Chalcopyrite Thin Film Materials for Photoelectrochemical Hydrogen Evolution from Water under Sunlight
Abstract
:1. Introduction
2. Hydrogen Evolution from Water Using Copper Chalcopyrite Photocathodes





3. Band Structure Modulation by Composition Control
| Photocathode [Electrolyte] | Published Year | Photocurrent Value at 0 VRHE | IPCE at 0 VRHE | Maximum HC-STH | Stability at 0 VRHE | Ref. |
|---|---|---|---|---|---|---|
| Pt/CdS/CIGS [0.1 M Na2SO4 pH 9.5] | 2010 | 12 mA cm−2 under Xe lamp | 59% at 600 nm (at −0.24 VRHE) | N.A. | Unstable | [41] |
| Pt/CdS/CIS [0.1 M Na2SO4 pH 13] | 2011 | 6 mA cm−2 under Xe lamp | >20% at 500~750 nm (at −0.37 VRHE in pH 4) | N.A. | N.A. | [48] |
| Pt/CuGa3Se5 [0.1 M Na2SO4 pH 9.5] | 2012 | 4.95 mA cm−2 under AM1.5G | 15% at 600 nm | 0.35% at 0.17 VRHE | N.A. | [58] |
| Pt/ZnS/CuGa3Se5 [0.1 M Na2SO4 pH 9.5] | 2012 | 4.35 mA cm−2 under AM1.5G | 25.4% at 520 nm | 0.25% at 0.1 VRHE | 25 h (at 0.1 VRHE) | [54] |
| Pt/CdS/CuGaSe2 [0.1 M Na2SO4 pH 9] | 2013 | 7.5 mA cm−2 under AM1.5G | 45% at 520 nm | 0.83% at 0.2 VRHE | >10 days | [42] |
| Pt/CdS/CGSe (powder, Ga/Cu = 2) [0.1 M Na2SO4 pH 9.5] | 2014 | 2.4 mA cm−2 under AM1.5D | N.A. | N.A. | 16 h (at 0.1 VRHE) | [43] |
| Pt/CdS/AgxCu1-xGaSe2 (x = 5.9%) [0.1 M Na2SO4 pH 9.5] | 2014 | 8.1 mA cm−2 under AM1.5G | 54% at 550 nm | 1.22% at 0.3 VRHE | 55 h | [45] |
| Pt/In2S3/CIS [0.1 M Na2SO4 pH 9] | 2014 | 15 mA cm−2 under AM1.5G | 37%–41% at 520–700 nm | 1.97% at 0.28 VRHE | N.A. | [59] |
| Pt/TiO2/CdS/CIS [0.1 M Na2HPO4 pH 10] | 2014 | 13.0 mA cm−2 under AM1.5G | 62% at 500–700 nm | 1.82% at 0.25 VRHE | 1 h | [49] |
| Pt/CdS/CuGa3Se5/ AgxCu1-xGaSe2 (x = 5.9%) [0.1 M Na2HPO4 pH 10] | 2015 | 8.79 mA cm−2 under AM1.5G | >57% at 520–600 nm | 1.81% at 0.36 VRHE | 20 days | [46] |
| Pt/Mo/Ti/Cu(In,Ga)Se2 [0.5 M Na2SO4 + 0.25 M Na2HPO4 + 0.25 M NaH2PO4 pH 6.8] | 2015 | 30 mA cm−2 under AM1.5G | N.A. | 8.5% at 0.38 VRHE | 10 days (with a decrease on a daily basis) | [47] |
4. PEC Properties of AGSe Photocathodes
4.1. Experimental Methods
4.2. Results and Discussion
4.2.1. PEC Properties of Gallium Selenide and AGSe Photocathodes



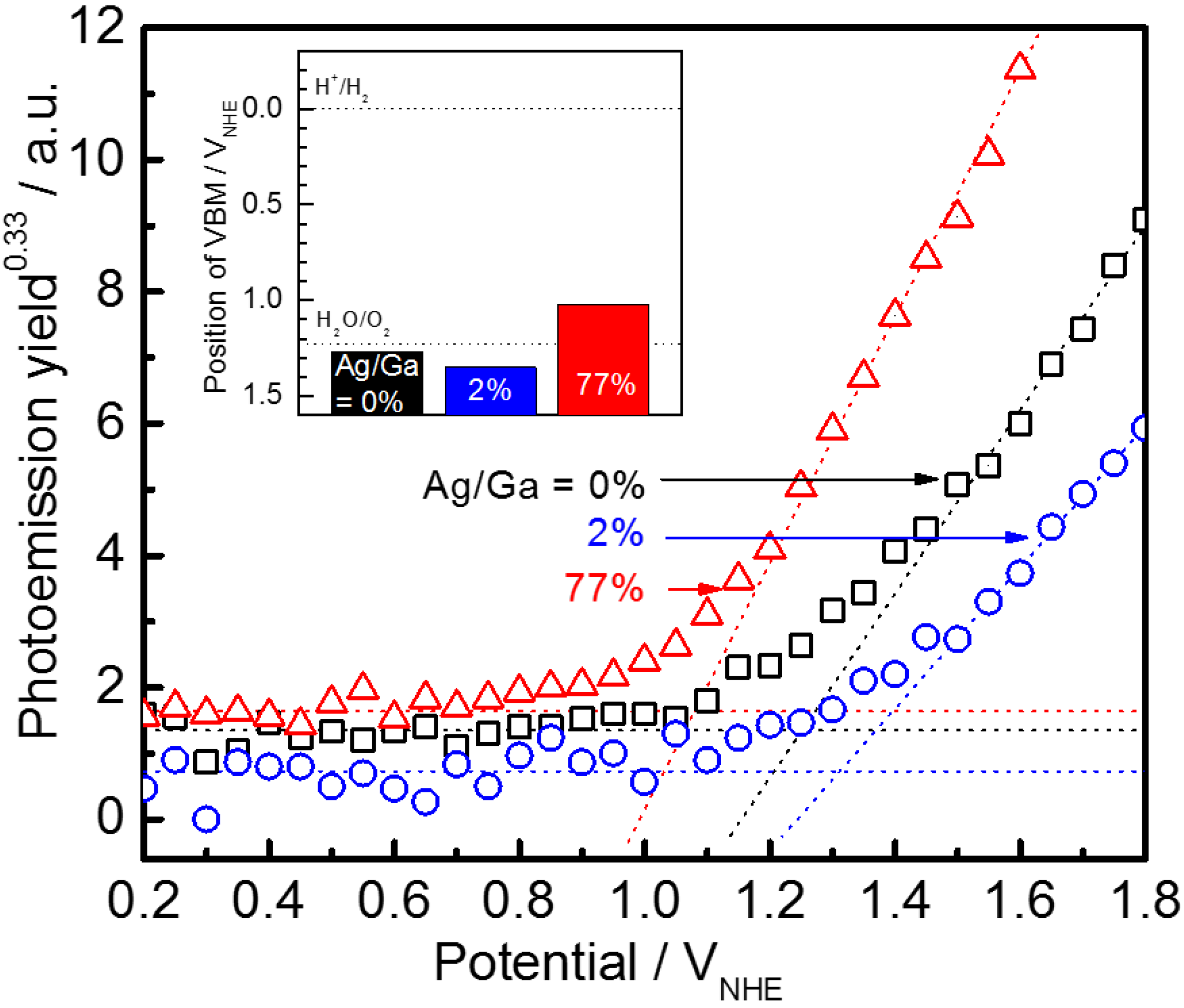
4.2.2. Effects of CdS Modification and AGSe Film Preparation under Partial Pressure of Hydrogen


5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, T.J.; Fjallstrom, V.; Sahlberg, M.; Edoff, M.; Edvinsson, T. A monolithic device for solar water splitting based on series interconnected thin film absorbers reaching over 10% solar-to-hydrogen efficiency. Energy Environ. Sci. 2013, 6, 3676–3683. [Google Scholar] [CrossRef]
- Yang, H. Bin; Miao, J.; Hung, S.; Huo, F.; Chen, H.M.; Liu, B.; Engineering, B.; Drive, N.; Road, R.; Science, M.; Avenue, N. Stable quantum dot photoelectrolysis cell for unassisted visible light solar. ACS Nano. 2014, 8, 10403–10413. [Google Scholar] [CrossRef] [PubMed]
- Cendula, P.; Tilley, S.D.; Gimenez, S.; Bisquert, J.; Schmid, M.; Grätzel, M.; Schumacher, J.O. Calculation of the energy band diagram of a photoelectrochemical water splitting cell. J. Phys. Chem. C 2014, 118, 29599–29607. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, S.; Sheehan, S.W.; Wang, D. Nanonet-based hematite heteronanostructures for efficient solar water splitting. J. Am. Chem. Soc. 2011, 133, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Y.; Mullen, T.J.; Lin, W.; Sun, L.; Yan, C.; Patten, T.E.; Wang, D.; Liu, G. Improving hematite’s solar water splitting efficiency by incorporating rare-earth upconversion nanomaterials. J. Phys. Chem. C 2012, 3188–3192. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Du, C.; Dai, P.; Zheng, Z.; Wang, D. Improving hematite-based photoelectrochemical water splitting with ultrathin TiO2 by atomic layer deposition. ACS Appl. Mater. Interfaces 2014, 6, 12005–12011. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Iwashina, K.; Kudo, A. Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation. PNAS 2012, 109, 11564–11569. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Ma, Y.; Oleynikov, P.; Domen, K.; Delaunay, J.-J. A conductive ZnO-ZnGaON nanowire-array-on-a-film photoanode for stable and efficient sunlight water splitting. Energy Environ. Sci. 2014, 7, 1693–1699. [Google Scholar] [CrossRef]
- Yokoyama, D.; Hashiguchi, H.; Maeda, K.; Minegishi, T.; Takata, T.; Abe, R.; Kubota, J.; Domen, K. Ta3N5 photoanodes for water splitting prepared by sputtering. Thin Solid Films 2011, 519, 2087–2092. [Google Scholar] [CrossRef]
- Li, Y.; Takata, T.; Cha, D.; Takanabe, K.; Minegishi, T.; Kubota, J.; Domen, K. Vertically aligned Ta3N5 nanorod arrays for solar-driven photoelectrochemical water splitting. Adv. Mater. 2013, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Urabe, H.; Hisatomi, T.; Minegishi, T.; Kubota, J.; Domen, K. Photoelectrochemical properties of SrNbO2N photoanodes for water oxidation fabricated by the particle transfer method. Faraday Discuss. 2014, 176, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Minegishi, T.; Clune, J.; Nakabayashi, M.; Hisatomi, T.; Nishiyama, H.; Katayama, M.; Shibata, N.; Kubota, J.; Yamada, T.; et al. Photoelectrochemical oxidation of water using BaTaO2N photoanodes prepared by particle transfer method. J. Am. Chem. Soc. 2015, 137, 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Dominey, R.N.; Lewis, N.S.; Bruce, J.A.; Bookbinder, D.C.; Wrighton, M.S. Improvement of photoelectrochemical hydrogen generation by surface modification of p-type silicon semiconductor photocathodes. J. Am. Chem. Soc. 1982, 104, 467–482. [Google Scholar] [CrossRef]
- Fan, R.; Dong, W.; Fang, L.; Zheng, F.; Su, X.; Zou, S.; Huang, J.; Wang, X.; Shen, M. Stable and efficient multi-crystalline n+p silicon photocathode for H2 production with pyramid-like surface nanostructure and thin Al2O3 protective layer. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Bae, D.; Pedersen, T.; Seger, B.; Malizia, M.; Kuznetsov, A.; Hansen, O.; Chorkendorff, I.; Vesborg, P.C. K. Back-illuminated Si photocathode: a combined experimental and theoretical study for photocatalytic hydrogen evolution. Energy Environ. Sci. 2015, 8, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, S.W.; Warren, E.L.; Putnam, M.C.; Santori, E.A.; Turner-Evans, D.; Kelzenberg, M.D.; Walter, M.G.; McKone, J.R.; Brunschwig, B.S.; Atwater, H.A.; et al. Photoelectrochemical hydrogen evolution using Si microwire arrays. J. Am. Chem. Soc. 2011, 133, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guio, C.G.; Tilley, S.D.; Vrubel, H.; Grätzel, M.; Hu, X. Hydrogen evolution from a copper(I) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hisatomi, T.; Watanabe, O.; Nakabayashi, M.; Shibata, N.; Domen, K.; Delaunay, J.-J. Positive onset potential and stability of Cu2O-based photocathodes in water splitting by atomic layer deposition of a Ga2O3 buffer layer. Energy Environ. Sci. 2015, 8. [Google Scholar] [CrossRef]
- Hu, S.; Shaner, M.R.; Beardslee, J.A.; Lichterman, M.; Brunschwig, B.S.; Lewis, N.S. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 2014, 344, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Takei, K.; Zhang, J.; Kapadia, R.; Zheng, M.; Chen, Y.Z.; Nah, J.; Matthews, T.S.; Chueh, Y.L.; Ager, J.W.; Javey, A. p-Type InP nanopillar photocathodes for efficient solar-driven hydrogen production. Angew. Chem. Int. Ed. 2012, 51, 10760–10764. [Google Scholar] [CrossRef] [PubMed]
- Coridan, R.H.; Arpin, K.A.; Brunschwig, B.S.; Braun, P.V.; Lewis, N.S. Photoelectrochemical behavior of hierarchically structured Si/WO3 core-shell tandem photoanodes. Nano Lett. 2014, 14, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Tang, J.; Yang, P. Zn-doped p-type gallium phosphide nanowire photocathodes from a surfactant-free solution synthesis. Nano Lett. 2012, 12, 5407–5411. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hisatomi, T.; Ma, G.; Iwanaga, A.; Minegishi, T.; Moriya, Y.; Katayama, M.; Kubota, J.; Domen, K. Improving the photoelectrochemical activity of La5Ti2CuS5O7 for hydrogen evolution by particle transfer and doping. Energy Environ. Sci. 2014, 7, 2239–2242. [Google Scholar] [CrossRef]
- Ma, G.; Liu, J.; Hisatomi, T.; Minegishi, T.; Moriya, Y.; Iwase, M.; Nishiyama, H.; Katayama, M.; Yamada, T.; Domen, K. Site-selective photodeposition of Pt on a particulate Sc-La5Ti2CuS5O7 photocathode: Evidence for one-dimensional charge transfer. Chem. Commun. 2015, 51, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1−x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 2004, 2, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Tsuji, I.; Kato, H. AgInZn7S9 solid solution photocatalyst for H2 evolution from aqueous solutions under visible light irradiation. Chem. Commun. 2002, 9, 1958–1959. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kudo, A. Visible-light-induced H2 evolution from an aqueous solution containing sulfide and sulfite over a ZnS-CuInS2-AgInS2 solid-solution photocatalyst. Angew. Chem. Int. Ed. 2005, 44, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hakari, Y.; Ikeda, S.; Jia, Q.; Iwase, A.; Kudo, A. Utilization of metal sulfide material of (CuGa)1–xZn2xS2 solid solution with visible light response in photocatalytic and photoelectrochemical solar water splitting systems. J. Phys. Chem. Lett. 2015, 1042–1047. [Google Scholar] [CrossRef]
- Kindyak, V.V.; Kindyak, A.S.; Gremenok, V.F.; Bodnar, I.V.; Rud’, Y.V.; Medvedkin, G.A. Optical properties of laser-evaporated CuGaSe2 films near and above the fundamental absorption edge. Thin Solid Films 1994, 250, 33–36. [Google Scholar] [CrossRef]
- Kisilev, A.; Marcu, V.; Cahen, D.; Schock, H.W.; Noufi, R. Photoelectrochemical characterization of CuGaSe2 and Cu(Ga, In)Se2 films. Sol. Cells 1990, 28, 57–67. [Google Scholar] [CrossRef]
- Cahen, D.; Chen, Y.W. n-CuInSe2 based photoelectrochemical cells: Improved, stable performance in aqueous polyiodide through rational surface and solution modifications. Appl. Phys. Lett. 1984, 45, 746–748. [Google Scholar] [CrossRef]
- Robbins, M.; Bachmann, K.J.; Lambrecht, V.G.; Thiel, F.A.; Thomson, J., Jr.; Vadimsky, R.G.; Menezes, S.; Heller, A.; Miller, B. CuInS2 liquid junction solar cells. J. Electrochem. Soc. Sol. State Sci. Technol. 1978, 125, 831–832. [Google Scholar]
- Valderrama, R.C.; Sebastian, P.J.; Enriquez, J.P.; Gamboa, S.A. Photoelectrochemical characterization of CIGS thin films for hydrogen production. Sol. Energy Mater. Sol. Cells 2005, 88, 145–155. [Google Scholar] [CrossRef]
- Marsen, B.; Cole, B.; Dorn, S.; Rocheleau, R.E.; Miller, E.L. Copper gallium diselenide photocathodes for solar photoelectrolysis. Sol. Hydrog. Nanotechnol. II 2007, 6650. [Google Scholar] [CrossRef]
- Wadia, C.; Alivisatos, A.P.; Kammen, D.M. Materials availability expands the opportunity for large-scale photovoltaics deployment. Environ. Sci. Technol. 2009, 43, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Marsen, B.; Cole, B.; Miller, E.L. Photoelectrolysis of water using thin copper gallium diselenide electrodes. Sol. Energy Mater. Sol. Cells 2008, 92, 1054–1058. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Maeda, K.; Katayama, M.; Kubota, J.; Yamada, A.; Konagai, M.; Domen, K. Photoelectrochemical water splitting using a Cu(In,Ga)Se2 thin film. Electrochem. commun. 2010, 12, 851–853. [Google Scholar] [CrossRef]
- Moriya, M.; Minegishi, T.; Kumagai, H.; Katayama, M.; Kubota, J.; Domen, K. Stable hydrogen evolution from CdS modified CuGaSe2 photoelectrode under visible light irradiation. J. Am. Chem. Soc. 2013, 135, 3733–3735. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Minegishi, T.; Moriya, Y.; Kubota, J.; Domen, K. Photoelectrochemical hydrogen evolution from water using copper gallium selenide electrodes prepared by a particle transfer method. J. Phys. Chem. C 2014, 118, 16386–16392. [Google Scholar] [CrossRef]
- Minegishi, T.; Nishimura, N.; Kubota, J.; Domen, K. Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting. Chem. Sci. 2013, 4, 1120–1124. [Google Scholar] [CrossRef]
- Zhang, L.; Minegishi, T.; Kubota, J.; Domen, K. Hydrogen evolution from water using AgxCu1−xGaSe2 photocathodes under visible light. Phys. Chem. Chem. Phys. 2014, 16, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Minegishi, T.; Nakabayashi, M.; Suzuki, Y.; Seki, K.; Shibata, N.; Kubota, J.; Domen, K. Durable hydrogen evolution from water driven by sunlight using (Ag,Cu)GaSe2 photocathodes modified with CdS and CuGa3Se5. Chem. Sci. 2015, 6, 894–901. [Google Scholar] [CrossRef]
- Kumagai, H.; Minegishi, T.; Sato, N.; Yamada, T.; Kubota, J.; Domen, K. Efficient solar hydrogen production from neutral electrolytes using surface-modified Cu(In,Ga)Se2 photocathodes. J. Mater. Chem. A 2015, 3, 8300–8307. [Google Scholar] [CrossRef]
- Ikeda, S.; Nakamura, T.; Lee, S.M.; Yagi, T.; Harada, T.; Minegishi, T.; Matsumura, M. Photoreduction of water by using modified CuInS2 electrodes. Chem. Sus. Chem. 2011, 4, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Minegishi, T.; Zhang, L.; Zhong, M.; Gunawan, R.; Nakabayashi, M.; Ma, G.; Hisatomi, T.; Katayama, M.; Ikeda, S.; et al. Enhancement of solar hydrogen evolution from water by surface modification with CdS and TiO2 on porous CuInS2 photocathodes prepared by an electrodeposition-sulfurization method. Angew. Chemie Int. Ed. 2014, 53, 11808–11812. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Minegishi, T.; Yokoyama, D.; Kubota, J.; Domen, K. Photoelectrochemical hydrogen production on Cu2ZnSnS4/Mo-mesh thin-film electrodes prepared by electroplating. Chem. Phys. Lett. 2011, 501, 619–622. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Jimbo, K.; Hisatomi, T.; Ma, G.; Katayama, M.; Kubota, J.; Katagiri, H.; Domen, K. H2 evolution from water on modified Cu2ZnSnS4 photoelectrode under solar light. Appl. Phys. Express 2010, 3. [Google Scholar] [CrossRef]
- Rovelli, L.; Tilley, S.D.; Sivula, K. Optimization and stabilization of electrodeposited Cu2ZnSnS4 photocathodes for solar water reduction. ACS Appl. Mater. Interfaces 2013, 5, 8018–8024. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.L.; Tell, B.; Kasper, H.M.; Schiavone, L.M. p-d Hybridization of the valence bands of I-III-VI2 compounds. Phys. Rev. B 1972, 5, 5003–5005. [Google Scholar] [CrossRef]
- Kim, J.; Minegishi, T.; Kobota, J.; Domen, K. Enhanced photoelectrochemical properties of CuGa3Se5 thin films for water splitting by the hydrogen mediated co-evaporation method. Energy Environ. Sci. 2012, 5, 6368–6374. [Google Scholar] [CrossRef]
- Hautier, G.; Ong, S.P.; Jain, A.; Moore, C.J.; Ceder, G. Accuracy of density functional theory in predicting formation energies of ternary oxides from binary oxides and its implication on phase stability. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85. [Google Scholar] [CrossRef]
- Simchi, H.; McCandless, B. An investigation of the surface properties of (Ag,Cu)(In,Ga)Se2 thin films. IEEE J. Photovoltaics 2012, 2, 519–523. [Google Scholar] [CrossRef]
- Zhang, X.; Kobayashi, T.; Kurokawa, Y.; Miyajima, S.; Yamada, A. Deposition of Ag(In,Ga)Se2 solar cells by a modified three-stage method using a low-temperature-deposited Ag-Se cap layer. Jpn. J. Appl. Phys. 2013, 52, 1–4. [Google Scholar]
- Kim, J.; Minegishi, T.; Kobota, J.; Domen, K. Investigation of Cu-deficient copper gallium selenide thin film as a photocathode for photoelectrochemical water splitting. Jpn. J. Appl. Phys. 2012, 51, 1–6. [Google Scholar] [CrossRef]
- Gunawan; Septina, W.; Ikeda, S.; Harada, T.; Minegishi, T.; Domen, K.; Matsumura, M. Platinum and indium sulfide-modified CuInS2 as efficient photocathodes for photoelectrochemical water splitting. Chem. Commun. 2014, 50, 8941–8943. [Google Scholar] [CrossRef]
- Chang, C.C.; Zeng, J.X.; Lan, S.M.; Uen, W.Y.; Liao, S.M.; Yang, T.N.; Ma, W.Y.; Chang, K.J. Fabrication of single-phase ε-GaSe films on Si(100) substrate by metal organic chemical vapor deposition. Thin Solid Films 2013, 542, 119–122. [Google Scholar] [CrossRef]
- Sano, M.; Miyamoto, K.; Kato, H.; Yao, T. Role of hydrogen in molecular beam epitaxy of ZnO. Jpn. J. Appl. Phys. 2004, 95, 5527–5531. [Google Scholar] [CrossRef]
- Chun, Y. Effect of atomic hydrogen in highly lattice-mismatched molecular beam epitaxy. J. Cryst. Growth 1995, 150, 497–502. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujishima, A.; Honda, K. Potential variation at the semiconductor-electrolyte interface through a change in pH of the solution. Chem. Lett. 1974, 3, 897–900. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, H.; Minegishi, T.; Domen, K. Chalcopyrite Thin Film Materials for Photoelectrochemical Hydrogen Evolution from Water under Sunlight. Coatings 2015, 5, 293-311. https://doi.org/10.3390/coatings5030293
Kaneko H, Minegishi T, Domen K. Chalcopyrite Thin Film Materials for Photoelectrochemical Hydrogen Evolution from Water under Sunlight. Coatings. 2015; 5(3):293-311. https://doi.org/10.3390/coatings5030293
Chicago/Turabian StyleKaneko, Hiroyuki, Tsutomu Minegishi, and Kazunari Domen. 2015. "Chalcopyrite Thin Film Materials for Photoelectrochemical Hydrogen Evolution from Water under Sunlight" Coatings 5, no. 3: 293-311. https://doi.org/10.3390/coatings5030293





