Fabrication of Nanodiamond Coating on Steel
Abstract
:1. Introduction
| Interlayer(s) | Diamond quality* | Reference |
|---|---|---|
| Si | Good | [2] |
| Si | Medium | [3] |
| Si | Very good | [4] |
| 3C-SiC | Medium | [5] |
| Metal-diamond composite | Good | [5] |
| SiC | Medium | [6] |
| Ni-diamond composite | Good | [6] |
| Cr-diamond composite | Good | [6] |
| W | Good | [7] |
| CrNi | No film | [8] |
| TiN | No film | [8] |
| Ti(C,N) | No film | [8] |
| (Ti,Al)N | No film | [8] |
| CrN | No film | [8] |
| WC/C | No film | [8] |
| TiN | Medium | [9] |
| TiN | Medium | [10] |
| Ti | Good | [11,12,13] |
| Cr | Good | [13,14] |
| ZrN | Medium | [14] |
| ZrC | Medium | [14] |
| TiC/Ti(C,N)/TiN | Medium | [14] |
| TiC | Good | [14] |
| Ni | Good | [15] |
| Ni/Ni-diamond composite | Good | [16] |
| N | Good | [17] |
| N (CrN) | Good | [16] |
| Cr | Good | [17] |
| CrC | Good | [18,19] |
| CrN | Good | [20,21,22,23,24,25,26,27] |
| B | Good | [26,27] |
| Ni/Cu/Ti | Medium | [28] |
2. Experimental Section
| Material Grown | Substrate Temperature | Deposition Time |
|---|---|---|
| MCD | 400 °C | 2 h |
| NCD | 400 °C | 4 h |
| CNTs | 500 °C | 2 h |
3. Results and Discussion
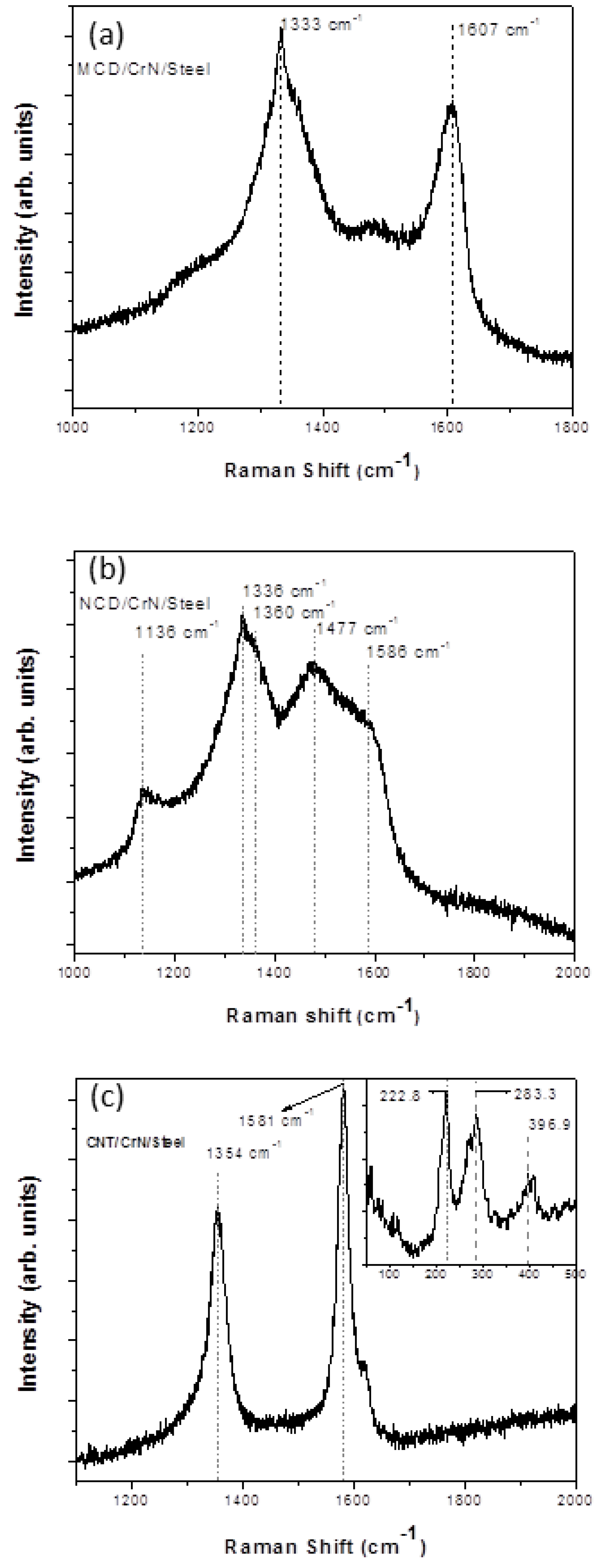


4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Neto, V.F.; Vaz, R.; Ali, N.; Oliveira, M.S.A.; Grácio, J.; Ghumman, C.A.A.; Teodoro, O.M.N.D. Carbon diffusion into steel during diamond chemical vapour deposition. Int. J. Nanomanufacturing 2008, 2, 192–203. [Google Scholar] [CrossRef]
- Chen, H.; Nielsen, M.L.; Gold, C.J.; Dillon, R.O.; DiGregorio, J.; Furtak, T. Growth of diamond films on stainless steel. Thin Solid Films 1992, 212, 169–172. [Google Scholar] [CrossRef]
- Fenker, M.; Ferber, H.; Füßer, H.-J.; Jörgensen, G.; Lahres, M.; Wolf, G.K. Deposition of CVD diamond onto ion beam modified ASP 23 cutting tools. Surf. Coatings Technol. 1998, 98, 1053–1059. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; van Enckevort, W.J.P.; Schermer, J.J.; ter Meulen, J.J. The applicability of ultra thin silicon films as interlayers for CVD diamond deposition on steels. Phys. Stat. Sol. A 2003, 195, 383–395. [Google Scholar] [CrossRef]
- Klages, C.-P.; Fryda, M.; Matthke, T.; Schäfer, L.; Dimigen, H. Diamond coatings and cBN coatings for tools. Int. J. Refract. Met. Hard Mater. 1998, 16, 171–176. [Google Scholar] [CrossRef]
- Schäfer, L.; Fryda, M.; Stolley, T.; Xiang, L.; Klages, C.-P. Chemical vapour deposition of polycrystalline diamond films on high-speed steel. Surf. Coatings Technol. 1999, 116–119, 447–451. [Google Scholar] [CrossRef]
- Ralchenko, V.G.; Smolin, A.A.; Pereverzev, V.G.; Obraztsova, E.D.; Korotoushenko, K.G.; Konov, V.I.; Lakhotkin, Y.V.; Loubnin, E.N. Diamond deposition on steel with CVD tungsten intermediate layer. Diam. Relat. Mater. 1995, 4, 754–758. [Google Scholar]
- Haubner, R.; Lux, B. Diamond deposition on steel substrates using intermediate layers. Int. J. Refract. Met. Hard Mater. 2006, 24, 380–386. [Google Scholar] [CrossRef]
- Weiser, P.S.; Prawer, S. Chemical vapour deposition of diamond onto iron based substrates—The use of barrier layers. Diam. Relat. Mater. 1995, 4, 710–713. [Google Scholar] [CrossRef]
- Lorenz, H.P. Investigation of TiN as an interlayer for diamond deposition on steel. Diam. Relat. Mater. 1995, 4, 1088–1092. [Google Scholar] [CrossRef]
- Fan, Q.H.; Fernandes, A.; Gracio, J. Diamond coating on steel with a titanium interlayer. Diam. Relat. Mater. 1998, 7, 603–606. [Google Scholar] [CrossRef]
- Fan, Q.H.; Fernandes, A.; Pereira, E.; Grácio, J. Adhesion of diamond coatings on steel and copper with a titanium interlayer. Diam. Relat. Mater. 1999, 8, 1549–1554. [Google Scholar] [CrossRef]
- Silva, F.J.G.; Baptista, A.P.M.; Pereira, E.; Teixeira, V.; Fan, Q.H.; Fernandes, A.J.S.; Costa, F.M. Microwave plasma chemical vapour deposition diamond nucleation on ferrous substrates with Ti and Cr interlayers. Diam. Relat. Mater. 2002, 11, 1617–1622. [Google Scholar] [CrossRef]
- Polini, R.; Mantini, F.P.; Braic, M.; Amar, M.; Ahmed, W.; Taylor, H. Effects of Ti- and Zr-based interlayer coatings on the hot filament chemical vapour deposition of diamond on high speed steel. Thin Solid Films 2006, 494, 116–122. [Google Scholar] [CrossRef]
- Lin, C.R.; Kuo, C.T. High adhesion and quality diamond films on steel substrate. Diam. Relat. Mater. 1998, 7, 903–907. [Google Scholar] [CrossRef]
- Borges, C.F.M.; Pfender, E.; Heberlein, J. Influence of nitrided and carbonitrided interlayers on enhanced nucleation of diamond on stainless steel 304. Diam. Relat. Mater. 2001, 10, 1983–1990. [Google Scholar] [CrossRef]
- Bareiβ, C.; Perle, M.; Rosiwal, S.M.; Singer, R.F. Diamond coating of steel at high temperatures in hot filament chemical vapour deposition (HFCVD) employing chromium interlayers. Diam. Relat. Mater. 2006, 15, 754–760. [Google Scholar] [CrossRef]
- Schwarz, S.; Musayev, Y.; Rosiwal, S.M.; Schaufler, C.; Singer, R.F.; Meerkamm, H. Diam. Relat. Mater. 2002, 11, 757–762. [CrossRef]
- Schwarz, S.; Rosiwal, S.M.; Musayev, Y.; Singer, R.F. High temperature diffusion chromizing as a successful method for CVD-diamond coating of steel—Part II. Diam. Relat. Mater. 2003, 12, 701–706. [Google Scholar]
- Fayer, A.; Glozman, O.; Hoffman, A. Deposition of continuous and well adhering diamond films on steel. Appl. Phys. Lett. 1995, 67, 2299–2301. [Google Scholar] [CrossRef]
- Glozman, O.; Berner, A.; Shechtman, D.; Hoffman, A. Influence of Cr-N interlayer properties on the initial stages of CVD diamond growth on steel substrates. Diam. Relat. Mater. 1998, 7, 597–602. [Google Scholar]
- Glozman, O.; Halperin, G.; Etsion, I.; Berner, A.; Shectman, D.; Lee, G.H.; Hoffman, A. Study of the wear behavior and adhesion of diamond films deposited on steel substrates by use of a Cr–N interlayer. Diam. Relat. Mater. 1999, 8, 859–864. [Google Scholar] [CrossRef]
- Glozman, O.; Hoffman, A. Adhesion improvement of diamond films on steel subtrates using chromium nitride interlayers. Diam. Relat. Mater. 1997, 6, 796–801. [Google Scholar] [CrossRef]
- Avigal, Y.; Glozman, O.; Etsion, I.; Halperin, G.; Hoffman, A. [100]-Textured diamond films for tribological applications. Diam. Relat. Mater. 1997, 6, 381–385. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Shankar, P.; Fleischer, W.; van Enckevort, W.J.P.; Schermer, J.J.; ter Meulen, J.J. CVD diamond deposition on steel using arc-plated chromium nitride interlayers. Diam. Relat. Mater. 2002, 11, 536–544. [Google Scholar] [CrossRef]
- Silva, F.J.G.; Fernandes, A.J.S.; Costa, F.M.; Baptista, A.P.M.; Pereira, E. A new interlayer approach for CVD diamond coating of steel substrates. Diam. Relat. Mater. 2004, 13, 828–833. [Google Scholar] [CrossRef]
- Silva, F.J.G.; Fernandes, A.J.S.; Costa, F.M.; Teixeira, V.; Baptista, A.P.M.; Pereira, E. Tribological behaviour of CVD diamond films on steel substrates. Wear 2003, 255, 846–853. [Google Scholar] [CrossRef]
- Silva, F.J.G.; Fernandes, A.J.S.; Costa, F.M.; Baptista, A.P.M.; Pereira, E. Unstressed PACVD diamond films on steel pre-coated with a composite multilayer. Surf. Coatings Technol. 2005, 191, 102–107. [Google Scholar] [CrossRef]
- Gupta, S.; Weiss, B.L.; Weiner, B.R.; Morell, G. Study of the electron field emission and microstructure correlation in nanocrystalline carbon thin films. J. Appl. Phys. 2001, 89, 5671–5675. [Google Scholar]
- Piazza, F.; Gonzalez, J.A.; Velazquez, R.; De Jesus, J.; Rosario, S.A.; Morell, G. Diamond film synthesis at low temperature. Diam. Relat. Mater. 2006, 15, 109–116. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Phil. Trans. R. Soc. Lond. A 2004, 362, 2477–2512. [Google Scholar]
- Islam, S.S.; Shah, K.A.; Mavi, H.S.; Shaukla, A.K.; Rath, S.; Harsh, S. Raman study on single-walled carbon nanotubes with different laser excitation energies. Bull. Mater. Sci. 2007, 30, 295–299. [Google Scholar]
- Bandow, S.; Asaka, S.; Saito, Y.; Rao, A.M.; Grigorian, L.; Richter, E.; Eklund, P.C. Effect of the growth temperature on the diameter distribution and chirality of single-wall carbon nanotubes. Phys. Rev. Lett. 1998, 80, 3779–3782. [Google Scholar]
- Kobayashi, Y.; Takayuki, T.; Ueno, Y.; Niwa, O.; Homma, Y.; Ogino, T. Extremely intense Raman signals from single-walled carbon nanotubes suspended between Si nanopillars. Chem. Phys. Lett. 2004, 386, 153–157. [Google Scholar]
- Wei, J.; Yung, K.P.; Tay, B.K. Formation of CNT bumps for interconnection applications. SIMTech Tech. Rep. 2009, 10, 76–79. [Google Scholar]
- Uebing, C.; Viefhaus, H.; Grabke, H.J. Formation of CrN surface compounds and surface precipitates on Fe-15%Cr-N single crystals. Appl. Surf. Sci. 1988, 32, 363–380. [Google Scholar] [CrossRef]
- Martin-Gil, J.; Matin-Gil, F.J.; Sarikaya, M.; Qian, M.; Jose-Yacaman, M.; Rubio, A. Evidence of a low compressibility carbon nitride with defect-zincblende structure. J. Appl. Phys. 1997, 81, 2555–2559. [Google Scholar]
- Keller, T.M.; Laskoski, M.; Osofsky, M.; Qadri, S.B. Carbon nanotube formation catalyzed by Ni nanoparticles in carbonaceous solid. Phys. Stat. Sol. A 2008, 205, 1585–1591. [Google Scholar]
- Neto, V.F.; Vaz, R.; Oliveira, M.S.A.; Grácio, J. CVD diamond-coated steel inserts for thermoplastic mould tools—Characterization and preliminary performance evaluation. J. Mater. Process. Technol. 2009, 209, 1085–1091. [Google Scholar]
- Neto, V.F.; Vaz, R.; Ali, N.; Oliveira, M.S.A.; Grácio, J. Performance of sub-micron diamond films coated on mould inserts for plastic injection moulding. J. Mater. Sci. 2008, 43, 3392–3399. [Google Scholar]
- Neto, V.F.; Vaz, R.; Ali, N.; Oliveira, M.S.A.; Grácio, J. Diamond coatings on 3D structured steel. Diam. Relat. Mater. 2008, 17, 1424–1428. [Google Scholar] [CrossRef]
- Neto, V.F.; Oliveira, M.S.A.; Ali, N.; Grácio, J. Time-modulated chemical vapour deposition diamonf on mould making 2738 steel. Vacuum 2008, 82, 1346–1349. [Google Scholar] [CrossRef]
- Haubner, R.; Sommer, D. Hot-filament diamond deposition with sulfur addition. Diam. Relat. Mater. 2003, 12, 298–305. [Google Scholar] [CrossRef]
- Oberlin, A. Carbonization and graphitization. Carbon 1984, 22, 521–541. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Velázquez, R.; Neto, V.F.; Uppireddi, K.; Weiner, B.R.; Morell, G. Fabrication of Nanodiamond Coating on Steel. Coatings 2013, 3, 243-252. https://doi.org/10.3390/coatings3040243
Velázquez R, Neto VF, Uppireddi K, Weiner BR, Morell G. Fabrication of Nanodiamond Coating on Steel. Coatings. 2013; 3(4):243-252. https://doi.org/10.3390/coatings3040243
Chicago/Turabian StyleVelázquez, Rafael, Victor F. Neto, Kishore Uppireddi, Brad R. Weiner, and Gerardo Morell. 2013. "Fabrication of Nanodiamond Coating on Steel" Coatings 3, no. 4: 243-252. https://doi.org/10.3390/coatings3040243




