Coating and Surface Treatments on Orthodontic Metallic Materials
Abstract
:1. Introduction
| Element | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Ti6Al4V |
|---|---|---|---|---|---|
| Nitrogen | 0.03 | 0.03 | 0.05 | 0.05 | 0.05 |
| Carbon | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Hydrogen | 0.015 | 0.015 | 0.015 | 0.015 | 0.0125 |
| Iron | 0.20 | 0.30 | 0.30 | 0.50 | 0.25 |
| Oxygen | 0.18 | 0.25 | 0.35 | 0.40 | 0.13 |
| Aluminium | -- | -- | -- | -- | 5.50–6.50 |
| Vanadium | -- | -- | -- | -- | 3.50–4.50 |
| Titanium | Balance | ||||
| Alloy/Element | AISI series | C | Cr | Ni | Mo | Mn | S | P | Si | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferritic | 4XX | 0.12 | 12–29 | <2 | -- | 1 | <0.03 | <0.04 | <1 | Balance |
| Martensitic | 4XX | 0.15–1 | 12–18 | >0.75 | -- | <1 | <0.03 | <0.04 | <1 | |
| Austenitic | 3XX | 0.02–0.05 | 17–20 | 8–12 | 2 (316–316L) | <2 | <0.015 | <0.04 | <1 | |
| Duplex | 2205 | <0.03 | 18–26 | 4.5–6.5 | 2.5–3.5 | <2 | <0.02 | <0.03 | <1 | |
| Precipitation-hardening | 630 (17–4) | 0.07 | 15.5–17.5 | 3-5 | 0.06 | 1.5 | 0.02 | 0.04 | 0.7 |
2. Coating Techniques and Materials
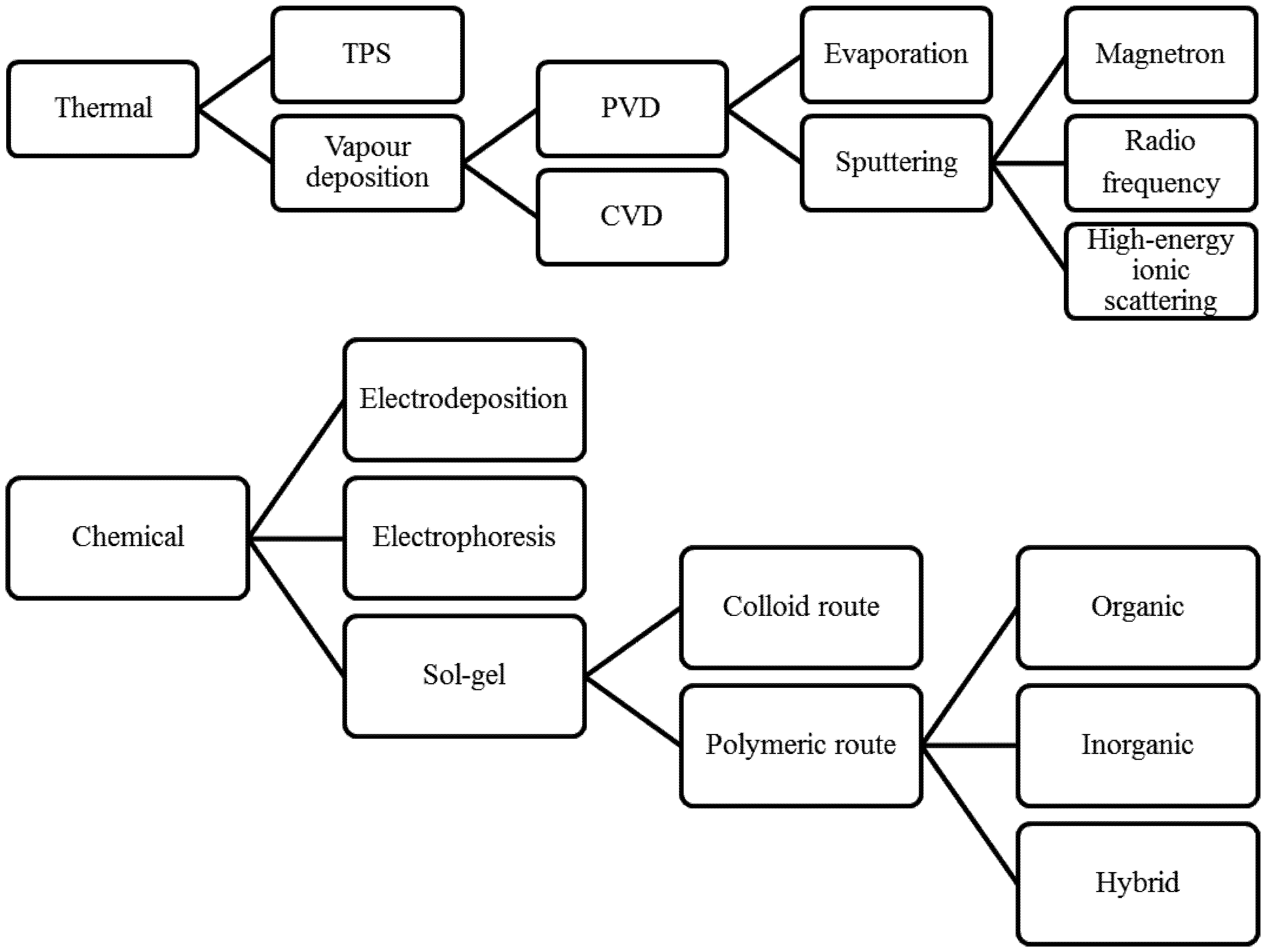
2.1. Thermal Methods
2.1.1. Thermal Plasma Spray (TPS)
2.1.2. Chemical Vapor Deposition (CVD)
2.1.3. Physical Vapor Deposition (PVD)
2.1.3.1. Evaporation
2.1.3.2. Physical Sputtering
2.2. Chemical Methods
2.2.1 Electrodeposition
2.3. Sol-Gel Method

3. Surface Modification
4. Conclusions
Acknowledgments
References
- Park, J.B.; Kim, Y.K. Metallic Biomaterials. In The Biomedical Engineering Handbook, 2nd; Bronzino, J.D., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- García, C. Bioactivación de metales de uso ortopédico mediante recubrimientos producidos por sol-gel. Ph.D. Thesis, Universidad Nacional de Colombia, Medellín, Colombia, 2004. [Google Scholar]
- Brantley, W.A.; Eliades, T. Orthodontic Materials: Scientific and Clinical Aspects; Thieme: New York, NY, USA, 2001. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef]
- El-Zohairy, M.A.; Mostafa, A.; Amin, A.; Abd El-Fattah, H.; Khalifa, S. Mandibular reconstruction using pectoralis major myocutaneous flap and titanium plates after ablative surgery for locally advanced tumors of the oral cavity. J. Egypt Natl. Canc. Inst. 2009, 21, 299–307. [Google Scholar]
- Lethaus, B.; Kessler, P.; Boeckman, R.; Poort, L.J.; Tolba, R. Reconstruction of a maxillary defect with a fibula graft and titanium mesh using CAD/CAM techniques. Head Face Med. 2010, 6, 16. [Google Scholar] [CrossRef]
- Stoetzer, M.; Rana, M.; von See, C.; Eckardt, A.M.; Gellrich, N.-C. Reconstruction of defects of maxillary sinus wall after removal of a huge odontogenic lesion using prebended 3D titanium-mesh and CAD/CAM technique. Head Face Med. 2011, 7, 21. [Google Scholar] [CrossRef]
- Nakajima, H.; Okabe, T. Titanium in dentistry: Development and research in the USA. Dent. Mater. J. 1996, 15, 77–90. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Luersen, M.A.; Borges, P.C. Nickel-titanium alloys: A systematic review. J. Orthod. 2012, 17, 71–82. [Google Scholar]
- Parvizi, F.; Rock, W.P. The load/deflection characteristics of thermally activated orthodontic archwires. Eur. J. Orthod. 2003, 25, 417–421. [Google Scholar] [CrossRef]
- ASTM F136-12a Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNS R56401). Available online: http://www.astm.org/Standards/F136.htmn (accessed on 19 December 2012).
- ASTM F67-06 Standard Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700). Available online: http://www.astm.org/Standards/F67.htm (accessed on 19 December 2012).
- Sfondrini, M.F.; Cacciafesta, V.; Maffia, E.; Massironi, S.; Scribante, A.; Alberti, G.; Biesuz, R.; Klersy, C. Chromium release from new stainless steel, recycled and nickel-free orthodontic brackets. Angle Orthod. 2009, 79, 361–367. [Google Scholar] [CrossRef]
- Kohl, R. Metallurgy in orthodontics. Angle Orthod. 1964, 34, 37–52. [Google Scholar]
- Roach, M. Base metal alloys used for dental restorations and implants. Dent. Clin. North Am. 2007, 51, 603–627. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Jang, S.G.; Oh, K.T.; Kim, J.G.; Kim, Y.S. Influences of passivating elements on the corrosion and biocompatibility of super stainless steels. J. Biomed. Mater. Res. B 2008, 86, 310–320. [Google Scholar]
- Anusavice, K.J. Phillips Ciencia de los Materiales Dentales, 11th ed; Elsevier España: Madrid, Spain, 2004. [Google Scholar]
- Izquierdo, P.P.; de Biasi, R.S.; Elias, C.N.; Nojima, L.I. Martensitic transformation of austenitic stainless steel orthodontic wires during intraoral exposure. Am. J. Orthod. Dentofacial. Orthop. 2010, 138. [Google Scholar] [CrossRef]
- Kocijan, A.; Conradi, M. The corrosion behaviour of austenitic and duplex stainless steels in artificial body fluids. Mater. Technol. 2010, 44, 21–24. [Google Scholar]
- Cardarelli, F. Materials Handbook: A Concise Desktop Reference, 2nd ed; Springer: London, UK, 2008. [Google Scholar]
- Craig, H. Stress Corrosion—New Approaches; ASTM International: West Conshohocken, PA, USA, 1976. [Google Scholar]
- Dionicio Padilla, E. Aplicaciones de los aceros inoxidables. Rev. Inst. Investig. Fac. Minas. Metal Cienc. Geogr. 1999, 2, 11–21. [Google Scholar]
- Mistakidis, I.; Gkantidis, N.; Topouzelis, N. Review of properties and clinical applications of orthodontic wires. Hell. Orthod. Rev. 2011, 14, 45–66. [Google Scholar]
- Oh, K.T.; Choo, S.U.; Kim, K.M.; Kim, K.N. A stainless steel bracket for orthodontic application. Eur. J. Orthod. 2005, 27, 237–244. [Google Scholar] [CrossRef]
- Soratur, S. Essentials of Dental Materials; Jaypee: New Delhi, India, 2002. [Google Scholar]
- Costa, M.T.; Lenza, M.A.; Gosch, C.S.; Costa, I.; Ribeiro-Dias, F. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J. Dent. Res. 2007, 86, 441–445. [Google Scholar] [CrossRef]
- Edie, J.W.; Andreasen, G.F.; Zaytoun, M.P. Surface corrosion of nitinol and stainless steel under clinical conditions. Angle Orthod. 1981, 51, 319–324. [Google Scholar]
- Kim, H.; Johnson, J.W. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999, 69, 39–44. [Google Scholar]
- Schmalz, G.; Arenholt-Bindslev, D. Biocompatibility of Dental Materials; Springer: Berlin, Germany, 2009. [Google Scholar]
- Javaherdashti, R. Microbiologically Influenced Corrosion. An Engineered Insight; Springer: London, UK, 2008. [Google Scholar]
- Anandkumar, B.; Maruthamuthu, S. Molecular identification and corrosion behaviour of manganese oxidizers on orthodontic wires. Curr. Sci. 2008, 94, 891–896. [Google Scholar]
- House, K.; Sernetz, F.; Dymock, D.; Sandy, J.R.; Ireland, A.J. Corrosion of orthodontic appliances—Should we care? Am. J. Orthod. Dentofacial Orthop. 2008, 133, 584–592. [Google Scholar] [CrossRef]
- Matos de Souza, R.; Macedo de Menezes, L. Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances. Angle Orthod. 2008, 78, 345–350. [Google Scholar] [CrossRef]
- Ehrnrooth, M.; Kerosuo, H. Face and neck dermatitis from a stainless steel orthodontic appliance. Angle Orthod. 2009, 79, 1194–1196. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Jacobsen, N.; Hensten-Pettersen, A. Changes in occupational health problems and adverse patient reactions in orthodontics from 1987 to 2000. Eur. J. Orthod. 2003, 25, 591–598. [Google Scholar] [CrossRef]
- Kanerva, L.; Sipiläinen-Malm, T.; Estlander, T.; Zitting, A.; Jolanki, R.; Tarvainen, K. Nickel release from metals, and a case of allergic contact dermatitis from stainless steel. Contact Dermatitis 1994, 31, 299–303. [Google Scholar] [CrossRef]
- Noble, J.; Ahing, S.I.; Karaiskos, N.E.; Wiltshire, W.A. Nickel allergy and orthodontics, a review and report of two cases. Br. Dent. J. 2008, 204, 297–300. [Google Scholar]
- Ağaoğlu, G.; Arun, T.; Izgi, B.; Yarat, A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001, 71, 375–379. [Google Scholar]
- Amini, F.; Borzabadi Farahani, A.; Jafari, A.; Rabbani, M. In vivo study of metal content of oral mucosa cells in patients with and without fixed orthodontic appliances. Orthod. Craniofac. Res. 2008, 11, 51–56. [Google Scholar] [CrossRef]
- Petoumenou, E.; Arndt, M.; Keilig, L.; Reimann, S.; Hoederath, H.; Eliades, T.; Jäger, A.; Bourauel, C. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 59–65. [Google Scholar] [CrossRef]
- Noble, J.; Ahing, S.I.; Karaiskos, N.E.; Wiltshire, W.A. Should I be concerned if a patient requiring orthodontic treatment has an allergy to nickel? J. Can. Dent. Assoc. 2008, 74, 897–898. [Google Scholar]
- Balenseifen, J.W.; Madonia, J.V. Study of dental plaque in orthodontic patients. J. Dent. Res. 1970, 49, 320–324. [Google Scholar] [CrossRef]
- Da Cunha, A.C.; Marquezan, M.; Freitas, A.O.; Nojima, L.I. Frictional resistance of orthodontic wires tied with 3 types of elastomeric ligatures. Braz. Oral Res. 2011, 25, 526–530. [Google Scholar] [CrossRef]
- Angolkar, P.V.; Kapila, S.; Duncanson, M.G., Jr.; Nanda, R.S. Evaluation of friction between ceramic brackets and orthodontic wires of four alloys. Am. J. Orthod. Dentofacial Orthop. 1990, 98, 499–506. [Google Scholar]
- Kapila, S.; Angolkar, P.V.; Duncanson, M.G., Jr.; Nanda, R.S. Evaluation of friction between edgewise stainless steel brackets and orthodontic wires of four alloys. Am. J. Orthod. Dentofacial Orthop. 1990, 98, 117–126. [Google Scholar] [CrossRef]
- Burrow, S.J. Friction and resistance to sliding in orthodontics: A critical review. Am. J. Orthod. Dentofacial Orthop. 2009, 135, 442–447. [Google Scholar] [CrossRef]
- Guerrero, A.P.; Guariza Filho, O.; Tanaka, O.; Camargo, E.S.; Vieira, S. Evaluation of frictional forces between ceramic brackets and archwires of different alloys compared with metal brackets. Braz. Oral Res. 2010, 24, 40–45. [Google Scholar] [CrossRef]
- Bandeira, A.M.; dos Santos, M.P.; Pulitini, G.; Elias, C.N.; da Costa, M.F. Influence of thermal or chemical degradation on the frictional force of an experimental coated NiTi wire. Angle Orthod. 2011, 81, 484–489. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Hirakuri, K.; Aoki, H. Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires. J. Mater. Sci. Mater. Med. 2007, 18, 2263–2268. [Google Scholar] [CrossRef]
- Demling, A.; Elter, C.; Heidenblut, T.; Bach, F.W.; Hahn, A.; Schwestka-Polly, R.; Stiesch, M.; Heuer, W. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur. J. Orthod. 2010, 32, 414–418. [Google Scholar] [CrossRef]
- Peláez-Vargas, A. Evaluación de la toxicidad in vitro, la adherencia y la nanotopografía de recubrimientos aplicados por sol-gel para implantes metálicos. Master’s Thesis, National University of Colombia, Medellín, Colombia, 2005. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Chu, P.K. Plasma-treated biomaterials. IEEE Trans. Plasma Sci. 2007, 35, 181–187. [Google Scholar] [CrossRef]
- Junker, R.; Manders, P.J.; Wolke, J.; Borisov, Y.; Braceras, I.; Jansen, J.A. Loaded microplasma-sprayed CaP-coated implants in vivo. J. Dent. Res. 2010, 89, 1489–1493. [Google Scholar] [CrossRef]
- Prymak, O.; Bogdansk, D.; Esenwein, S.A.; Köller, M.; Epple, M. NiTi shape memory alloys coated with calcium phosphate by plasma-spraying. Chemical and biological properties. Materialwiss. Werkstofftech. 2004, 35, 346–351. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K. A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Park, J.H.; Sudarshan, T.S. Chemical Vapor Deposition; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Tripi, T.R.; Bonaccorso, A.; Condorelli, G.G. Fabrication of hard coatings on NiTi instruments. J. Endod. 2003, 29, 132–134. [Google Scholar] [CrossRef]
- Tripi, T.R.; Bonaccorso, A.; Rapisarda, E.; Tripi, V.; Condorelli, G.G.; Marino, R.; Fragalà, I. Depositions of nitrogen on NiTi instruments. J. Endod. 2002, 28, 497–500. [Google Scholar] [CrossRef]
- Borges, C.F.; Magne, P.; Pfender, E.; Heberlein, J. Dental diamond burs made with a new technology. J. Prosthet. Dent. 1999, 82, 73–79. [Google Scholar] [CrossRef]
- Lima, L.M.; Motisuki, C.; dos Santos-Pinto, L.; dos Santos-Pinto, A.; Corat, E.J. Cutting characteristics of dental diamond burs made with CVD technology. Braz. Oral Res. 2006, 20, 155–161. [Google Scholar]
- Mattox, D.M. Handbook of Physical Vapor Deposition Processing, 2nd ed; Elsevier: Burlington, VT, USA, 2010. [Google Scholar]
- Ryu, H.S.; Bae, I.H.; Lee, K.G.; Hwang, H.S.; Lee, K.H.; Koh, J.T.; Cho, J.H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Krishnan, V.; Ravikumar, K.K.; Sukumaran, K.; Kumar, K.J. In vitro evaluation of physical vapor deposition coated beta titanium orthodontic archwires. Angle Orthod. 2012, 82, 22–29. [Google Scholar] [CrossRef]
- Krishnan, V.; Krishnan, A.; Remya, R.; Ravikumar, K.K.; Nair, S.A.; Shibli, S.M.; Varma, H.K.; Sukumaran, K.; Kumar, K.J. Development and evaluation of two PVD-coated beta-titanium orthodontic archwires for fluoride-induced corrosion protection. Acta Biomater. 2011, 7, 1913–1927. [Google Scholar]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials. Synthesis, Properties and Applications, 2nd ed; World Scientific Publishing: Singapore, 2011. [Google Scholar]
- Vásquez, A.; Damborenea, J. Ciencia e Ingeniería de la Superficie de los Materiales Metálicos; CSIC-Dpto. de Publicaciones: Madrid, Spain, 2001. [Google Scholar]
- Shah, A.G.; Shetty, P.C.; Ramachandra, C.S.; Bhat, N.S.; Laxmikanth, S.M. In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. Angle Orthod. 2011, 81, 1028–1035. [Google Scholar] [CrossRef]
- Ozeki, K.; Yuhta, T.; Aoki, H.; Fukui, Y. Inhibition of Ni release from NiTi alloy by hydroxyapatite, alumina, and titanium sputtered coatings. Biomed. Mater. Eng. 2003, 13, 271–279. [Google Scholar]
- Ozeki, K.; Yuhta, T.; Aoki, H.; Asaoka, T.; Daisaku, T.; Fukui, Y. Deterioration in the superelasticity of Ti sputter coated on NiTi orthodontic wire. Biomed. Mater. Eng. 2003, 13, 355–362. [Google Scholar]
- Surmenev, R.A.; Ryabtseva, M.A.; Shesterikov, E.V.; Pichugin, V.F.; Peitschm, T.; Epple, M. The release of nickel from nickel-titanium (NiTi) is strongly reduced by a sub-micrometer thin layer of calcium phosphate deposited by rf-magnetron sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1233–1239. [Google Scholar] [CrossRef]
- Grainger, S.; Blunt, J. Engineering Coatings. Design and Application, 2nd ed; Plastics Design Library: Cambridge, UK, 1998. [Google Scholar]
- Redlich, M.; Gorodnev, A.; Feldman, Y.; Kaplan-Ashiri, I.; Tenne, R.; Fleischer, N.; Genut, M.; Feuerstein, N. Friction reduction and wear resistance of electro-co-deposited inorganic fullerene-like WS2 coating for improved stainless steel orthodontic wires. J. Mater. Res. 2008, 23, 2909–2915. [Google Scholar] [CrossRef]
- Samorodnitzky-Naveh, G.R.; Redlich, M.; Rapoport, L.; Feldman, Y.; Tenne, R. Inorganic fullerene-like tungsten disulfide nanocoating for friction reduction of nickel-titanium alloys. Nanomedicine (Lond.) 2009, 4, 943–950. [Google Scholar] [CrossRef]
- Zein El Abedin, S.; Welz-Biermann, U.; Endres, F. A study on the electrodeposition of tantalum on NiTi alloy in an ionic liquid and corrosion behaviour of the coated alloy. Electrochem. Commun. 2005, 7, 941–946. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Bescher, E.P. Physical properties of sol-gel coatings. J. Sol-Gel Sci. Technol. 2000, 19, 23–29. [Google Scholar] [CrossRef]
- Patil, K.R.; Hwang, Y.K.; Kim, M.J.; Chang, J.S.; Park, S.E. Preparation of thin films comprising palladium nanoparticles by a solid-liquid interface reaction technique. J. Colloid Interface Sci. 2004, 276, 333–338. [Google Scholar] [CrossRef]
- Cable, M.; Parker, J.M. High-Performance Glasses; Springer: New York, NY, USA, 1992. [Google Scholar]
- Bach, H.; Krause, D. Thin Films on Glass; Springer: Berlin, Germany, 2003. [Google Scholar]
- Chun, M.J.; Shim, E.; Kho, E.H.; Park, K.J.; Jung, J.; Kim, J.M.; Kim, B.; Lee, K.H.; Cho, D.L.; Bai, D.H.; et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007, 77, 483–488. [Google Scholar] [CrossRef]
- Rendón Arias, L.A.; Cano Correa, G.A.; peláez Vargas, A.; Jaramillo Vallejo, P.M.; García Garcia, C.; Góez, Y.M. Evaluación in vitro de la resistencia friccional entre brackets cerámicos y arcos de acero inoxidable con y sin recubrimiento vítreo aplicado por el método Sol-Gel. Rev. Fac. Odontol. Univ. Antioq. 2008, 20, 58–71. [Google Scholar]
- Horiuchi, Y.; Horiuchi, M.; Hanawa, T.; Soma, K. Effect of surface modification on the photocatalysis of Ti-Ni alloy in orthodontics. Dent. Mater. J. 2007, 26, 924–929. [Google Scholar] [CrossRef]
- Narayan, R. Biomedical Materials; Springer: New York, NY, USA, 2009. [Google Scholar]
- De Franco, D.J.; Spiller, R.E., Jr.; von Fraunhofer, J.A. Frictional resistances using Teflon-coated ligatures with various bracket-archwire combinations. Angle Orthod. 1995, 65, 63–72. [Google Scholar]
- Neumann, P.; Bourauel, C.; Jager, A. Corrosion and permanent fracture resistance of coated and conventional orthodontic wires. J. Mater. Sci. Mater. Med. 2002, 13, 141–147. [Google Scholar] [CrossRef]
- Husmann, P.; Bourauel, C.; Wessinger, M.; Jäger, A. The frictional behavior of coated guiding archwires. J. Orofac. Orthop. 2002, 63, 199–211. [Google Scholar] [CrossRef]
- Farronato, G.; Maijer, R.; Caria, M.P.; Esposito, L.; Alberzoni, D.; Cacciatore, G. The effect of Teflon coating on the resistance to sliding of orthodontic archwires. Eur. J. Orthod. 2012, 34, 410–417. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Mizoguchi, I. Effects of a diamond-like carbon coating on the frictional properties of orthodontic wires. Angle Orthod. 2011, 81, 141–148. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Nakagaki, S.; Endo, K.; Mizoguchi, I. Frictional and mechanical properties of diamond-like carbon-coated orthodontic brackets. Eur. J. Orthod. 2011. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arango, S.; Peláez-Vargas, A.; García, C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings 2013, 3, 1-15. https://doi.org/10.3390/coatings3010001
Arango S, Peláez-Vargas A, García C. Coating and Surface Treatments on Orthodontic Metallic Materials. Coatings. 2013; 3(1):1-15. https://doi.org/10.3390/coatings3010001
Chicago/Turabian StyleArango, Santiago, Alejandro Peláez-Vargas, and Claudia García. 2013. "Coating and Surface Treatments on Orthodontic Metallic Materials" Coatings 3, no. 1: 1-15. https://doi.org/10.3390/coatings3010001





