Photocatalytic Activity of Reactively Sputtered Titania Coatings Deposited Using a Full Face Erosion Magnetron
Abstract
:1. Introduction
2. Experimental

3. Results
3.1. Coating Structures, Compositions and Deposition Rates
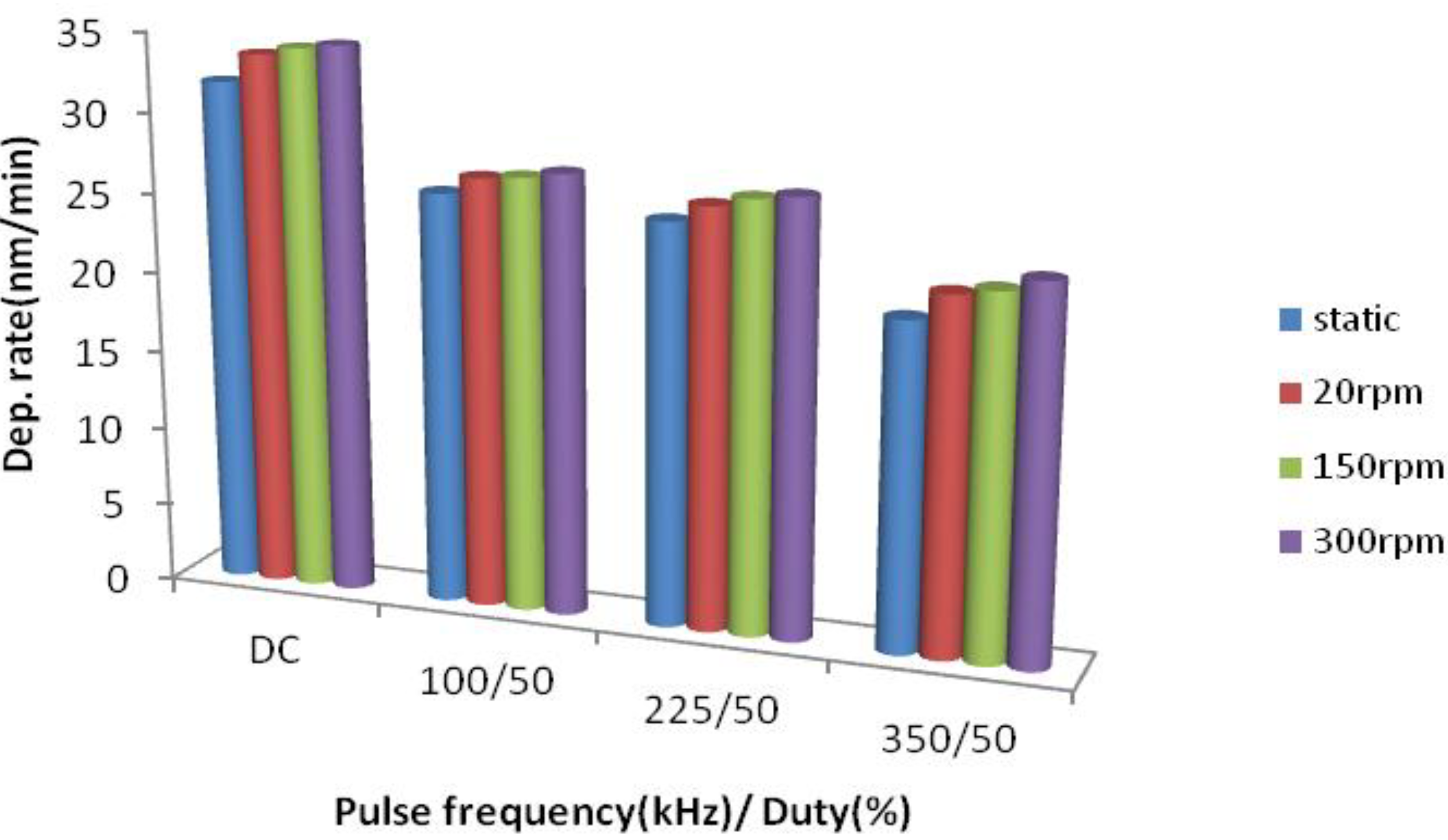
3.1.1. As-Deposited Coatings
| Sample No | Frequency, (kHz) | FFE Oscillation Speed, rpm | Deposition Rate, nm/min | Ra Value, nm | Post-annealed Coatings | |||
|---|---|---|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | 700 °C | |||||
| S1 | DC | – | 31.8 | 9 | AM | AM | RU | RU |
| S2 | DC | 20 | 33.6 | 11 | AM | AM | RU | RU |
| S3 | DC | 150 | 34.1 | 15 | AM | AM | RU | RU |
| S4 | DC | 300 | 34.4 | 28 | AM | AN | AN | AN |
| S5 | 100 | – | 25.8 | 17 | AM | AM | AN | AN/RU |
| S6 | 100 | 20 | 26.9 | 20 | AM | AN | AN | AN |
| S7 | 100 | 150 | 27.1 | 21 | AM | AN | AN | AN |
| S8 | 100 | 300 | 27.5 | 19 | AM | AN/RU | AN/RU | AN/RU |
| S9 | 225 | – | 25.1 | 27 | AM | AN | AN | AN/RU |
| S10 | 225 | 20 | 26.2 | 29 | AM | AN/RU | AN/RU | AN/RU |
| S11 | 225 | 150 | 26.8 | 29 | AM | AN/RU | AN/RU | AN/RU |
| S12 | 225 | 300 | 27.1 | 30 | AM | AN/RU | AN/RU | AN/RU |
| S13 | 350 | – | 20.4 | 15 | AM | AM | RU/AN | RU |
| S14 | 350 | 20 | 22.1 | 9 | AM | AM | RU | RU |
| S15 | 350 | 150 | 22.5 | 11 | AM | AM | RU | RU |
| S16 | 350 | 300 | 23.3 | 12 | AM | AM | RU | RU |


3.1.2. Annealed Coatings



3.2. Assessment of Photocatalytic Activity



| Sample No | Predominant Structure at 600 °C | MeO gradient, UV | Normalised MeO UV rate, arbitrary units(min/mW·cm−2) | MeO gradient, Fl | Normalised MeO Fl rate, arbitrary units(min/mW·cm−2) |
|---|---|---|---|---|---|
| S1 | RU | 0.030 | 0.008 | 0.020 | 0.003 |
| S2 | RU | 0.032 | 0.008 | 0.022 | 0.004 |
| S3 | RU | 0.035 | 0.009 | 0.025 | 0.004 |
| S4 | AN | 0.142 | 0.036 | 0.073 | 0.012 |
| S5 | AN | 0.131 | 0.033 | 0.067 | 0.011 |
| S6 | AN | 0.156 | 0.039 | 0.089 | 0.015 |
| S7 | AN | 0.158 | 0.040 | 0.090 | 0.015 |
| S8 | AN/RU | 0.121 | 0.030 | 0.058 | 0.010 |
| S9 | AN | 0.158 | 0.040 | 0.096 | 0.016 |
| S10 | AN/RU | 0.159 | 0.040 | 0.096 | 0.016 |
| S11 | AN/RU | 0.160 | 0.040 | 0.096 | 0.016 |
| S12 | AN/RU | 0.157 | 0.039 | 0.094 | 0.016 |
| S13 | RU/AN | 0.110 | 0.028 | 0.075 | 0.013 |
| S14 | RU | 0.099 | 0.025 | 0.049 | 0.008 |
| S15 | RU | 0.097 | 0.024 | 0.051 | 0.009 |
| S16 | RU | 0.095 | 0.024 | 0.051 | 0.009 |

4. Discussion


5. Conclusions
Conflicts of Interest
References
- Gloss, D.; Frach, P.; Zywitzki, O.; Klinkenberg, S.; Gottfried, C. Photocatalytic titanium dioxide thin films prepared by reactive pulse magnetron sputtering at low temperature. Surf. Coat. Technol. 2005, 200, 967–971. [Google Scholar] [CrossRef]
- Minemoto, T.; Negami, T.; Nishiwaki, S.; Takakuraa, H.; Hamakawa, Y. Preparation of Zn1−xMgxO films by radio frequency magnetron sputtering. Thin Solid Films 2000, 372, 173–176. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kluth, O.; Rech, B.; Houben, L.; Wieder, S.; Schöpe, G.; Beneking, C.; Wagner, H.; Löffl, A.; Schock, H.W. Texture etched ZnO:Al coated glass substrates for silicon based thin film solar cells. Thin Solid Films 1999, 351, 247–253. [Google Scholar] [CrossRef]
- Fortunato, E.; Nunes, P.; Costa, D.; Brida, D.; Ferreira, I.; Martins, R. Characterisation of aluminium doped zinc oxide thin films deposited on polymeric substrates. Vacuum 2002, 64, 233–236. [Google Scholar] [CrossRef]
- Szyszka, B. Transparent and conductive aluminum doped zinc oxide films prepared by mid-frequency reactive magnetron sputtering. Thin Solid Films 1999, 251, 164–169. [Google Scholar] [CrossRef]
- Ding, X.Z.; Liu, X.H. Correlation between anatase-to-rutile transformation and grain growth in nanocrystalline titania powders. J. Mat. Res. 1998, 13, 2556–2559. [Google Scholar] [CrossRef]
- Sicha, J.; Musil, J.; Meissner, M.; Cerstvy, R. Nanostructure of photocatalytic TiO2 films sputtered at temperatures below 200 °C. Appl. Surf. Sci. 2008, 254, 3793–3800. [Google Scholar] [CrossRef]
- Rawat, R.S.; Aggarwal, V.; Hassan, M.; Lee, P.; Springham, S.V.; Tan, T.L.; Lee, S. Nano-phase titanium dioxide thin film deposited by repetitive plasma focus: Ion irradiation and annealing based phase transformation and agglomeration. Appl. Surf. Sci. 2008, 255, 2932–2941. [Google Scholar] [CrossRef]
- Bendavid, A.; Martin, P.J.; Preston, E.W. The effect of pulsed direct current substrate bias on the properties of titanium dioxide thin films deposited by filtered cathodic vacuum arc deposition. Thin Solid Films 2008, 517, 494–499. [Google Scholar] [CrossRef]
- Long, H.; Yang, G.; Chen, A.; Li, Y.; Lu, P. Growth and characteristics of laser deposited anatase and rutile TiO2 films on Si substrates. Thin Solid Films 2008, 517, 745–749. [Google Scholar] [CrossRef]
- Srivatsa, K.M.K.; Bera, M.; Basu, A. Pure brookite titania crystals with large surface area deposited by plasma enhanced chemical vapour deposition technique. Thin Solid Films 2008, 516, 7443–7446. [Google Scholar] [CrossRef]
- Fusi, M.; Russo, V.; Casari, C.S.; Bassi, A.L.; Bottani, C.E. Titanium oxide nanostructured films by reactive pulsed laser deposition. Appl. Surf. Sci. 2009, 255, 5334–5337. [Google Scholar] [CrossRef]
- Ribarsky, M.W. Titaniumm dioxide (TiO2) (Rutile). Handb. Opt. Constants Solids 1997, 1, 795–804. [Google Scholar]
- Henderson, P.S.; Kelly, P.J.; Arnell, R.D.; Bäcker, H.; Bradley, J.W. Investigation into the properties of titanium based films deposited using pulsed magnetron sputtering. Surf. Coat. Technol. 2003, 174, 779–783. [Google Scholar]
- Kelly, P.J.; Arnell, R.D. The influence of deposition parameters on the structure of Al, Zr and W coatings deposited by closed-field unbalanced magnetron sputtering. Surf. Coat. Technol. 1996, 86–87, 425–431. [Google Scholar] [CrossRef]
- Kelly, P.J.; Bradley, J.W. Pulsed magnetron sputtering—Process overview and applications. J. Opto. Adv. Mat. 2009, 11, 1101–1107. [Google Scholar]
- Yates, H.M.; Brook, L.A.; Ditta, I.B.; Evans, P.; Foster, H.A.; Sheel, D.W.; Steele, A. Photo-induced self-cleaning and biocidal behaviour of titania and copper oxide multilayers. J. Photochem. Photobiol. A Chem. 2008, 197, 197–205. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhu, S.; Wang, F. Fe-doped photocatalytic TiO2 film prepared by pulsed DC reactive magnetron sputtering. J. Vac. Sci. Technol. 2003, A21, 1877–1882. [Google Scholar]
- Kok, Y.N.; Kelly, P.J. Properties of pulsed magnetron sputtered TiO2 coatings grown under different magnetron configurations and power deliver modes. Plasma Proc. Polym. 2007, 4, 299–304. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Q.; Lian, J. Visible-light photocatalytic activity of nitrogen-doped TiO2 thin film prepared by pulsed laser deposition. Appl. Surf. Sci. 2008, 254, 4620–4625. [Google Scholar] [CrossRef]
- Rampaul, A.; Parkin, I.P.; O’Neill, S.A.; de Souza, J.; Mills, A.; Elliot, N. Titania and tungsten doped titania thin films on glass; active photocatalysts. Polyhedron 2003, 22, 35–44. [Google Scholar] [CrossRef]
- Park, S.E.; Joo, H.; Kang, J.W. Effect of impurities in TiO2 thin films on trichloroethylene conversion. Sol. Energy Mater. Sol. Cells 2004, 83, 39–53. [Google Scholar] [CrossRef]
- Ratova, M.; Kelly, P.J.; West, G.; Lordanova, I. Enhanced properties magnetron sputtered photocatalytic coatings via transition metal doping. Surf. Coat. Technol. 2013, 228, 544–549. [Google Scholar] [CrossRef]
- Kelly, P.J.; West, G.; Kok, Y.N.; Bradley, J.W.; Swindells, I.; Clarke, G.C.B. A comparison of the characteristics of planar and cylindrical magnetrons operating in pulsed DC and AC modes. Surf. Coat. Technol. 2007, 202, 952–956. [Google Scholar] [CrossRef]
- Fernández, A.; Lassaletta, G.; Jiménez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Env. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, Z.; Chen, F.; Zhang, J.; Chen, H.; Anpo, M.; Huang, J.; Zhang, L. Hydrothermal doping method for preparation of Cr3+-TiO2 photocatalysts with concentration gradient distribution of Cr3+. Appl. Catal. B Env. 2006, 62, 329–325. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, C.; Yoon, J. Developing a testing method for antimicrobial efficacy on TiO2 photocatalytic products. Environ. Eng. Res. 2008, 13, 136–140. [Google Scholar] [CrossRef]
- Evans, P.; Pemble, M.E.; Sheel, D.W.; Yates, H.M. Multifunctional self-cleaning thermochromic films by atmospheric pressure chemical vapour deposition. J. Photochem. Photobiol. A Chem. 2007, 189, 387–397. [Google Scholar] [CrossRef]
- Evans, P.; English, T.; Hammond, D.; Pemble, M.E.; Sheel, D.W. The role of SiO2 barrier layers in determining the structure and photocatalytic activity of TiO2 films deposited on stainless steel. Appl. Catal. A Gen. 2007, 321, 140–146. [Google Scholar] [CrossRef]
- Kenanakis, G.; Giannakoudakis, Z.; Vernardou, D.; Savvakis, C.; Katsarakis, N. Photocatalytic degradation of stearic acid by ZnO thin films and nanostructures deposited by different chemical routes. J. Catal. Today 2010, 151, 34–38. [Google Scholar] [CrossRef]
- Brook, L.A.; Evans, P.; Foster, H.A.; Pembleb, M.E.; Steele, A.; Sheel, D.W.; Yates, H.M. Highly bioactive silver and silver/titania composite films grown by chemical vapour deposition. J. Photochem. Photobiol. A Chem. 2007, 187, 53–63. [Google Scholar] [CrossRef]
- Evans, P.; Pemble, M.E.; Sheel, D.W. Precursor-directed control of crystalline type in atmospheric pressure CVD growth of TiO2 on stainless steel. J. Chem. Mat. 2006, 18, 5750–5755. [Google Scholar] [CrossRef]
- Guettai, N.; Amar, H.A. Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension, Part I, Parametric Study. Desalination 2005, 185, 427–437. [Google Scholar]
- Hazan, R.; Sreekantan, S.; Lockman, Z.; Mat, I. A study on photocatalytic degradation of methyl orange using carbon doped TiO2 nanotubes. J. Fund. Sci. 2010, 96, 355–365. [Google Scholar]
- Carcel, R.A.; Andronic, L.; Duta, A. Cd2+ modified TiO2 for methyl orange photodegradation. Rev. Roum. Chim. 2009, 54, 309–312. [Google Scholar]
- Kim, S.; Ehrman, S.H. Photocatalytic activity of a surface-modified anatase and rutile titania nanoparticle mixture. J. Col. Interf. Sci. 2009, 338, 304–307. [Google Scholar] [CrossRef]
- Onifade, A.A.; Kelly, P.J. The influence of deposition parameters on the structure and properties of magnetron-sputtered titania coatings. Thin Solid Films 2006, 494, 8–12. [Google Scholar] [CrossRef]
- Ohno, S.; Takasawa, N.; Sato, Y.; Yoshikawa, M.; Suzuki, K.; Frach, P.; Shugesato, Y. Photocatalytic TiO2 films deposited by reactive magnetron sputtering with unipolar pulsing and plasma emission control systems. Thin Solid Films 2006, 496, 126–130. [Google Scholar] [CrossRef]
- Evans, P.; Mantke, S.; Mills, A.; Robinson, A.; Sheel, D.W. A comparative study of three techniques for determining photocatalytic activity. J. Photochem. Photobiol. A Chem. 2007, 188, 387–391. [Google Scholar] [CrossRef]
- Mills, A.; McGrady, M. A study of new photocatalyst indicator inks. J. Photochem. Photobiol. A Chem. 2008, 193, 228–236. [Google Scholar] [CrossRef]
- Mills, A.; Hepburn, J.; McFarlane, M. A novel, fast-responding, indicator ink for thin film photocatalytic surfaces. ACS Appl. Mat. Interf. 2009, 1, 1163–1165. [Google Scholar] [CrossRef]
- Coutinho, C.A.; Gupta, V.K. Photocatalytic degradation of methyl orange using polymer-titania microcomposites. J. Col. Interf. Sci. 2009, 333, 64. [Google Scholar]
- Bates, R.I.; Arnell, R.D. Alloy coatings by dual magnetron sputter barrel plating. Surf. Coat. Technol. 1994, 68–69, 686–690. [Google Scholar] [CrossRef]
- Zywitzki, O.; Modes, T.; Frach, P.; Gloss, D. Effect of Structure and Roughness on Photocatalytic Properties of TiO2. In Proceedings of European Materials Research Society (EMRS): European Materials Research Society (E-MRS) Spring Meeting, Strasbourg, France, 28 May–1 June 2007.
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Films 1994, 251, 72–79. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farahani, N.; Kelly, P.J.; West, G.; Hill, C.; Vishnyakov, V. Photocatalytic Activity of Reactively Sputtered Titania Coatings Deposited Using a Full Face Erosion Magnetron. Coatings 2013, 3, 177-193. https://doi.org/10.3390/coatings3040177
Farahani N, Kelly PJ, West G, Hill C, Vishnyakov V. Photocatalytic Activity of Reactively Sputtered Titania Coatings Deposited Using a Full Face Erosion Magnetron. Coatings. 2013; 3(4):177-193. https://doi.org/10.3390/coatings3040177
Chicago/Turabian StyleFarahani, Nick, Peter J. Kelly, Glen West, Claire Hill, and Vladimir Vishnyakov. 2013. "Photocatalytic Activity of Reactively Sputtered Titania Coatings Deposited Using a Full Face Erosion Magnetron" Coatings 3, no. 4: 177-193. https://doi.org/10.3390/coatings3040177






