Ni-Al and NiO-Al Composite Coatings by Combustion-Assisted Flame Spraying
Abstract
:1. State of the Art
| Coating Process | Typical Coating Thickness | Coating Material | Characteristics | Examples |
|---|---|---|---|---|
| PVD | 1–5 μm | Ti(C,N) | Wear resistance | Machine tools |
| CVD | 1–50 μm | SiC | Wear resistance | Fibre coatings |
| Thermal Spray Coating | 0.04–3 mm | Ceramic & Metallic alloys | Wear resistance, Corrosion resistance | Bearings, axles |
| Hard Chromium Plating | 10–100 μm | Chrome | Wear resistance | Rolls |
| Weld Overlay | 0.5–5 mm | Steel, Stellite | Wear resistance | Valves |
| Galvanisation | 1–5 μm | Zinc | Corrosion resistance | Steel sheet |
| Braze Overlay | 10–100 μm | Ni-Cr-B-Si alloys | Very hard, dense surface | Shafts |
1.1. Flame Spray Coatings
1.2. Nickel Aluminide Coatings
| Properties | Applications |
|---|---|
| Generally low cost | Turbo charger rotors in diesel engine trucks |
| Good high temperature oxidation resistance, excellent strength at high strain rates | Die materials for isothermal forging; Moulding material for glass processing |
| Resistance to carburizing and oxidizing atmospheres | Fixture material for heat treatment of auto parts in high temperature furnaces |
| High temperature strength, good oxidation & corrosion resistance | Rollers for steel slab heating furnaces |
| Excellent vibration and cavitation resistance in water | Hydro turbine rotors |
| Low & high temperature strength for cutting tools | Cutting tools |
| Superior strength & creep resistance | Turbine blades vanes for jet engines. |
1.3. Combustion Synthesis (CS)
1.4. SHS of Nickel Aluminides
2. Experimental
| Sample # | wt% NiO | wt% Ni | wt% Al |
|---|---|---|---|
| 1 | 58.14 | – | 41.86 |
| 2 | – | 42.1 | 57.9 |
| 3 | – | 59.3 | 40.7 |
| 4 | – | 65.1 | 34.9 |
| 5 | – | 86.8 | 13.2 |

3. Results and Discussion
3.1. Reactions and Formation of Intermetallic Phases by CAFSY Flame Spraying
| NiO + Al system | Ni + Al system |
| Al +3NiO → 3Ni + Al2O3 | Al + Ni → NiAl |
| 3Al + Ni → NiAl3 | 3Al + Ni → NiAl3 |
| 4Al+3O2 → 2Al2O3 | 3Al + 2Ni → Ni2Al3 |
| Al2O3 +NiO → NiAl2O4 | 2Ni+O2 → 2NiO |
| 4Al+3O2 → 2Al2O3 | |
| Al2O3 +NiO → NiAl2O4 | |
| Al + 3Ni → Ni3Al |
| Reaction | Gibbs free energy of formation ΔGf° (kJ·mol−1) |
|---|---|
| Ni +Al → NiAl | −133.0 |
| Ni+NiAl3→ Ni2Al3 | −144.1 |
| 2Ni +3Al → Ni2Al3 | −311.0 |
| Ni + 3Al → NiAl3 | −166.8 |
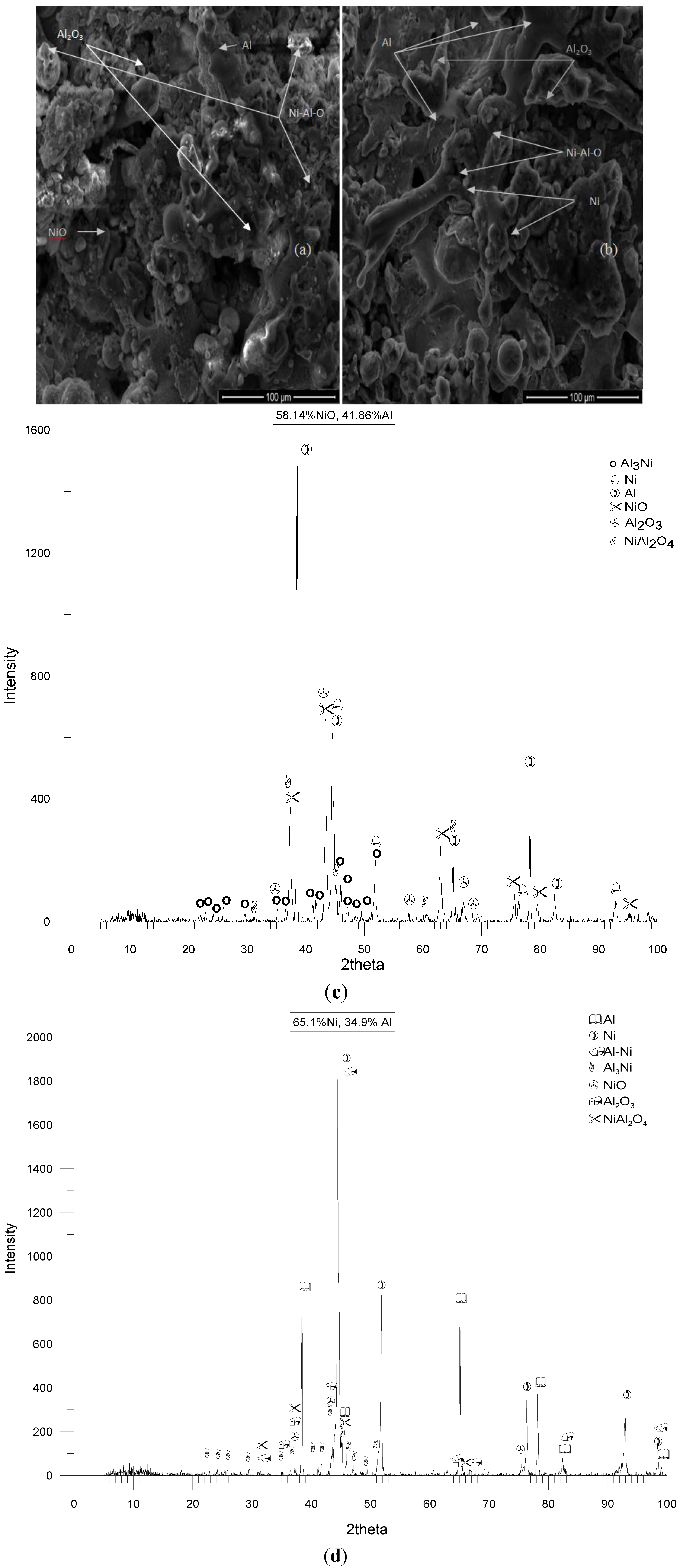



Particle Size Effect

3.2. Properties of the Ni-Al Coatings as Functions of the Spraying Conditions
3.2.1. Effect of Spray Distance

3.2.2. Effect of O2/C2H2 Ratio

3.2.3. Influence of Substrate Temperature during Thermal Spraying

3.2.4. Initial Powder Mixture Composition


3.3. Summary of Most Promising Coatings
| Sample | Porosity, % | Roughness, μm | Adhesion Strength, MPa | Erosion rate, mg/min |
|---|---|---|---|---|
| Commercial Ni-Al Coating | 4.5 ± 1.0 | 6.4 ± 0.5 | 41.0 ± 1.0 | 6 |
| CAFSY Coating J5 | 3.0 ± 0.5 | 7.2 ± 0.5 | 45.5 ± 1.0 | 2 |
| CAFSY Coating J6 | 2.0 ± 0.8 | 7.0 ± 0.6 | 49.5 ± 1.0 | 8 |
4. Conclusions
Conflicts of Interest
References
- Goward, G.W. Progress in coatings for gas turbines airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Davis, J.R. Handbook of Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Ozturk, A.; Cetegen, B.M. Modeling of precipitate formation in precursor droplets injected axially into an oxygen/acetylene combustion flame. Mater. Sci. Eng. A 2006, 422, 163–175. [Google Scholar] [CrossRef]
- An Introduction to Thermal Spraying; Sulzer Metco: Winterthur, Switzerland, 2011.
- Eskner, M.; Sandström, R. Measurement of the ductile-to-brittle transition temperature in a nickel aluminide coating by a miniaturised disc bending test technique. Surf. Coat. Technol. 2003, 165, 71–80. [Google Scholar] [CrossRef]
- Kohlscheen, J.; Stock, H.R. Deposition of silicon enriched nickel aluminide coatings on internally cooled airfoils. Surf. Coat. Technol. 2008, 203, 476–479. [Google Scholar] [CrossRef]
- Uyulgan, B.; Dokumaci, E.; Celik, E.; Kayatekin, I.; Ak Azem, N.F.; Ozdemir, I.; Toparli, M. Wear behaviour of thermal flame sprayed FeCr coatings on plain carbon steel substrate. J. Mater. Process. Technol. 2007, 190, 204–210. [Google Scholar] [CrossRef]
- Chaithanya, M. Processing & Characterization of Ni-Al Coating on Metal Substrates. Master’s Thesis, National Institute of Technology, Rourkela, India, 2007. [Google Scholar]
- Malik, A.U.; Ahmad, R.; Ahmad, S.; Ahmad, S. High temperature oxidation behaviour of nickel aluminide coated mild steel. Anti-Corros. Methods Mater. 1991, 38, 4–10. [Google Scholar] [CrossRef]
- Dey, G.K. Physical metallurgy of nickel aluminides. Sadhana 2003, 28, 247–262. [Google Scholar] [CrossRef]
- Brandl, W.; Marginean, G.; Maghet, D.; Utu, D. Effects of specimen treatment and surface preparation on the isothermal oxidation behaviour of the HVOF-sprayed MCrAlY coatings. Surf. Coat. Technol. 2004, 188–189, 20–26. [Google Scholar] [CrossRef]
- Brandl, W.; Crabke, H.J.; Toma, D.; Krüger, J. The oxidation behaviour of sprayed MCrAlY coatings. Surf. Coat. Technol. 1996, 86–87, 41–47. [Google Scholar] [CrossRef]
- Brandl, W.; Toma, D.; Krüger, J.; Grabke, H.J.; Matthäus, G. The oxidation behavior of HVOF thermal sprayed MCrAlY coating. Surf. Coat. Technol. 1997, 94–95, 21–26. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyanb, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Sierra, C.; Vázquez, A.J. Dry sliding wear behaviour of nickel aluminides coatings produced by self-propagating high-temperature synthesis. Intermetallics 2006, 14, 848–852. [Google Scholar] [CrossRef]
- Morsi, K. Review: Reaction synthesis processing of Ni–Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Moore, J.J.; Feng, H.J. Combustion synthesis of advanced materials: Part 1. Reaction parameters. Progr. Mater. Sci. 1995, 39, 243–273. [Google Scholar] [CrossRef]
- Mimani, T.; Patil, K.C. Solution combustion synthesis of nanoscale oxides and their composite. Mater. Phys. Mech. 2001, 4, 134–137. [Google Scholar]
- Biswas, A.; Roy, S.K. Comparison between the microstructural evolutions of two modes of SHS of NiAl: Key to a common reaction mechanism. Acta Mater. 2004, 52, 257–270. [Google Scholar] [CrossRef]
- Zhu, L.; He, J.; Yan, D.; Dong, Y.; Zhang, J.; Li, X.; Liao, H. Atmospheric reactive plasma sprayed Fe–Al2O3–FeAl2O4 composite coating and its property evaluation. Appl. Surf. Sci. 2011, 257, 10282–10288. [Google Scholar] [CrossRef]
- Yeh, C.L. Combustion Synthesis: Principles and Applications. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Kramer, E.J., Mahajan, S., Veyssiere, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; p. 18. [Google Scholar]
- Talako, T.; Ilyuschenko, A.; Letsko, A. SHS powders for thermal spray coating. KONA Powder Part. J. 2009, 55–72. [Google Scholar]
- Yang, Y.; Yan, D.; Dong, Y.; Wang, L.; Chen, X.; Zhang, J.; He, J.; Li, X. In situ nanostructured ceramic matrix composite coating prepared by reactive plasma spraying micro-sized Al–Fe2O3 composite powders. J. Alloys Compd. 2011, 509, L90–L94. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Swindeman, C.J.; Seals, R.D. Reactive spraying of nickel-aluminide coatings. J. Therm. Spray Technol. 1997, 6, 335–344. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Swindeman, C.J.; Seals, R.D. Application of reaction synthesis principles to thermal spray coatings. J. Mater. Sci. 1997, 32, 3315–3325. [Google Scholar] [CrossRef]
- Tsunekawa, Y.; Okumiya, M.; Gotoh, K.; Nakamura, T.; Niimi, I. Synthesis of iron aluminide matrix in situ composites from elemental powders by reactive low-pressure plasma spraying. Mater. Sci. Eng. A 1992, 159, 253–259. [Google Scholar] [CrossRef]
- Leica AS, version 3.1. software for optical microscopy, Leica microscopy DMLM. Leica Microsystems Wetzlar GmbH: Wetzlar, Germany, 1999.
- Rog, G.; Borchardt, G.; Wellen, M.; Lose, W.J. Determination of the activities in the (Ni + Al) alloys in the temperature range 870 K to 920 K by a solid-state galvanic cell using a CaF2 electrolyte. Chem. Thermodyn. 2003, 35, 261–268. [Google Scholar] [CrossRef]
- Itin, V.I.; Naiborodenko, Y.S. High Temperature Synthesis of Intermetallic Compounds; Publishing House of Tomsk University: Tomsk, Russia, 1989. (In Russian) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xanthopoulou, G.; Marinou, A.; Vekinis, G.; Lekatou, A.; Vardavoulias, M. Ni-Al and NiO-Al Composite Coatings by Combustion-Assisted Flame Spraying. Coatings 2014, 4, 231-252. https://doi.org/10.3390/coatings4020231
Xanthopoulou G, Marinou A, Vekinis G, Lekatou A, Vardavoulias M. Ni-Al and NiO-Al Composite Coatings by Combustion-Assisted Flame Spraying. Coatings. 2014; 4(2):231-252. https://doi.org/10.3390/coatings4020231
Chicago/Turabian StyleXanthopoulou, Galina, Amalia Marinou, George Vekinis, Aggeliki Lekatou, and Michalis Vardavoulias. 2014. "Ni-Al and NiO-Al Composite Coatings by Combustion-Assisted Flame Spraying" Coatings 4, no. 2: 231-252. https://doi.org/10.3390/coatings4020231





