxinemia and Septic shock): Study protocol for a randomized controlled trial. Trials 2014, 15, 218. [Google Scholar] [CrossRef]
- Iba, T.; Fowler, L. Is polymyxin B-immobilized fiber column ineffective for septic shock? A discussion on the press release for EUPHRATES trial. J. Intensive Care. [CrossRef] [PubMed]
- Bernard, G.R.; Vincent, J.L.; Laterre, P.F.; LaRosa, S.P.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. PROWESS-SHOCK Study Group. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, G.A.; Driscoll, D.; Schwarzbach, H.; Kinscherf, R. Krill oil-in-water emulsion protects against lipopolysaccharide-induced proinflammatory activation of macrophages in vitro. Mar. Drugs 2017, 15, 74. [Google Scholar] [CrossRef]
- Driscoll, D.F. Pharmaceutical and clinical aspects of lipid injectable emulsions. JPEN 2017, 41, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Elsom, R.L.; Calkder, P.C.; Griffin, B.A.; Harris, W.S.; Jebb, S.A.; Lovegrove, J.A.; Moore, C.S.; Riemersma, R.A.; Sanders, T.A. UK Food Strandards Agency Workshop Report: The effects of the dietary n6/n3 fatty acid ratio on cardiovascular health. Br. J. Nutr. 2007, 98, 1305–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, A.R.; Rössler, S.; Litz, R.J.; Stehr, S.N.; Heller, S.C.; Koch, R.; Koch, T. Omega-3 fatty acids improve the diagnosis-related outcome. Crit. Care Med. 2006, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.W.; Thul, P.; Czarnetzki, H.D.; Morlion, B.J.; Kemen, M.; Jauch, K.W. Evaluation of clinical safety and beneficial effects of a fish oil-containing emulsion (Lipoplus, MLF541): Data from a prospective, randomized, multicenter trial. Crit. Care Med. 2007, 35, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Nehne, J.; Peterss, H.; Franke, R.; Bistrian, B.R.; Niemann, W. The influence of medium-chain triglycerides on the stability of all-in-one formulations. Int. J. Pharm. 2002, 240, 1–10. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Vural, J.M.; Carpentier, Y.A.; Deckelbaum, R.J. Incorporation of medium chain triacylglycerols into phospholipid bilayers: Effect of long chain triacyglycerols, cholesterol, and cholesterol esters. J. Lipid Res. 1996, 37, 773–786. [Google Scholar] [PubMed]
- Washington, C.; David, S.S. Aging effects in parenteral fat emulsions: The role of fatty acids. Int. J. Pharm. 1987, 39, 33–37. [Google Scholar] [CrossRef]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Pharmaceutical Function | Therapeutic Function | |
|---|---|---|---|
| 1 | Ceftolozane | --- | β-lactam antibiotic |
| 2 | Tazobactam | --- | β-lactamase inhibitor |
| 3 | krill oil phospholipids | Emulsifier | binds endotoxin; 2nd source of omega-3 fatty acids |
| 4 | enriched fish oil triglycerides | drug vehicle | major source of omega-3 fatty acids |
| 5 | medium chain triglyceride (MCT) oil | drug vehicle | improves lipid clearance |
| 6 | glycerol | tonicity agent | --- |
| 7 | sodium oleate | co-emulsifier | binds endotoxin? |
| 8 | alpha-tocopherol | antioxidant | antioxidant delivery? |
| 9 | sterile water for injection | drug vehicle | --- |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driscoll, D.F. Potentially Important Therapeutic Interactions between Antibiotics, and a Specially Engineered Emulsion Drug Vehicle Containing Krill-Oil-Based Phospholipids and Omega-3 Fatty Acids. Antibiotics 2018, 7, 22. https://doi.org/10.3390/antibiotics7010022
Driscoll DF. Potentially Important Therapeutic Interactions between Antibiotics, and a Specially Engineered Emulsion Drug Vehicle Containing Krill-Oil-Based Phospholipids and Omega-3 Fatty Acids. Antibiotics. 2018; 7(1):22. https://doi.org/10.3390/antibiotics7010022
Chicago/Turabian StyleDriscoll, David F. 2018. "Potentially Important Therapeutic Interactions between Antibiotics, and a Specially Engineered Emulsion Drug Vehicle Containing Krill-Oil-Based Phospholipids and Omega-3 Fatty Acids" Antibiotics 7, no. 1: 22. https://doi.org/10.3390/antibiotics7010022




