Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process and Reaction Principle
2.2.1. Synthesis of Epoxy Modified Silicate Emulsion
2.2.2. Formulation and Preparation of the Zinc-Rich Coating
2.3. Measurement and Characterization
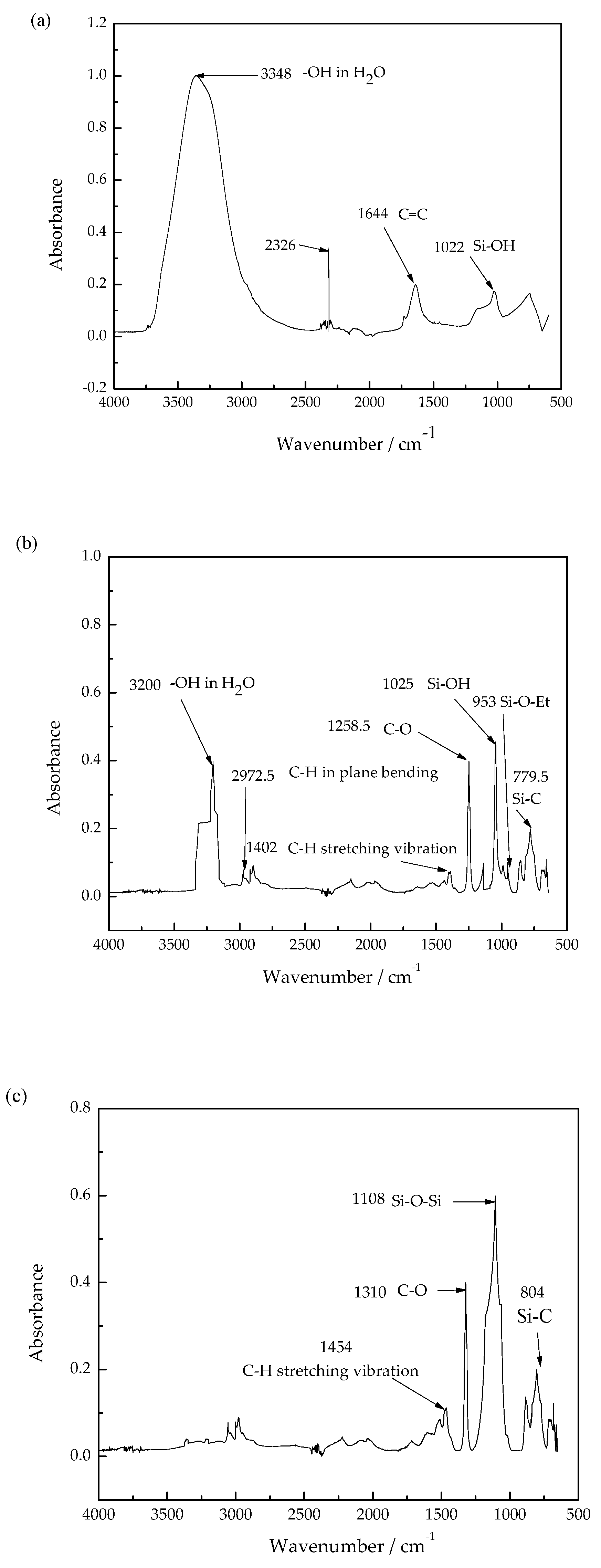
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Determination of Solid Content
2.3.3. Basic Mechanical Properties
2.3.4. Film Thickness Measurement
2.3.5. Immersion Test in 3.5% NaCl Solution
2.3.6. Electrochemical Test
3. Results
3.1. Emulsion Properties
3.2. Chemical Structure
3.3. Basic Properties of the Silicate Coatings
3.4. Corrosion Performance of the Coatings
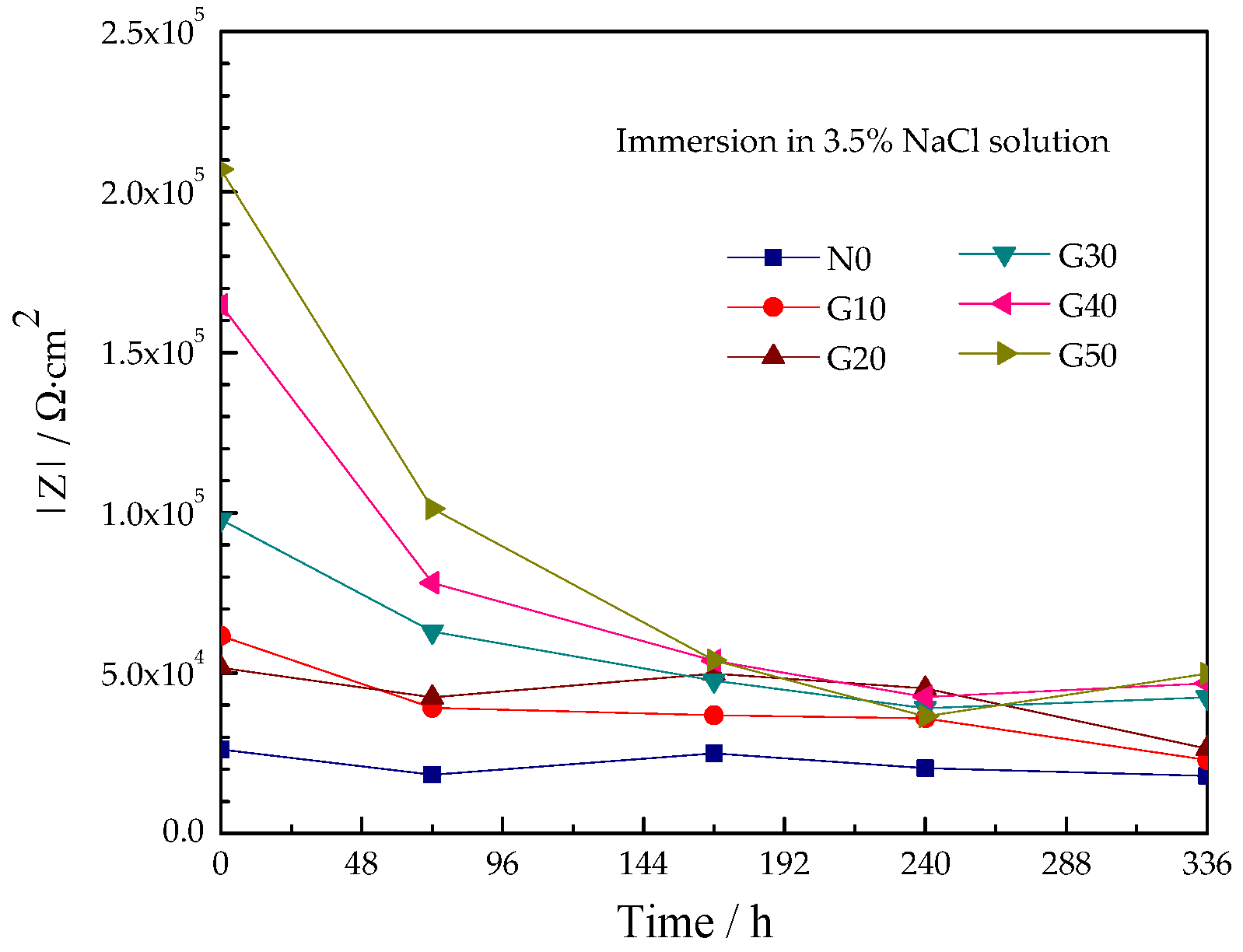
3.4.1. Effect of Epoxy Content on Corrosive Performance
3.4.2. Corrosive Performance of Coatings with Immersion Time
3.4.3. Analysis of Electrochemical Parameters of Coating Equivalent Circuit
3.5. Anticorrosive Mechanism of Zinc Rich Coatings
4. Conclusions
- Epoxy modified silicate emulsions were successfully synthesized. The viscosity and solid content of the modified emulsion increased with epoxy content.
- Compared with the silicate coating, epoxy modified silicate coatings had the best adhesion (grade 1), lower hardness and higher impact resistance, were more compact and had better durability.
- The impedance of the silicate zinc-rich coating was 3 × 104 Ω⋅cm2 and increased with the epoxy content in the modified silicate zinc-rich coating, so that the G50 coating was only about 2 × 105 Ω⋅cm2. Modification with epoxy did not harm the sacrificial anode effect of zinc powder in the coating.
- Epoxy modified silicate zinc-rich coatings had a lower capacitance of the coating (i.e., lower water absorption and better insulating effect than those of the silicate coatings). Thus, they can provide longer lifetime protection than silicate ones.
Author Contributions
Funding
Conflicts of Interest
References
- Munger, C.G.; Vincent, L.D. Corrosion Prevention by Protective Coatings, 2nd ed.; NACE Publishing: Houston, TX, USA, 1999. [Google Scholar]
- Shreepathi, S.; Bajaj, P.; Mallik, B.P. Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: Role of Zn content on corrosion protection mechanism. Electrochim. Acta. 2010, 55, 5129–5134. [Google Scholar] [CrossRef]
- Merlatti, C.; Perrin, F.X.; Aragon, E.; Margaillan, A. Evaluation of physico-chemical changes in sub-layers of multi-layer anticorrosive marine paint systems: Plasticizer and solvent release. Prog. Org. Coat. 2008, 61, 53–62. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of electrochemical action of zinc-rich paints by addition of nanoparticulate zinc. Corros. Sci. 2013, 66, 380. [Google Scholar] [CrossRef]
- Marchebois, H.; Savall, C.; Bernard, J.; Touzain, S. Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta 2004, 49, 2945. [Google Scholar] [CrossRef]
- Zeng, D.F.; Tao, N.W.; Jiang, S.W.; Wang, J.N. NORSOK M-501 test method for heavy-duty anticorrosive marine coatings. Coat. Ind. 2015, 45, 51. [Google Scholar]
- Del Amo, B.; Romagnoli, R.; Deyá, C.; González, J.A. High performance water-based paints with non-toxic anticorrosive pigments. Prog. Org. Coat. 2002, 45, 389–397. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.Q.; Wei, T.; Xiao, C.J. Research status and prospects of water-based anticorrosive coatings. Shanghai Coat. 2012, 50, 37–41. [Google Scholar]
- Chen, L.; Wang, Z.Q.; Wei, T.; Xiao, C.J. Research on the modification of water-borne inorganic zinc-rich coatings and its performances. Adv. Mater. Res. 2015, 1095, 626–630. [Google Scholar] [CrossRef]
- Izquierdo, M.; Novoa, X.R.; Pena, G.; Espada, L. The mechanism of protection of zinc-rich inorganic coatings. A study based on electrochemical impedance spectroscopy (EIS). Mater. Sci. Forum 1992, 111–112, 257–268. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Ebrahimi, M.; Sharif, F.; Attar, M.M.; Faridi, H.R. Synthesis and evaluating corrosion protection effects of emeraldine base PAni/clay nanocomposite as a barrier pigment in zinc-rich ethyl silicate primer. Prog. Org. Coat. 2011, 70, 39–44. [Google Scholar] [CrossRef]
- Yun, T.H.; Park, J.H.; Kim, J.S.; Park, J.M. Effect of the surface modification of zinc powders with organosilanes on the corrosion resistance of a zinc pigmented organic coating. Prog. Org. Coat. 2014, 77, 1780–1788. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Feng, Y.; Keyao, Z. Development and Construction of New Waterborne Zinc-rich Coatings. Shanghai Coatings 2006, 44, 4–8. [Google Scholar]
- Wu, G.M.; Kong, Z.W.; Chen, J.; Huo, S.P.; Liu, G.F. Preparation and properties of waterborne polyurethane/epoxy resin composite coating from anionic terpene-based polyol dispersion. Prog. Org. Coat. 2014, 77, 315–321. [Google Scholar] [CrossRef]
- Shengchuang, S. Study on Preparation and Performance of Waterborne Silicate Inorganic Zinc-Rich Anticorrosive Coatings. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2017. [Google Scholar]
- Yanyun, L. Research on Water-Based Inorganic Zinc-Rich Coatings. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2008. [Google Scholar]
- Ross, T.K.; Wolstenholme, J. Anti-corrosion properties of zinc dust paints. Corros. Sci. 1977, 17, 341–351. [Google Scholar] [CrossRef]
- GB/T 1720-1979 Method of Test for Adhesion of Paint Films; China Petroleum and Chemical Industry Federation: Beijing, China, 1980.
- GB/T 6739-2006 Paints and Varnishes-Determination of Film Hardness by Pencil Test; China Petroleum and Chemical Industry Federation: Beijing, China, 2007.
- GB/T 1732-1993 Determination of Impact Resistance of Film; China Petroleum and Chemical Industry Federation: Beijing, China, 1993.
- Qi, Y.H.; Zhang, Z.P.; Miao, M.; Zhang, X.Z. Studies on estimating methods of polarization performance for coated steel in seawater. Mater. Sci. Forum 2010, 654–656, 2418–2421. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Wang, X.T.; Hou, B.R. Surface modification of nano-ZrO2 nanoparticles with styrene coupling agent and its effect on the corrosion behaviour of epoxy coating. Chin. J. Oceanol. Limnol. 2014, 32, 1163–1171. [Google Scholar] [CrossRef]
- Shuyan, Z.H.; Xinhong, T.O.; Fuchun, L.I.; Jinyu, W.E.; En-Hou, H.A.; Xiaohui, L.I.; Lin, Y.A. Corrosion Resistance of Three Zinc-rich Epoxy Coatings. J. Chin. Soc. Corros. Prot. 2019, 39, 563–570. [Google Scholar]














| Raw Materials/wt.% | G10 | G20 | G30 | G40 | G50 |
|---|---|---|---|---|---|
| E51 | 9.5 | 18.1 | 26 | 33.3 | 40 |
| LT550 | 5.0 | 9.5 | 13 | 16.7 | 20 |
| E777-2 | 85.5 | 72.4 | 61 | 50 | 40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qi, Y.; Zhao, X.; Zhang, Z. Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution. Coatings 2020, 10, 444. https://doi.org/10.3390/coatings10050444
Wang J, Qi Y, Zhao X, Zhang Z. Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution. Coatings. 2020; 10(5):444. https://doi.org/10.3390/coatings10050444
Chicago/Turabian StyleWang, Jingtao, Yuhong Qi, Xu Zhao, and Zhanping Zhang. 2020. "Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution" Coatings 10, no. 5: 444. https://doi.org/10.3390/coatings10050444





