Molecularly Imprinted Polymer/Metal Organic Framework Based Chemical Sensors
Abstract
:1. Introduction
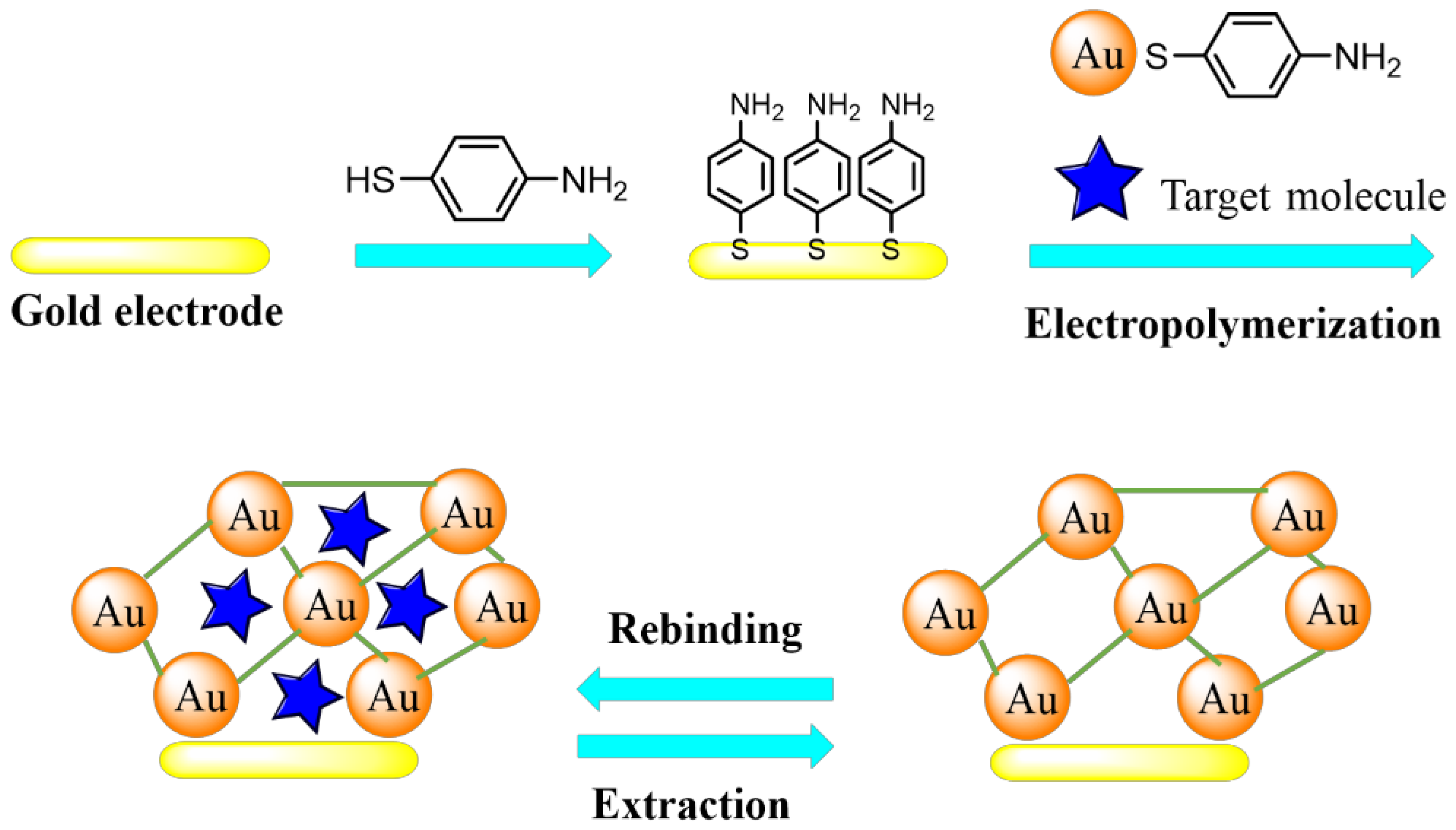
2. Sensors Based on Molecularly Imprinted MOF Thin Films
2.1. Fabrication of Molecularly Imprinted MOF Thin Films
2.2. Electrochemical Sensors Based on Molecularly Imprinted MOF Thin Films
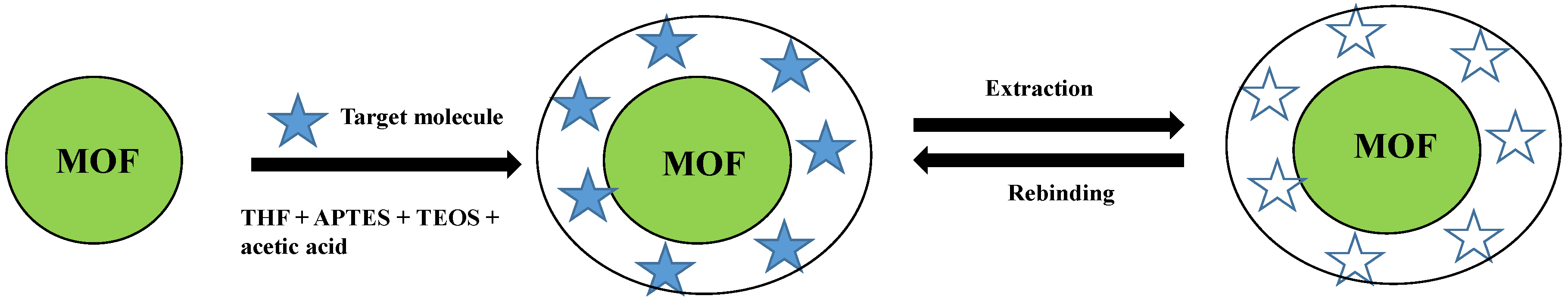
3. Sensors Based on Molecularly Imprinted Core-Shell Nanoparticles Using MOF as a Core
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. A functional monomer is not enough: Principal component analysis of the influence of template complexation in pre-polymerization mixtures on imprinted polymer recognition and morphology. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, Y.; Kong, L.; Zhou, T.; Shi, G. A novel composite of SiO2-coated graphene oxide and molecularly imprinted polymers for electrochemical sensing dopamine. Biosens. Bioelectron. 2013, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing–Synthesis, characterisation and application. Sens. Actuators B-Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, Z.B.; Zeng, Y.; Zhou, T.; Shi, G. A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens. Bioelectron. 2014, 52, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Viveiros, R.; Bonifácio, V.D.B.; Aguiar-Ricardo, A.; Casimiro, T. Supercritical fluid technology as a new strategy for the development of semi-covalent molecularly imprinted materials. RSC Adv. 2012, 2, 5075–5079. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Xu, Y.; Du, X.; Sun, X.; Sun, L.; Wang, H.; Zhao, Q.; Yu, A.; Zhang, H.; et al. Determination of fluoroquinolone antibiotics in environmental water samples based on magnetic molecularly imprinted polymer extraction followed by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 662, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Soares da Silva, M.; Vão, E.R.; Temtem, M.; Mafra, L.; Caldeira, J.; Aguiar-Ricardo, A.; Casimiro, T. Clean synthesis of molecular recognition polymeric materials with chiral sensing capability using supercritical fluid technology. Application as HPLC stationary phases. Biosens. Bioelectron. 2010, 25, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Soares da Silva, M.; Casimiro, T.; Afonso, C.A.M. Isolation, analytical quantification and seasonal variation of labdanolic acid from the Portuguese-grown Cistus ladaniferus. Ind. Crops Prod. 2014, 60, 226–232. [Google Scholar]
- Mattiasson, B.; Ye, L. Molecularly Imprinted Polymers in Biotechnology; Series: Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2015; Volume 150. [Google Scholar]
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, S.; Chen, L.; Wei, Y.; Xiong, H. Recent advances in molecularly imprinted polymers in food analysis. J. Appl. Polym. Sci. 2014, 131, 40766. [Google Scholar] [CrossRef]
- Soares da Silva, M.; Viveiros, R.; Coelho, M.; Aguiar-Ricardo, A.; Correia, I.J.; Casimiro, T. Supercritical CO2-assisted preparation of a PMMA composite membrane for bisphenol A recognition in aqueous environment. Chem. Eng. Sci. 2012, 68, 94–100. [Google Scholar] [CrossRef]
- Puoci, F.; Cirillo, G.; Curcio, M.; Iemma, F.; Parisi, O.I.; Spizzirri, U.G.; Picci, N. Molecularly Imprinted Polymers (PIMs) in Biomedical Applications, Biopolymers; Magdy Elnashar, M., Ed.; InTech: Rijeka, Croatia, 2010; Available online: http://www.intechopen.com/books/biopolymers/molecularly-imprinted-polymers-for-biomedical-applications (accessed on 21 August 2016). [CrossRef]
- Soares da Silva, M.; Viveiros, R.; Morgado, P.I.; Aguiar Ricardo, A.; Correia, I.J.; Casimiro, T. Development of 2-(dimethylamino)ethyl methacrylate-based molecular recognition devices for controlled drug delivery using supercritical fluid technology. Int. J. Pharm. 2011, 416, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.; Fard, S.E.; Mohammadi, A.; Abdouss, M.; Ganjali, M.R.; Norouzi, P.; Safaraliee, L. Molecularly imprinted polymer based potentiometric sensor for the determination of hydroxyzine in tablets and biological fluids. Anal. Chim. Acta 2008, 612, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Si, S.; Yang, Z. A highly selective photoelectrochemical biosensor for uric acid based on core–shell Fe3O4@C nanoparticle and molecularly imprinted TiO2. Biosens. Bioelectron. 2015, 65, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Li, H.; Guan, H.; Zhao, J.; Majeed, S.; Anjum, S.; Liang, F.; Xu, G. Electrochemical cholesterol sensor based on carbon nanotube @ molecularly imprinted polymer modified ceramic carbon electrode. Biosens. Bioelectron. 2013, 47, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Cao, Y.Y.; Wang, X.M.; Fang, G.Z.; Wang, S. Prussian blue mediated amplification combined with signal enhancement of ordered mesoporous carbon for ultrasensitive and specific quantification of metolcarb by a three-dimensional molecularly imprinted electrochemical sensor. Biosens. Bioelectron. 2015, 64, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Uzek, R.; Senel, S.; Say, R.; Denizli, A. Chiral recognition of proteins having L-histidine residues on the surface with lanthanide ion complex incorporated-molecularly imprinted fluorescent nanoparticles. Mater. Sci. Eng. C 2013, 33, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Piri-Moghadam, H. Electroentrapment of polyaniline in [3-(2,3-epoxypropoxy)propyl]trimethoxysilane-derived xerogel: A facile methodology towards molecularly imprinted xerogels. Chromatographia 2014, 77, 1185–1194. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Liu, G.; Pan, M.; Wang, X.; Kong, L.; He, X.; Wang, S. Electrochemical sensor based on molecularly imprinted polymer film via sol-gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens. Bioelectron. 2013, 47, 475–481. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.M.; Segatelli, M.G.; Tarley, C.R. Hybrid molecularly imprinted poly(methacrylic acid-TRIM)-silica chemically modified with (3-glycidyloxypropyl)trimethoxysilane for the extraction of folic acid in aqueous medium. Mater. Sci. Eng. C 2016, 59, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, K.; Wu, D.; Wang, J.; Sun, B. A novel quantum dots-labeled on the surface of molecularly imprinted polymer for turn-off optosensing of dicyandiamide in dairy products. Biosens. Bioelectron. 2016, 77, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Viveiros, R.; Moro, A.; Lima, J.C.; Bonifácio, V.D.B.; Casimiro, T. Supercritical CO2-assisted synthesis of an ultrasensitive amphibious quantum dot-molecularly imprinted sensor. RSC Adv. 2014, 4, 63338–63341. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Guo, Y.; Qi, J. A Ag-molecularly imprinted polymer composite for efficient surface-enhanced Raman scattering activities under a low-energy laser. Analyst 2015, 140, 3239–3243. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Chen, B.L.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 adsorption in a microporous metal-organic framework with open metal sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2011, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2011, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic framework for sensing applications. TrAC 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal-Organic Frameworks. ChemPlusChem 2016, 81, 675. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.-H.; Bansal, V.; Kumar, P.A.; Deep, A. Practical utilization of nanocrystal metal organic framework biosensor for parathion specific recognition. Microchem. J. 2016, 128, 102–107. [Google Scholar] [CrossRef]
- Riskin, M.; Tel-Vered, R.; Bourenko, T.; Granot, E.; Willner, I. Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: Development of an electrochemical TNT sensor based on p-donor-acceptor interactions. J. Am. Chem. Soc. 2008, 130, 9726–9733. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Cristea, C.; Vocanson, F.; Săndulescu, R.; Jaffrezic-Renault, N. Electrochemical sensor for the detection of estradiol based on electropolymerized molecularly imprinted polythioaniline film with signal amplification using gold nanoparticles. Electrochem. Commun. 2015, 59, 36–39. [Google Scholar] [CrossRef]
- Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins 2015, 7, 3540–3553. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Florea, A.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Zhang, A.; Săndulescu, R.; Lagarde, F.; Jaffrezic-Renault, N. 1,3,5-Trinitrotoluene detection by a molecularly imprinted polymersensor based on electropolymerization of amicroporous-metal-organic framework. Sens. Actuators B Chem. 2015, 207, 960–966. [Google Scholar] [CrossRef]
- Florea, A.; Guo, Z.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Dzyadevych, S.; Săndulescu, R.; Jaffrezic-Renault, N. Anticancer drug detection using a highly sensitive molecularly imprinted electrochemical sensor based on an electropolymerized microporous metal organic framework. Talanta 2015, 138, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.T.; Jaffrezic-Renault, N. Molecularly imprinted polymer based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Săndulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metalorganic framework for tetracycline detection in honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Braganca, A.; Araujo, M.; Bonifácio, V.D.B. Polyurea dendrimer-perylene self-imprinted nanoshells for trace explosives detection. Part. Part. Syst. Charact. 2015, 32, 98–103. [Google Scholar] [CrossRef]
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Deng, Q.; Fang, G.; Wang, J.; Pan, M.; Wang, S.; Pu, Y. Metal-organic frameworks supported surface-imprinted nanoparticles for the sensitive detection of metolcarb. Biosens. Bioelectron. 2016, 79, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Deng, Q.; Fang, G.; Gu, D.; Yang, Y.; Wang, S. Upconversion fluorescence metal-organic frameworks thermosensitive imprinted polymer for enrichment and sensing protein. Biosens. Bioelectron. 2016, 79, 341–346. [Google Scholar] [CrossRef] [PubMed]
| Target | Transduction Type | Reference | |||||
|---|---|---|---|---|---|---|---|
| Electrochemistry | Quartz Microbalance | Fluorescence | |||||
| Detection Limit | Dynamic Range | Detection Limit | Dynamic Range | Detection Limit | Dynamic Range | ||
| estradiol | 0.3 pg/L | 1 pg/L to 1 µg/L | [37] | ||||
| aflatoxin | 0.9 pg/L | 1 pg/L to 1 mg/L | [38] | ||||
| 1,3,5-trinitrotoluene | 0.9 pg/L | 1 pg/L to 1 µg/L | [39] | ||||
| gemcitabine | 0.9 pg/L | 1.1 pg/L to 11.4 µg/L | [40] | ||||
| glyphosate | 0.8 pg/L | 1 pg/L to 1 µg/L | [41] | ||||
| tetracycline | 44.4 pg/L | 9 pg/L to 10 µg/L | [42] | ||||
| metalocarb | 0.069 mg/L | 0.1–0.9 mg/L | [45] | ||||
| hemoglobine | 0.062 mg/mL | 0–0.6 mg/mL | [46] | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Florea, A.; Jiang, M.; Mei, Y.; Zhang, W.; Zhang, A.; Săndulescu, R.; Jaffrezic-Renault, N. Molecularly Imprinted Polymer/Metal Organic Framework Based Chemical Sensors. Coatings 2016, 6, 42. https://doi.org/10.3390/coatings6040042
Guo Z, Florea A, Jiang M, Mei Y, Zhang W, Zhang A, Săndulescu R, Jaffrezic-Renault N. Molecularly Imprinted Polymer/Metal Organic Framework Based Chemical Sensors. Coatings. 2016; 6(4):42. https://doi.org/10.3390/coatings6040042
Chicago/Turabian StyleGuo, Zhenzhong, Anca Florea, Mengjuan Jiang, Yong Mei, Weiying Zhang, Aidong Zhang, Robert Săndulescu, and Nicole Jaffrezic-Renault. 2016. "Molecularly Imprinted Polymer/Metal Organic Framework Based Chemical Sensors" Coatings 6, no. 4: 42. https://doi.org/10.3390/coatings6040042





