Current Status and Future Prospects of Applying Bioinspired Superhydrophobic Materials for Conservation of Stone Artworks
Abstract
:1. Introduction
2. Basic Principles of Wetting States and Superhydrophobicity
2.1. Wetting Properties and Basic Models
2.1.1. Smooth Surface
2.1.2. Rough Surface
2.1.3. Dynamic Wetting Behaviors
3. Fabrication of Superhydrophobic Surfaces
3.1. Chemicals
3.1.1. Silica Nanoparticles
3.1.2. Polydimethylsiloxane (PDMS)
3.1.3. Silanes and Silicones
3.1.4. Titania Nanoparticles
3.2. Fabrication Methods
3.2.1. Sol-gel
3.2.2. Layer-by-Layer Assembly
3.2.3. Solution Immersion
3.2.4. Spray
3.2.5. Other Methods
4. Superhydrophobic Materials Proposed for Stone Conservation
4.1. Working Mechanisms of Superhydrophobic Materials for Stone Conservation
4.2. Materials Proposed for Stone Conservation
5. Droplet Impact on Superhydrophobic surfaces and Wetting Stability
6. Conclusion and Future Prospects
- Development of more types of superamphiphobic compounds for the varied types of stone materials and varied conditions of application, e.g., high and low porous substrates, smooth- and rough-textured stones, dry and humid environment, etc.
- In-depth evaluation of the properties of the newly synthesized products, as well as of the coated surfaces. Understanding the durability of the superamphiphobicity against chemical corrosion, water immersion, mechanical abrasion, UV exposure, etc., is the first step, and the evaluation of the stability and performance durability of the coated surfaces under harsh, open-air environmental conditions (UV, rain, pollution, etc.) is also desired;
- Understanding the variation of the physicochemical properties of different stone materials after coating with the new products through the evaluation of some important parameters such as vapor diffusivity, porosity, and surface color;
- Development of superamphiphobic, self-cleaning coatings with an anti-biofouling property which can prevent micro-biological growth on outdoor stone surfaces. The interest in this topic is increasing, but few studies have been found in the literature.
Author Contributions
Funding
Conflicts of Interest
References
- Ruedrich, J.; Kirchner, D.; Siegesmund, S. Physical weathering of building stones induced by freeze-thaw action: A laboratory long-term study. Environ. Earth. Sci. 2011, 63, 1573–1586. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Marzal, R.M.; Scherer, G.W. Advances in understanding damage by salt crystallization. Acc. Chem. Res. 2010, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, C. Contribution of atmospheric deposition to the formation of damage layers. Sci. Total Environ. 1995, 167, 49–55. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, O. Process of biodegradation: General mechanisms. In Plant Biology for Cultural Heritage; Caneva, G., Nugari, M.P., Salvadori, O., Eds.; The Getty Conservation Institute: Los Angeles, CA, USA, 2009; Chapter 2; pp. 15–55. [Google Scholar]
- Doehne, E.; Price, C.A. Stone Conservation an Overview of Current Research, 2nd ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2010; pp. 20–24. [Google Scholar]
- Amoroso, G.G.; Camaiti, M. Scienza dei Materiali e Restauro; Alinea Editrice: Florence, Italy, 1997; pp. 119–142. [Google Scholar]
- EN 16581:2014. Conservation of Cultural Heritage—Surface Protection for Porous Inorganic Materials—Laboratory Test Methods for the Evaluation of the Performance of Water Repellent Products; BSI: London, UK, 2014. [Google Scholar]
- Charola, A.E. Water-repellent treatments for building stones: A practical overview. APT Bull. 1995, 26, 10–17. [Google Scholar] [CrossRef]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005; Chapter 1; pp. 1–11. [Google Scholar]
- Tabasso, L.M. Acrylic polymers for the conservation of stone: Advantages and drawbacks. APT Bull. 1995, 26, 17–21. [Google Scholar] [CrossRef]
- Ciardelli, F.; Aglietto, M.; Castelvetro, V.; Toniolo, L.; Chiantore, O. Fluorinated polymeric materials for the protection of monumental buildings. Macromol. Symp. 2000, 152, 211–222. [Google Scholar] [CrossRef]
- Piacenti, F.; Pasetti, A.; Matteoli, U.; Strepparola, E. Method for Protecting Stone Materials from Atmospheric Agents by Means of Perfluoropolyether Derivatives. U.S. Patent 4745009A, 17 May 1988. [Google Scholar]
- Piacenti, F.; Camaiti, M. Synthesis and characterization of fluorinated polyetheric amides. J. Fluor. Chem. 1994, 68, 227–235. [Google Scholar] [CrossRef]
- Mazzola, M.; Frediani, P.; Bracci, S.; Salvini, A. New strategy for the synthesis of partially fluorinated acrylic polymers as possible materials for the protection of stone monuments. Eur. Polym. J. 2003, 39, 1995–2003. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Ershad-Langroudi, A. Polymeric coatings for protection of historic monument: Opportunities and challenges. J. Appl. Polym. Sci. 2009, 112, 2535–2551. [Google Scholar] [CrossRef]
- Striani, R.; Frigione, M.; Corcione, C.E. UV-cured siloxane-modified methacrylic system containing hydroxyapatite as potential protective coating for carbonate stones. Prog. Org. Coat. 2013, 76, 1236–1242. [Google Scholar]
- Camaiti, M.; Brizi, L.; Bortolotti, V.; Papacchini, A.; Salvini, A.; Fantazzini, P. An environmental friendly fluorinated oligoamide for producing nonwetting coatings with high performance on porous surfaces. ACS Appl. Mater. Interfaces 2017, 42, 37279–37288. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Oligoamide grafted with perfluoropolyether blocks: A potential protective coating for stone materials. Prog. Org. Coat. 2017, 111, 164–174. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Facile design of “sticky” near superamphiphobic surfaces on highly porous substrate. Mater. Design 2018, 153, 139–152. [Google Scholar] [CrossRef]
- Lazzari, M.; Aglietto, M.; Castelvetro, V.; Chiantore, O. Photochemical stability of partially fluorinated acrylic protective coatings IV. Copolymers of 2,2,2-trifluoroethyl methacrylate and methyl α-trifluoromethyl acrylate with vinyl ethers. Polym. Degrad. Stab. 2003, 79, 345–351. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part II: Photo-oxidative and salts induced weathering of acrylic-silicone mixtures. Polym. Degrad. Stab. 2007, 92, 335–351. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Gu, J. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Cappitelli, F.; Principi, P.; Pedrazzani, R.; Toniolo, L.; Sorlini, C. Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Sci. Total Environ. 2007, 385, 172–181. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Hansen, W.R.; Autumn, K. Evidence for self-cleaning in gecko setae. Proc. Natl. Acad. Sci. USA 2005, 102, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Shirtcliffe, N.; McHale, G.; Newton, M. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2011, 161, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Celia, E.; Darmanin, T.; De Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Wen, L.; Tian, Y.; Jiang, L. Bio-inspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Ed. 2015, 54, 3387–3399. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Repellent materials. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling J. Bioadhesion Biofilm Res. 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, M.; Chen, J.; Wang, J.; Song, Y.; Jiang, L. Anti-icing surfaces based on enhanced self-propelled jump of condensed water microdroplets. Chem. Commun. 2013, 49, 4516–4518. [Google Scholar] [CrossRef] [PubMed]
- Ragesh, P.; Ganesh, V.A.; Nair, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, H.; Wang, X.; Lin, T. A superamphiphobic coating with an ammonia-triggered transition to superhydrophilic and superoleophobic for oil-water separation. Angew. Chem. Int. Ed. 2015, 54, 4527–4530. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Reick, F.G. Substrate Coated with Super-Hydrophobic Layers. U.S. Patent 3931428, 6 January 1976. [Google Scholar]
- Wenzel, R.N. Resistance of solid surface to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Reyssat, M.; Yeomans, J.M.; Quere, D. Impairment of fakir drops. Europhys. Lett. 2008, 81, 26006. [Google Scholar] [CrossRef]
- Bartolo, D.; Bouamrirene, F.; Verneuil, E.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplets: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.C.; Bhushan, B. Dynamic effects induced transition of droplets on biomimetic superhydrophobic surfaces. Langmuir 2009, 25, 9208–9218. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Stein, T.; Whyman, G.; Erlich, M.; Musin, A.; Machavariani, V.; Aurbach, D. Characterization of rough surfaces with vibrated drops. Phys. Chem. Chem. Phys. 2008, 10, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Koishi, T.; Yasuoka, K.; Fujikawa, S.; Ebisuzaki, T.; Zeng, X. Coexistence and transition between Cassie and Wenzel state on pillared hydrophobic surface. Proc. Natl. Acad. Sci. USA 2009, 106, 8435–8440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furmidge, C.G.L. Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Bayer, I.S.; Megaridis, C.M.; Zhang, J.; Gamota, D.; Biswas, A. Analysis and surface energy estimation of various model polymeric surfaces using contact angle hysteresis. J. Adhes. Sci. Technol. 2007, 21, 1439–1467. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Artus, G.R.J.; Jung, S.; Zimmermann, J.; Gautschi, H.P.; Marquardt, K.; Seeger, S. Silicone nanofilaments and their application as superhydrophobic coatings. Adv. Mater. 2006, 18, 2758–2762. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Fujiwara, K.; Kamegawa, T.; Mori, K.; Yamashita, H. An efficient method for the creation of a superhydrophobic surface: Ethylene polymerization over self-assembled colloidal silica nanoparticles incorporating single-site Cr-oxide catalysts. J. Mater. Chem. 2011, 21, 8543–8546. [Google Scholar] [CrossRef]
- Hsieh, C.; Wu, F.; Chen, W. Superhydrophobicity and superoleophobicity from hierarchical silica sphere stacking layers. Mater. Chem. Phys. 2010, 121, 14–21. [Google Scholar] [CrossRef]
- Liang, J.; Wang, L.; He, L.; Sun, S. Pyridine-containing block copolymer/silica core–shell nanoparticles for one-step preparation of superhydrophobic surfaces. Phys. Chem. Chem. Phys. 2013, 15, 10921–10929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Gao, P.; Ma, H.; Wei, Q. Superhydrophobic hybridfilms prepared from silica nanoparticles and ionic liquids via layer-by-layer self-assembly. Thin Solid Films 2014, 570, 27–32. [Google Scholar] [CrossRef]
- Bayiati, P.; Tserepi, A.; Gogolides, E.; Misiakos, K. Selective plasma-induced deposition of fluorocarbon films on metal surfaces for actuation in microfluidics. J. Vac. Sci. Technol. A 2004, 22, 1546–1551. [Google Scholar] [CrossRef]
- Tserepi, A.D.; Vlachopoulou, M.-E.; Gogolides, E. Nanotexturing of poly(dimethylsiloxane) in plasmas for creating robust super-hydrophobic surfaces. Nanotechnology 2006, 17, 3977–3983. [Google Scholar] [CrossRef]
- Sun, M.H.; Luo, C.X.; Xu, L.P.; Ji, H.; Qi, O.Y.; Yu, D.P.; Chen, Y. Artificial lotus leaf by nanocasting. Langmuir 2005, 21, 8978–8991. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Huang, B.; Zhong, M. Fabrication of superhydrophobic and heat-insulating antimony doped tin oxide/polyurethane films by cast replica micromolding. J. Colloid Interface Sci. 2009, 336, 268–272. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Akbulut, O.; Hu, J.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Influence of production method, silicone type and thickness on silicon rubber superhydrophobic coatings. Prog. Org. Coat. 2018, 90, 291–295. [Google Scholar] [CrossRef]
- Joo, J.; Choun, M.; Kim, K.; Uhm, S.; Kim, Y.D.; Lee, J. Controlled water flooding of polymer electrolyte fuel cells applying superhydrophobic gas diffusion layer. Curr. Appl. Phys. 2014, 14, 1374–1379. [Google Scholar] [CrossRef]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-inspired titanium dioxide materials with special wettability and their applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Byanvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. A review on synthesis of nano-TiO2 via different methods. J. Nanostruct. 2013, 3, 1–9. [Google Scholar]
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic surfaces with photocatalytic self-cleaning properties by nanocomposite coating of TiO2 and polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhou, S.; Gu, G.; Wu, L. A facile and large-area fabrication method of superhydrophobic self-cleaning fluorinated polysiloxane/TiO2 nanocomposite coatings with long-term durability. J. Mater. Chem. 2011, 21, 6161–6164. [Google Scholar] [CrossRef]
- Darmanin, T.; De Givenchy, E.T.; Amigoni, S.; Guittard, F. Superhydrophobic surfaces by electrochemical processes. Adv. Mater. 2013, 25, 1378–1394. [Google Scholar] [CrossRef]
- Pozzato, A.; DalZilio, S.; Fois, G.; Vendramin, D.; Mistura, G.; Belotti, M.; Chen, Y.; Natali, M. Superhydrophobic surfaces fabricated by nanoimprint lithography. Microelectron. Eng. 2006, 83, 884–888. [Google Scholar] [CrossRef]
- Woodward, I.; Schofield, W.C.E.; Roucoules, V.; Badyal, J.P.S. Super-hydrophobic surfaces produced by palsma fluorination of polybutadiene films. Langmuir 2013, 19, 3432–3438. [Google Scholar] [CrossRef]
- Liao, K.; Wan, A.; Batteas, J.D.; Bergbreiter, D.E. Superhydrophobic surfaces formed using layer-by-layer self-assembly with aminated multiwall carbon nanotubes. Langmuir 2008, 24, 4245–4253. [Google Scholar] [CrossRef]
- Wu, X.; Fu, Q.; Kumar, D.; Ho, J.W.C.; Kanhere, P.; Zhou, H.; Chen, Z. Mechanically robust superhydrophobic and superoleophobic coatings derived by sol–gel method. Mater. Design 2006, 89, 1302–1309. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silica nanoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 877–879. [Google Scholar] [CrossRef] [Green Version]
- Amigoni, S.; De Givenchy, E.T.; Dufay, M.; Guittard, F. Covalent layer-by-layer assembled superhydrophobic organic−inorganic hybrid films. Langmuir 2009, 25, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, X.; He, J. Self-cleaning antireflective coatings assembled from peculiar mesoporous silica nanoparticles. Langmuir 2010, 26, 13528–132534. [Google Scholar] [CrossRef] [PubMed]
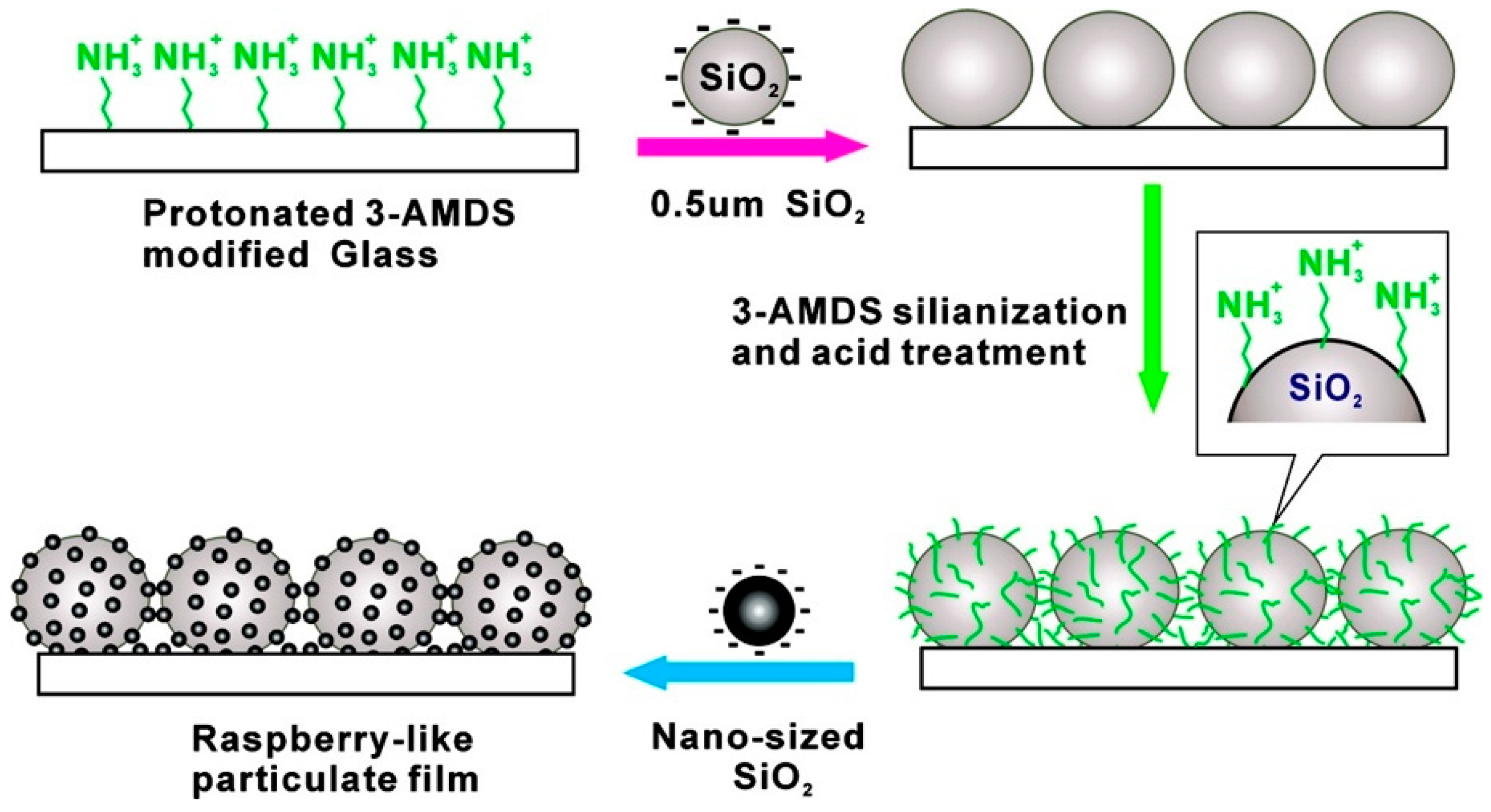
- Tsai, H.; Lee, Y. Facile method to fabricate raspberry-like particulate films for superhydrophobic surfaces. Langmuir 2007, 23, 12687–12692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, H.; Fang, Z.; E, Y.; Wu, T.; Chen, F. Superhydrophobic and conductive properties of carbon nanotubes/polybenzoxazine nanocomposites coated ramie fabric prepared by solution-immersion process. Appl. Surf. Sci. 2014, 309, 218–224. [Google Scholar] [CrossRef]
- Xua, J.; Xu, J.; Cao, Y.; Ji, X.; Yan, Y. Fabrication of non-flaking, superhydrophobic surfaces using a one-step solution-immersion process on copper foams. Appl. Surf. Sci. 2013, 286, 220–227. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. All spray processes for the fabrication of robust, self-healing, superhydrophobic coatings. Adv. Mater. 2014, 26, 3344–3348. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Liu, X.; Zhou, F. Spray-coated fluorine-free superhydrophobic coatings with easy repairability and applicability. ACS Appl. Mater. Interfaces 2009, 1, 1656–1661. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yuan, X. Facile preparation of superhydrophobic coating by spraying a fluorinated acrylic random copolymer micelle solution. Soft Matter 2013, 9, 1005–1009. [Google Scholar] [CrossRef]
- Facio, D.S.; Mosquera, M.J. Simple strategy for producing superhydrophobic nanocomposite coatings in situ on a building substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef]
- Aslanidou, D.; Panayiotou, C.; Karapanagiotis, I. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater Design 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Kota, K.A.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Tuteja, A.; Choi, W.; Ma, M.L.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, Y.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Bioinspired surfaces with superamphiphobic properties: Concepts, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1707415. [Google Scholar] [CrossRef]
- Cao, Y. Stone Artifacts Conservation: Synthesis and Study of New Partially Fluorinated Compounds. Ph.D. Thesis, University of Florence, Florence, Italy, 31 October 2018. [Google Scholar]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles–polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331, 158–165. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing …. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Antonini, C.; Amirfazli, A.; Marengo, M. Drop impact and wettability: From hydrophilic to superhydrophobic surfaces. Phys. Fluids 2012, 24, 102104. [Google Scholar] [CrossRef]
- Gauthier, A.; Symon, S.; Clanet, C.; Quéré, D. Water impacting on superhydrophobic macrotextures. Nat. Commun. 2015, 6, 8001. [Google Scholar] [CrossRef]
- Khojasteh, D.; Kazerooni, M.; Salarian, S.; Kamali, R. Droplet impact on superhydrophobic surfaces: A review of recent developments. J. Ind. Eng. Chem. 2016, 42, 1–14. [Google Scholar] [CrossRef]
- Cao, M.; Guo, D.; Yu, C.; Li, K.; Liu, M.; Jiang, L. Water-repellent properties of superhydrophobic and lubricant- infused “slippery” surfaces: A brief study on the functions and applications. ACS Appl. Mater. Interfaces 2016, 8, 3615–3623. [Google Scholar] [CrossRef]
- De Ruiter, J.; Soto, D.; Varanasi, K.K. Self-peeling of impacting droplets. Nat. Phys. 2018, 14, 35–39. [Google Scholar] [CrossRef]
- Xia, Z.; Xiao, Y.; Yang, Z.; Li, L.; Wang, S.; Liu, X.; Tian, Y. Droplet impact on the super-hydrophobic surface with micro-pillar arrays fabricated by hybrid laser ablation and silanization process. Materials 2019, 12, 765. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Wang, T.; Zhang, L.; Jiang, Y. Droplet impacting dynamics on wettable, rough and slippery oil-infuse surfaces. J. Mech. Sci. Technol. 2020, 34, 219–228. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z. Bouncing droplets on nonsuperhydrophobic surfaces. Phys. Rev. E 2010, 82, 016308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ruiter, J.; Lagraauw, R.; Van den Ende, D.; Mugele, F. Wettability-independent bouncing on flat surfaces mediated by thin air films. Nat. Phys. 2014, 11, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Bahadur, V.; Zhong, S.; Shang, W.; Li, R.; Ruud, J.; Yamada, M.; Ge, L.; Dhinojwala, A.; Sohal, M. Temperature dependent droplet impact dynamics on flat and textured surfaces. Appl. Phys. Lett. 2012, 100, 111601. [Google Scholar] [CrossRef]
- Richard, D.; Clanet, C.; Quéré, D. Surface phenomena: Contact time of a bouncing drop. Nature 2002, 417, 811. [Google Scholar] [CrossRef] [PubMed]
- Clanet, C.; Béguin, C.; Richard, D.; Quéré, D. Maximal deformation of an impacting drop. J. Fluid Mech. 2004, 517, 199–208. [Google Scholar] [CrossRef]
- Bird, J.C.; Dhiman, R.; Kwon, H.-M.; Varanasi, K.K. Reducing the contact time of a bouncing drop. Nature 2013, 503, 385–388. [Google Scholar] [CrossRef]
- Ukiwe, C.; Kwok, D.Y. On the maximum spreading diameter of impacting droplets on well-prepared solid surfaces. Langmuir 2005, 21, 666–673. [Google Scholar] [CrossRef]
- Asai, A.; Shioya, M.; Hirasawa, J.; Okazaki, T. Impact of an ink drop on paper. J. Imaging Sci. Technol. 1993, 37, 205–207. [Google Scholar]
- Li, X.Y.; Mao, L.Q.; Ma, X.H. Dynamic behavior of water droplet impact on microtextured surfaces: The effect of geometrical parameters on anisotropic wetting and the maximum spreading diameter. Langmuir 2013, 29, 1129–1138. [Google Scholar] [CrossRef]
- Moevius, L.; Liu, Y.; Wang, Z.; Yeomans, J.M. Pancake bouncing: Simulations and theory and experimental verification. Langmuir 2014, 30, 13021–13032. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisensee, P.B.; Tian, J.; Miljkovic, N.; King, W.P. Water droplet impact on elastic superhydrophobic surfaces. Sci. Rep. 2016, 6, 30328. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Bhushan, B. Dynamic effects of bouncing water droplets on superhydrophobic surfaces. Langmuir 2008, 24, 6262–6269. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Nam, Y.; Lastakowski, H.; Hur, J.I.; Shin, S.; Biance, A.-L.; Pirat, C.; ‘‘CJ’’ Kim, C.-J.; Ybert, C. Two types of Cassie-to-Wenzel wetting transitions on superhydrophobic surfaces during drop impact. Soft Matter 2015, 11, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Bi, P.; Li, H.; Zhao, G.; Ran, M.; Cao, L.; Guo, H.; Xue, Y. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef]
- Heinonena, S.; Huttunen-Saarivirta, E.; Nikkanen, J.-P.; Raulio, M.; Priha, O.; Laakso, J.; Storgårds, E.; Levänen, E. Antibacterial properties and chemical stability of superhydrophobic silver-containing surface produced by sol–gel route. Colloid. Surf. A 2014, 453, 149–161. [Google Scholar] [CrossRef]
- Du, C.; He, X.; Tian, F.; Bai, X.; Yuan, C. Preparation of superhydrophobic steel surfaces with chemical stability and corrosion. Coatings 2019, 9, 398. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Guo, Z. Biomimetic super durable and stable surfaces with superhydrophobicity. J. Mater. Chem. A 2018, 6, 16731–16768. [Google Scholar] [CrossRef]
- Wang, C.-F.; Wang, Y.-T.; Tung, P.-H.; Kuo, S.-W.; Lin, C.-H.; Sheen, Y.-C.; Chang, F.-C. Stable superhydrophobic polybenzoxazine surfaces over a wide pH range. Langmuir 2006, 22, 8289–8292. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.; Bayer, I.; Loth, E. Adhesion Strength and superhydrophobicity of polyurethane/organoclay nanocomposite coatings. J. Appl. Polym. Sci. 2012, 125, E445–E452. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of rare-earth oxide ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. Superhydrophobic fluorinated oligomers as protective agents for outdoor stone artworks. J. Cult. Herit. 2020. [Google Scholar] [CrossRef]








| Product | Chemical Composition | Static CA (°) | Hysteresis (CAH) |
|---|---|---|---|
| Untreated | – | 14 ± 2 | – |
| BS290 | Silane, siloxane mixture from Wacker | 131 ± 11 | 22 ± 3 |
| UCA-TP | TES 40 EN, PMDS, and surfactant | 140 ± 3 | 13 ± 1 |
| UCA-TPS | TES 40 EN, PMDS, surfactant, and colloidal silica particles | 149 ± 2 | 7 ± 1 |
| Absorption Time (h) | Amount of Water Absorbed (g) | Inhibition Efficacy (%) | |
|---|---|---|---|
| Before Hydrophobization | After Hydrophobization | ||
| 0.5 | 6.92 | 0.09 | 99 |
| 1 | 9.79 | 0.15 | 98 |
| 2 | 10.12 | 0.26 | 97 |
| 24 | 10.30 | 0.80 | 92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Salvini, A.; Camaiti, M. Current Status and Future Prospects of Applying Bioinspired Superhydrophobic Materials for Conservation of Stone Artworks. Coatings 2020, 10, 353. https://doi.org/10.3390/coatings10040353
Cao Y, Salvini A, Camaiti M. Current Status and Future Prospects of Applying Bioinspired Superhydrophobic Materials for Conservation of Stone Artworks. Coatings. 2020; 10(4):353. https://doi.org/10.3390/coatings10040353
Chicago/Turabian StyleCao, Yijian, Antonella Salvini, and Mara Camaiti. 2020. "Current Status and Future Prospects of Applying Bioinspired Superhydrophobic Materials for Conservation of Stone Artworks" Coatings 10, no. 4: 353. https://doi.org/10.3390/coatings10040353




