Electrochemical Synthesis of Nitro-Chitosan and Its Performance in Chromium Removal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrodeposition

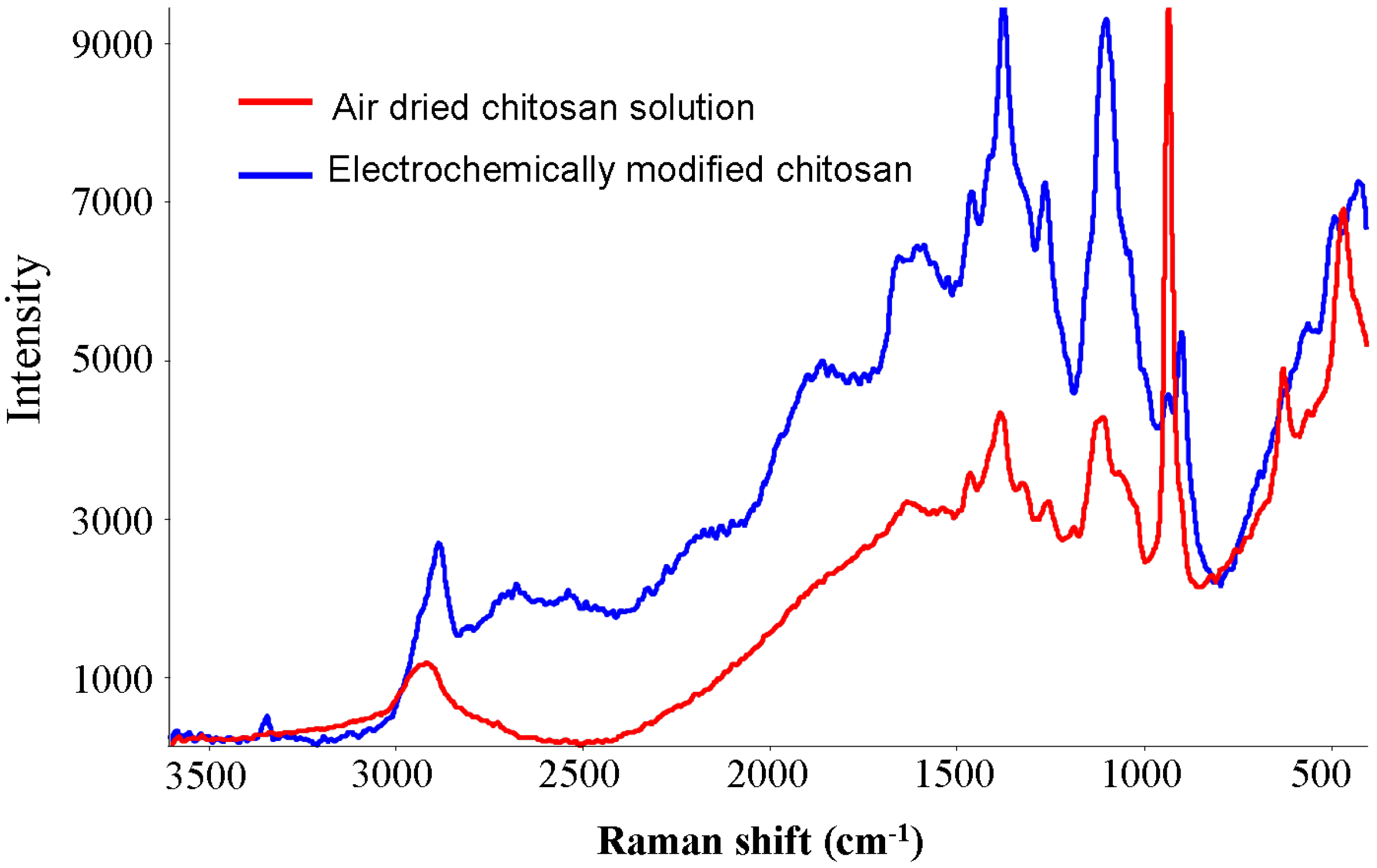
2.2. Characterization
| Peak (cm−1) | Assignment |
|---|---|
| 1663 | combination of amide I C=O stretching, asymmetric NO2 stretching and OH deformation vibrations |
| 1585 | NH bending frequency |
| 1442 | C–C stretching vibration |
| 1380 | combination of CH3 deformation and CO stretching frequencies |
| 1203 | combination of NH deformation and symmetric NO2 stretching vibrations |
| 1154, 1020 | symmetric and asymmetric stretching vibrations of C–O–C respectively |
| 970 | amine oxide |
| 805 | O–H deformation |


2.3. Equilibrium Adsorption Isotherms


2.4. Effect of pH on Adsorption of Chromate
2.5. Sorption Kinetics


3. Experimental Section
3.1. Electrodeposition of Chitosan
3.2. FTIR
3.3. Raman Spectroscopy
3.4. XPS
3.5. Absorption Experiments
3.6. DCP-AES
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mulligen, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-removal process during groundwater remediation by a zerovalent iron permeable reactive barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Kumari, A.; Fatinathan, S.; Ng, P.W. Adsorption of chromium from aqueous solution using chitosan beads. Adsorption 2006, 12, 249–257. [Google Scholar] [CrossRef]
- Baroni, P.; Vieira, R.S.; Meneghetti, E.; da Silva, M.G.C.; Beppu, M.M. Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J. Hazard. Mater. 2008, 152, 1155–1163. [Google Scholar] [CrossRef]
- Loyaux-lawniczak, S.; Lecomte, P. Chromium in a polluted groundwater: Redox process and immobilization in soils. Environ. Sci. Technol. 2001, 35, 1350–1357. [Google Scholar] [CrossRef]
- Wang, J.; Peng, R.; Bao, Z.; Yang, J.; Chen, S.; Liu, Q. Quick Removal of Chromium (VI) Ions from Aqueous Solution by Chitosan and Ferrous Ions. In Proceedings of IEEE 4th International Conference on Bioinformatics and Biomedical Engineering (ICBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Maniukiewicz, W.; Wojtasz-pajak, A. Determination of Hydrogel chitosan membrane structure. Polish Chitin Soc. Monogr. XI 2006, 113–121. [Google Scholar]
- Nunthanid, J.; Puttipipatkhanachorn, S.; Yamamoto, K.; Peck, G.E. Physical properties and molecular behavior of chitosan films. Drug Dev. Ind. Pharm. 2001, 27, 143–157. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Separ. Purif. Tech. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Rojas, G.; Silva, J.; Flores, J.A.; Rodriguez, A.; Ly, M.; Maldonado, H. Adsorption of chromium onto cross-linked chitosan. Separ. Purif. Tech. 2005, 44, 31–36. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Halada, G.P.; Jha, P.; Nelson, K.; Zhao, W.; Korach, C.S.; Neiman, A.; Lee, S.J.; Mintzer, E. Formation and characterization of chitosan-based coatings on stainless steel. In Biomaterials; Kulshrestha, A.S., Mahapatro, A., Henderson, L.A., Eds.; ACS symposium series; American Chemical Society: Washington DC, USA, 2010; pp. 159–171. [Google Scholar]
- Fernendes, R.; Wu, L.Q.; Chen, T.; Yi, H.; Rubloff, G.W.; Ghodssi, R.; Bentley, W.E.; Payne, G.F. Electrochemically induced deposition of a polysaccharide hydrogel onto a patterned surface. Langmuir 2003, 19, 4058–4062. [Google Scholar]
- Beard, B.C. Cellulose nitrate as a binding energy reference in N(1s) XPS studies of nitrogen-containing organic molecules. Appl. Surf. Sci. 1990, 45, 221–227. [Google Scholar] [CrossRef]
- Berger, P.; Vel Leitner, N.K.; Dore, M.; Legube, B. Ozone and hydroxyl radicals induced oxidation of glycine. Water Res. 1999, 33, 433–441. [Google Scholar]
- Gad, Y.H. Preparation and characterization of poly(2-acrylamido-2-methypropane sulfonic acid)/chitosan hydrogel using gamma irradiation and its application in wastewater treatment. Radiat. Phys. Chem. 2008, 77, 1101–1107. [Google Scholar] [CrossRef]
- Hena, S. Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer. J. Hazard. Mater. 2010, 181, 474–479. [Google Scholar] [CrossRef]
- Verma, B.M.; Krishnaiah, A.; Talbott, J.L.; Edgar, S.D. Removal of Hexavalent Chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef]
- Aydin, Y.A.; Aksoy, N.D. Adsorption of chromium on Chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Aksu, Z.; Gonen, F.; Demircan, Z. Biosorption of chromium(VI) ions by Mowital B3OH resin immobilized activated sludge in a packed bed: comparison with granular activated carbon. Process Biochem. 2002, 38, 175–186. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Zhang, Z.; Liu, Y.; Xiao, L.; Shi, X. Adsorption of Chromium (VI) on a novel quaternized chitosan resin. J. Appl. Polym. Sci. 2003, 90, 505–510. [Google Scholar] [CrossRef]
- Baran, A.; Bicak, E.; Baysal, S.H.; Onal, S. Comparative studies on the adsorption of Cr(VI) ions on to various sorbents. Bioresour. Technol. 2006, 98, 661–665. [Google Scholar]
- Sabharwal, S.; Ramnani, S.P. Adsorption behavior of Cr(VI) onto radiation crosslinked chitosan and its possible application for the treatment of wastewater containing Cr(VI). React. Funct. Polym. 2006, 66, 902–909. [Google Scholar] [CrossRef]
- Hu, Xin-jiang; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.; Bao, Z.; Zeng, X.; Chen, A.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, Kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- OMNIC for Nicolet Almega, version 7.3 service pack 1; Thermo Electron Corporation: Waltham, MA, USA, 2006.
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan(chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jha, P.K.; Halada, G.P.; McLennan, S.M. Electrochemical Synthesis of Nitro-Chitosan and Its Performance in Chromium Removal. Coatings 2013, 3, 140-152. https://doi.org/10.3390/coatings3030140
Jha PK, Halada GP, McLennan SM. Electrochemical Synthesis of Nitro-Chitosan and Its Performance in Chromium Removal. Coatings. 2013; 3(3):140-152. https://doi.org/10.3390/coatings3030140
Chicago/Turabian StyleJha, Prashant K., Gary P. Halada, and Scott M. McLennan. 2013. "Electrochemical Synthesis of Nitro-Chitosan and Its Performance in Chromium Removal" Coatings 3, no. 3: 140-152. https://doi.org/10.3390/coatings3030140




