Translation Effects in Fluorine Doped Tin Oxide Thin Film Properties by Atmospheric Pressure Chemical Vapour Deposition
Abstract
:1. Introduction
2. Experimental Section
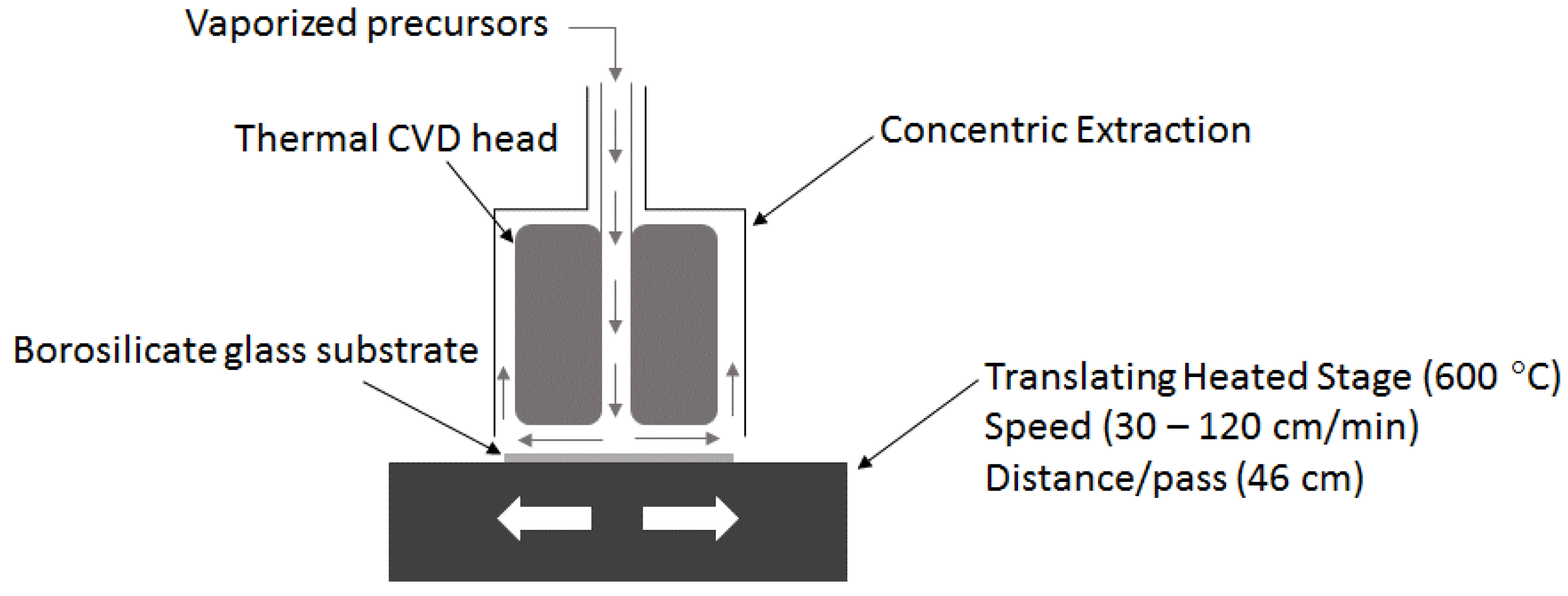
2.1. Deposition of Thin Films
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beneking, C.; Rech, B.; Wieder, S.; Kluth, O.; Wagner, H.; Frammelsberger, W.; Geyer, R.; Lechner, P.; Rubel, H.; Schade, H. Recent developments of silicon thin film solar cells on glass substrates. Thin Solid Films 1999, 351, 241–246. [Google Scholar] [CrossRef]
- Tiwari, A.N.; Khrypunov, G.; Kurdzesau, F.; Bätzner, D.L.; Romeo, A.; Zogg, H. CdTe solar cell in a novel configuration. Prog. Photovolt. Res. Appl. 2004, 12, 33–38. [Google Scholar] [CrossRef]
- Lewis, B.G.; Paine, D.C. Applications and processing of transparent conducting oxides. Mater. Res. Soc. Bull. 2000, 25, 22–27. [Google Scholar] [CrossRef]
- Edwards, P.P.; Porch, A.; Jones, M.O.; Morgan, D.V.; Perks, R.M. Basic materials physics of transparent conducting oxides. Dalton Trans. 2004, 19, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Baraton, M.-I. Transparent conductive oxide materials: financial stakes and technological challenges. Int. J. Nanotech. 2009, 6, 776–784. [Google Scholar] [CrossRef]
- Sheel, D.W.; Yates, H.M.; Evans, P.; Dagkaldiran, U.; Gordijn, A.; Finger, F.; Remes, Z.; Vanecek, M. Atmospheric pressure chemical vapour deposition of F doped SnO2 for optimum performance solar cells. Thin Solid Films 2009, 517, 3061–3065. [Google Scholar] [CrossRef]
- Noor, N.; Chew, C.K.T.; Bhachu, D.S.; Waugh, M.R.; Carmalt, C.J.; Parkin, I.P. Influencing FTO thin film growth with thin seeding layers: A route to microstructural modification. J. Mater. Chem. C 2015, 3, 9359–9368. [Google Scholar] [CrossRef]
- Vernardou, D.; Pemble, M.E.; Sheel, D.W. In-situ Fourier transform infrared spectroscopy gas phase studies of vanadium (IV) oxide coating by atmospheric pressure chemical vapour deposition using vanadyl (IV) acetylacetonate. Thin Solid Films 2008, 516, 4502–4507. [Google Scholar] [CrossRef]
- Springer, J.; Poruba, A.; Vanecek, M. Improved three-dimensional optical model for thin-film silicon solar cells. J. Appl. Phys. 2004, 90, 5329–5337. [Google Scholar] [CrossRef]
- Yates, H.M.; Evans, P.; Sheel, D.W.; Nicolay, S.; Ding, L.; Ballif, C. High-performance tandem silicon solar cells on F:SnO2. Surf. Coat Technol. 2013, 230, 228–233. [Google Scholar] [CrossRef]
- Yates, H.M.; Evans, P.; Sheel, D.W.; Nicolay, S.; Ding, L.; Ballif, C. The development of high performance SnO2:F as TCOs for thin film silicon solar cells. Surf. Coat. Technol. 2012, 213, 167–174. [Google Scholar] [CrossRef]
- Mehmood, U.; Afzaal, M.; Al-Ahmed, A.; Yates, H.M.; Hakeem, A.S. Effect of transparent conductive oxide (TCO) film properties on the photovoltaic performance of dye-sensitized solar cells: A move towards 11% efficiency. IEEE J. Photovol. 2016. submitted. [Google Scholar]
- Agashe, C.; Hüpkes, J.; Schöpe, G.; Berginski, M. Physical properties of highly oriented spray-deposited fluorine-doped tin dioxide films as transparent conductor. Sol. Energy Mater. Sol. Cells 2009, 93, 1256–1262. [Google Scholar] [CrossRef]
- Illiberi, A.; Kniknie, B.; Deelan, J.V.; Steijvers, H.L.A.H.; Habets, D.; Simons, P.J.P.M.; Janssen, A.C.; Beckers, E.H.A. Industrial high-rate (14 nm/s) deposition of low resistive and transparent ZnOx:Al films on glass. Sol. Energy Mater. Sol. Cells 2011, 95, 1955–1959. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, D.H.; Win, S.Z.; Li, S.Y.; Chen, Y.P. Electrical and optical properties of F-doped textured SnO2 films deposited by APCVD. Sol. Energy Mater. Sol. Cells 1996, 40, 371–380. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, D.H.; Chen, Y.P.; Li, S.Y.; Ma, J.; Zong, F.J. Large scale fluorine doped textured transparent conducting SnO2 films deposited by APCVD. Proc. Soc. Photo. Opt. Instrum. 1996, 2897, 104–112. [Google Scholar]
- Yates, H.M.; Evans, P.; Sheel, D.W.; Dagkaldiran, U.; Gordijn, A.; Finger, F.; Remes, Z.; Vanecek, M. Optimum performance solar cells using atmospheric pressure chemical vapour deposition deposited TCOs. Int. J. Nanotechnol. 2009, 6, 816–827. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Barrett, C.S.; Massalski, T.B. Structure of Metals; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Agashe, C.; Takwale, T.; Bhide, V.; Mahamuni, S.; Kulkarni, S.K. Effect of Sn incorporation on the growth mechanism of sprayed SnO2 films. J. Appl. Phys. 1991, 70, 7382–7386. [Google Scholar] [CrossRef]
- Smith, A.; Laurent, J.-M.; Smith, D.S.; Bonnet, J.-P.; Clemente, R.R. Relation between solution chemistry and morphology of SnO2-based thin films deposited by a pyrosol process. Thin Solid Films 1995, 266, 20–30. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, X.L.; Liu, W.W.; Zhang, X.H.; Wang, J.V.; Pyrah, L.; Sanderson, K.D.; Ramsey, P.M.; Hirata, M.; Tsuri, K. Influence of preferred orientation on the electrical conductivity of fluorine-doped tin oxide films. Sci. Rep. 2014, 4, 3679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Zhong, X.H.; Wang, J.N. Significant roughness enhancement of fluorine-doped tin oxide films with low resistivity and high transparency by using HNO3 addition. RSC Adv. 2015, 5, 52174–52182. [Google Scholar] [CrossRef]
- Yates, H.M.; Evans, P.; Sheel, D.W. The influence of F-doping in SnO2 thin films. Phys. Procedia 2013, 46, 159–166. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, X.L.; Zhang, X.H.; Wang, J.N.; Pyrah, L.; Sanderson, K.D.; Ramsey, P.M.; Hirata, M.; Tsuri, K. Morphology control of fluorine-doped tin oxide thin films for enhanced light trapping. Sol. Energy Mater. Sol. Cells 2015, 132, 578–588. [Google Scholar] [CrossRef]
- Canestraro, C.D.; Oliveira, M.M.; Valaski, R.; da Silva, M.V.S.; David, D.G.F.; Pepe, I.; da Silva, A.F.; Roman, L.S.; Persson, C. Strong inter-conduction-band absorption in heavily fluorine doped tin oxide. Appl. Surf. Sci. 2008, 255, 1874–1879. [Google Scholar] [CrossRef]
- Proscia, J.; Gordon, R.G. Properties of fluorine-doped tin oxide films produced by atmospheric pressure chemical vapor deposition from tetramethyltin, bromotrifluoromethane and oxygen. Thin Solid Films 1992, 214, 175–187. [Google Scholar] [CrossRef]
- Oshima, M.; Takemoto, Y.; Yoshino, K. Optical and electrical characterization of FTO films grown by spray pyrolysis method. Phys. Status Solidi (c) 2009, 6, 1124–1126. [Google Scholar] [CrossRef]
- Van den Donker, M.N.; Gordijn, A.; Stiebig, H.; Finger, F.; Rech, B.; Stannowski, B.; Bartl, R.; Hamers, E.A.G.; Schlatmann, R.; Jongerden, G.J. Flexible amorphous and microcrystalline silicon tandem solar modules in the temporary superstrate concept. Sol. Energy Mater. Sol. Cells 2007, 91, 572–580. [Google Scholar] [CrossRef]
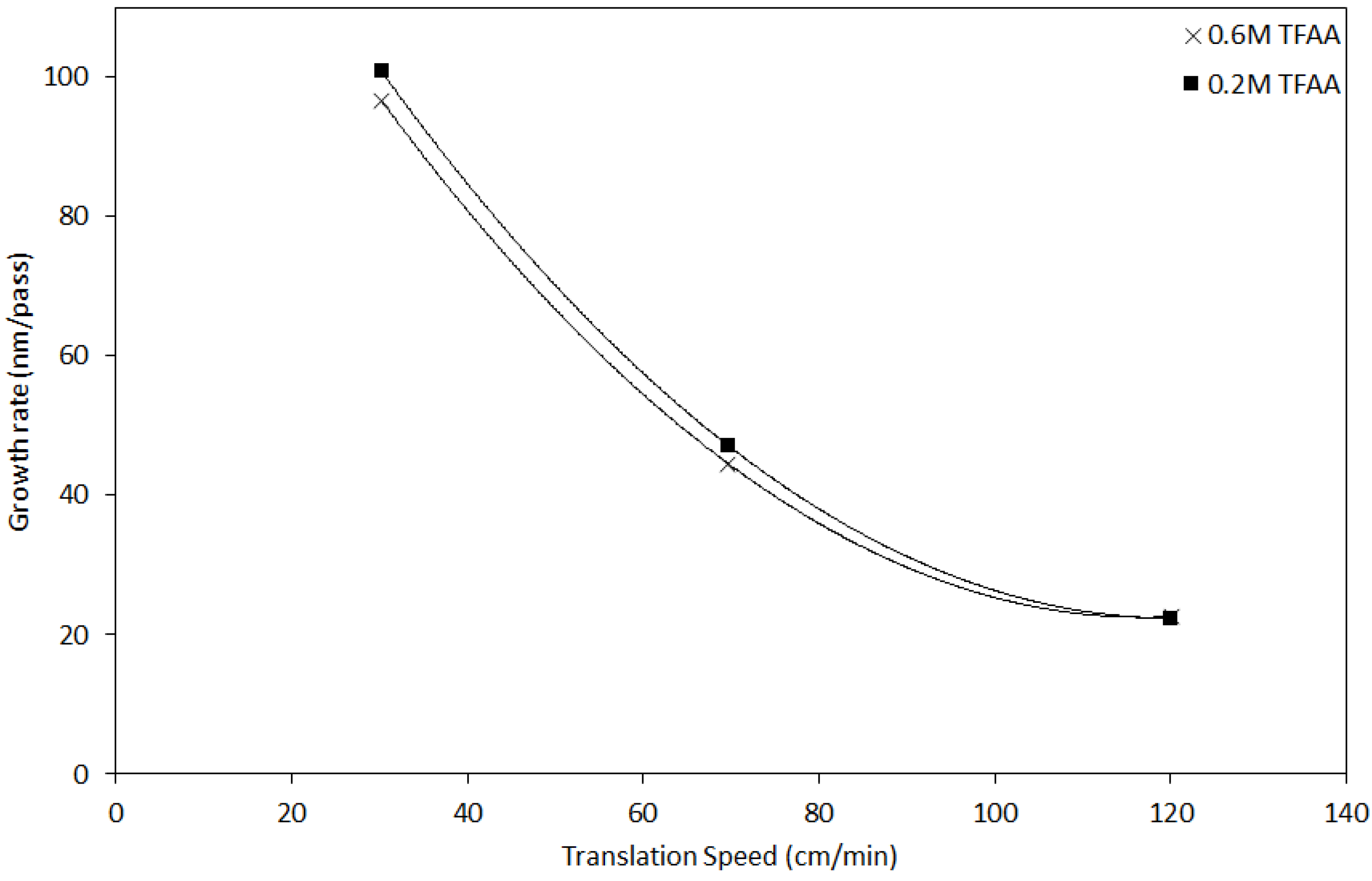


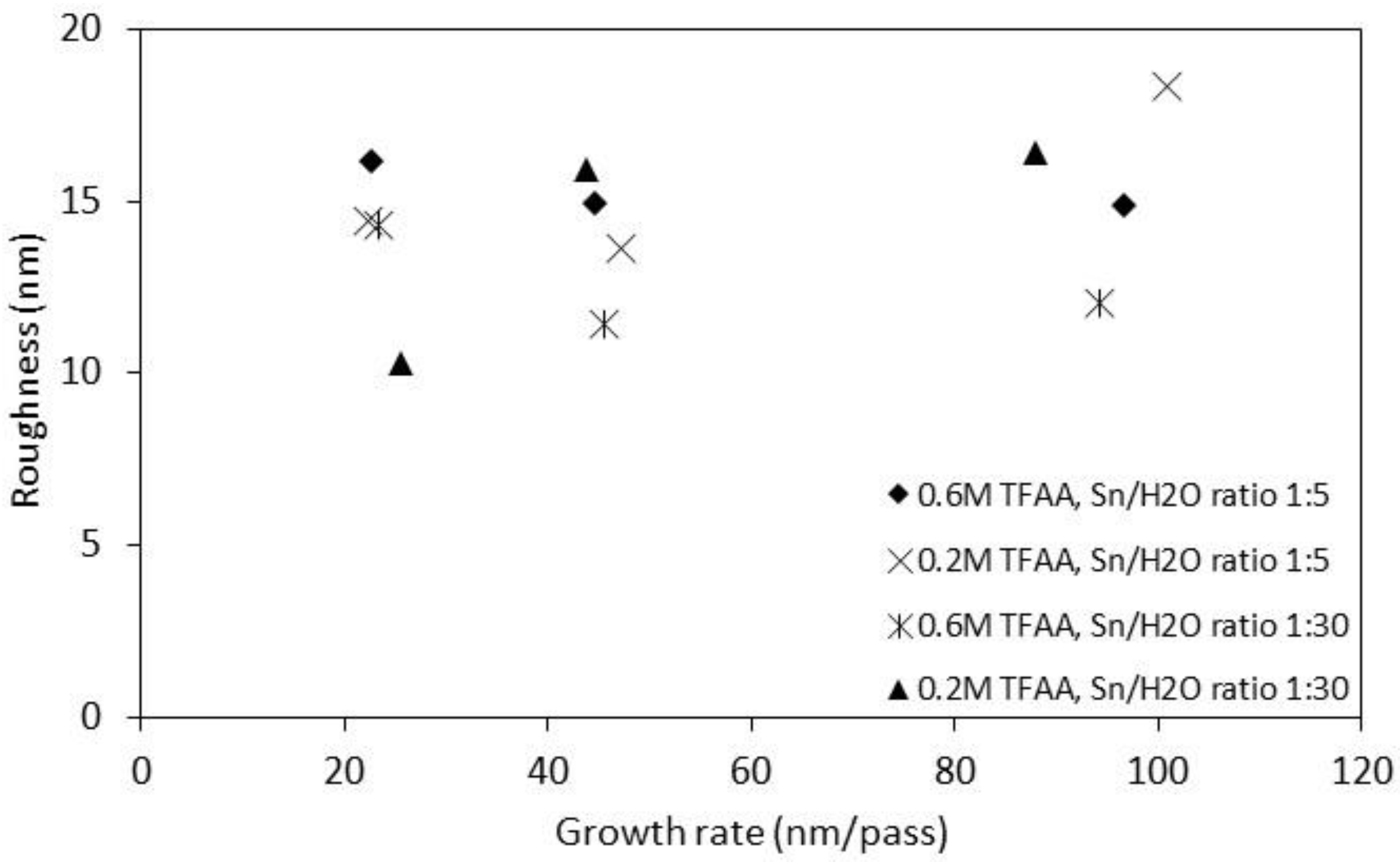
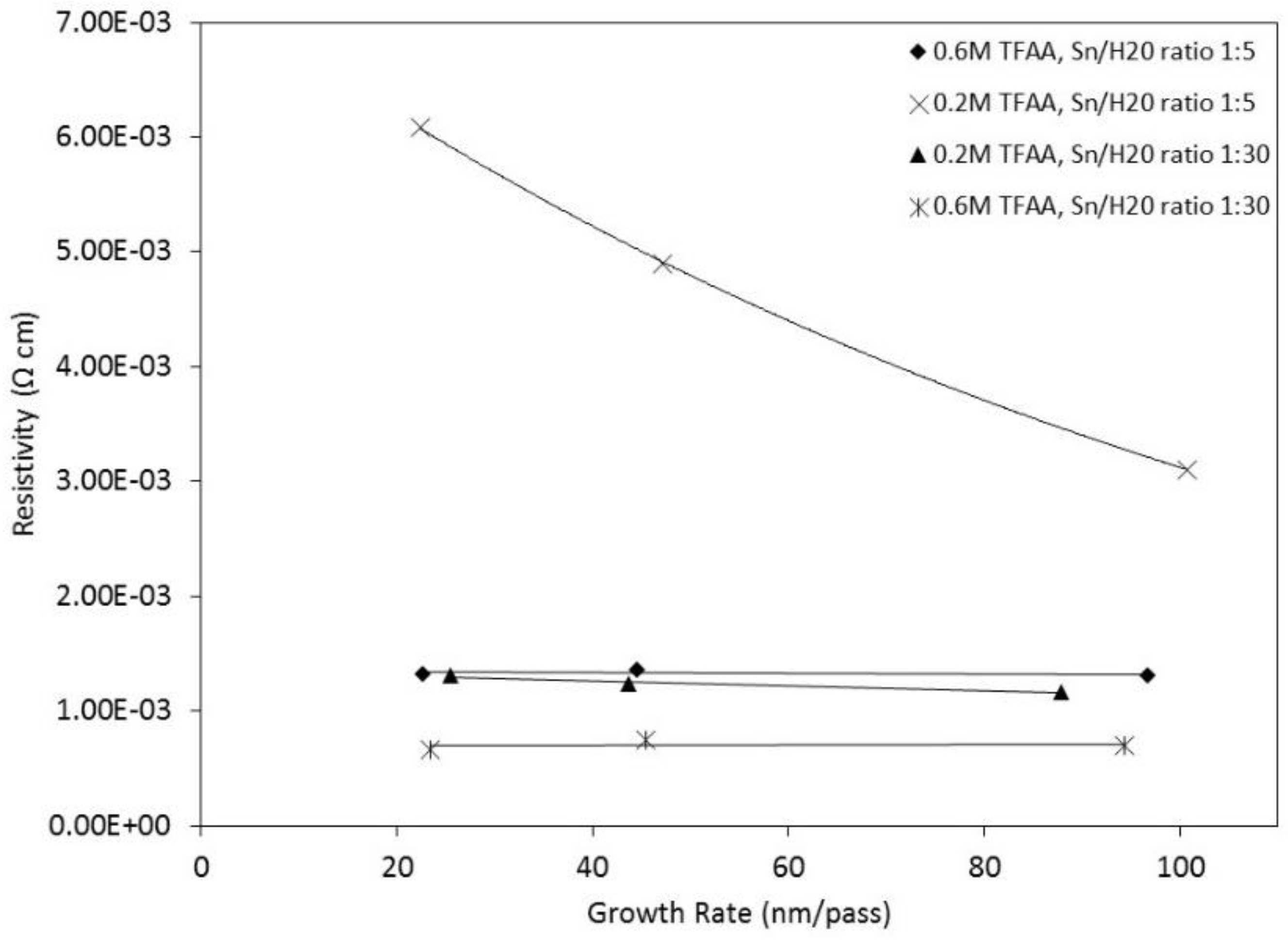
| Sample No. | Sn/H2O Ratio | TFAA Concentration(M) | Translation Rate (cm/min) | d (μm) | RMS Roughness (nm) | Gr (nm/pass) | p/×10−3 (Ω cm) |
| S1 | 1:5 | 0.6 | 30 | 0.386 ± 0.012 | 15 | 96.62 | 1.31 |
| S2 | 1:5 | 0.6 | 70 | 0.356 ± 0.02 | 15 | 44.54 | 1.36 |
| S3 | 1:5 | 0.6 | 120 | 0.361 ± 0.026 | 16 | 22.59 | 1.32 |
| S4 | 1:5 | 0.2 | 30 | 0.403 ± 0.02 | 18 | 100.8 | 3.1 |
| S5 | 1:5 | 0.2 | 70 | 0.377 ± 0.037 | 14 | 47.14 | 4.9 |
| S6 | 1:5 | 0.2 | 120 | 0.358 ± 0.016 | 14 | 22.38 | 6.09 |
| S7 | 1:30 | 0.6 | 30 | 0.377 ± 0.015 | 12 | 94.31 | 0.7 |
| S8 | 1:30 | 0.6 | 70 | 0.364 ± 0.018 | 11 | 45.5 | 0.65 |
| S9 | 1:30 | 0.6 | 120 | 0.373 ± 0.012 | 14 | 23.36 | 0.75 |
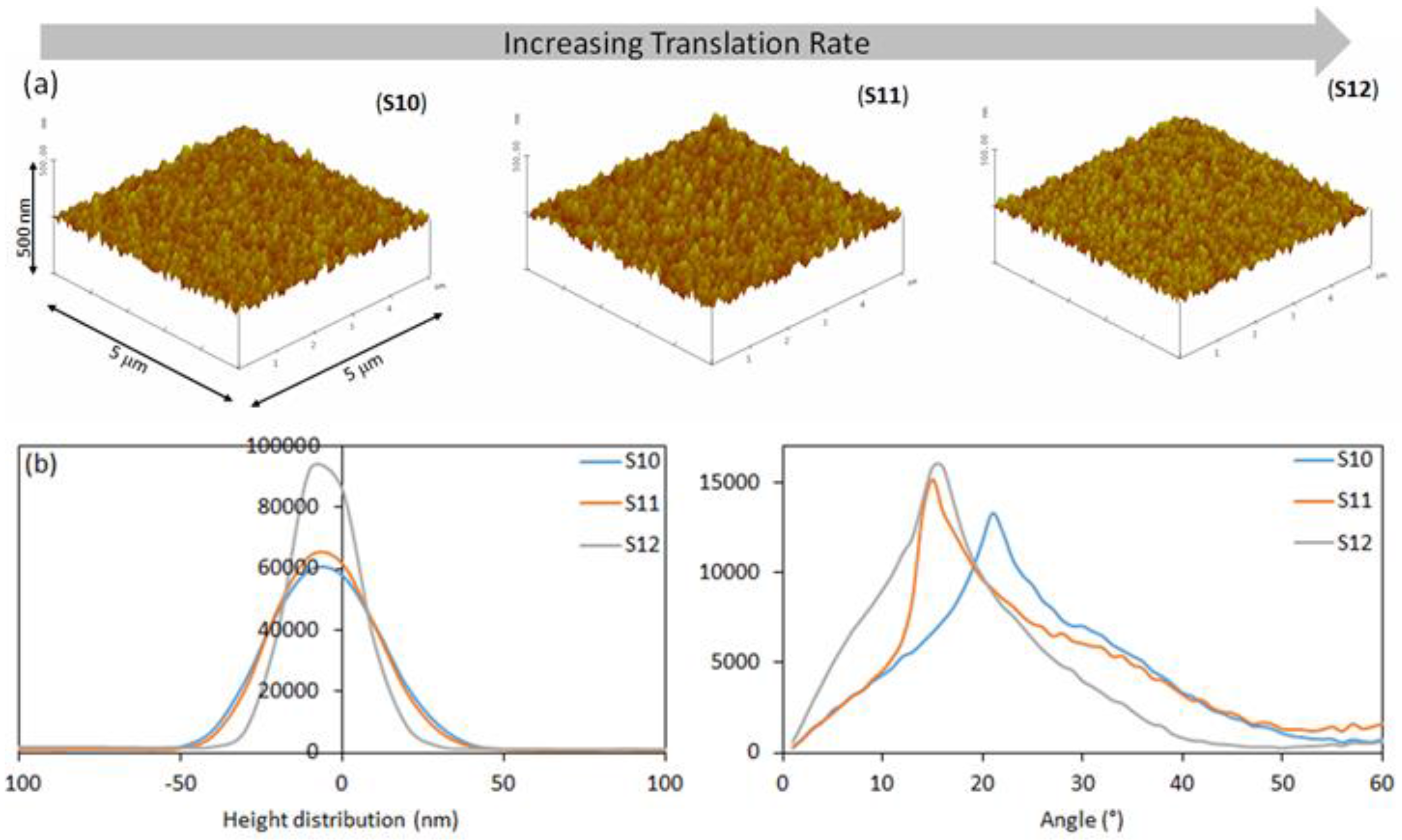
| S10 | 1:30 | 0.2 | 30 | 0.351 ±0.025 | 16 | 87.84 | 1.16 |
| S11 | 1:30 | 0.2 | 70 | 0.350 ± 0.045 | 16 | 43.72 | 1.24 |
| S12 | 1:30 | 0.2 | 120 | 0.407 ± 0.014 | 11 | 25.42 | 1.31 |
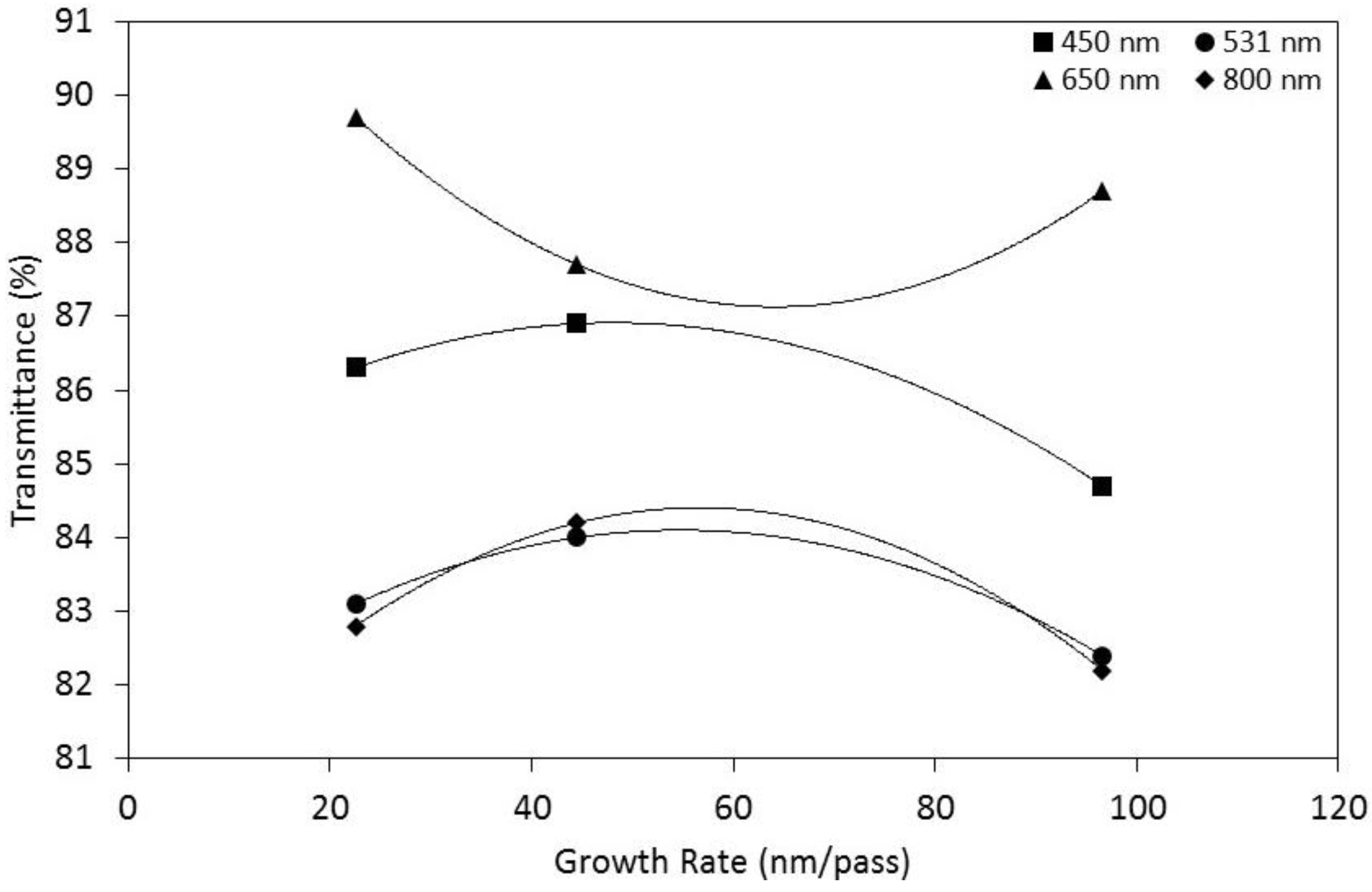
| Sample No. | µ (cm2/V·s) | N/×1020 (cm−3) | H (%) 450 nm | T (%) 450 nm | T (%) 531 nm | T (%) 650 nm | T (%) 800 nm |
| S1 | 17 | 3.3 | 1.3 | 84.7 | 82.4 | 88.7 | 82.2 |
| S2 | 20 | 2.6 | 0.8 | 86.9 | 84 | 87.7 | 84.2 |
| S3 | 8.9 | 7.1 | 0.9 | 86.3 | 83.1 | 89.7 | 82.8 |
| S4 | 13 | 1.6 | 2 | 83.8 | 86.5 | 85.1 | 85.2 |
| S5 | 21 | 0.69 | 1.1 | 84.1 | 83.9 | 88.4 | 83.9 |
| S6 | 19 | 0.58 | 1.2 | 86.4 | 82.2 | 89.1 | 80 |
| S7 | 21 | 4.7 | 0.6 | 85.2 | 83.6 | 87.5 | 81.8 |
| S8 | 22 | 4.2 | 0.8 | 85.4 | 83.9 | 87.1 | 83.5 |
| S9 | 21 | 3.9 | 0.3 | 85.7 | 81.8 | 86.9 | 82.3 |
| S10 | 18 | 3.3 | 1.2 | 84.9 | 84.2 | 88.8 | 82.4 |
| S11 | 13 | 4.7 | 1.2 | 85.3 | 84.9 | 88.2 | 82.1 |
| S12 | 22 | 2.3 | 1 | 83.9 | 85.3 | 86 | 86.9 |
| Sample No. | Grain Size (nm) | TC (110) | TC (101) | TC (200) | TC (211) |
|---|---|---|---|---|---|
| S1 | 37 | 0.24 | 0.02 | 3.50 | 0.24 |
| S2 | 37 | 0.16 | 0.02 | 3.62 | 0.20 |
| S3 | 36 | 0.12 | 0.01 | 3.81 | 0.06 |
| S4 | 29 | 0.77 | 0.06 | 2.86 | 0.31 |
| S5 | 30 | 0.94 | 0.07 | 2.62 | 0.38 |
| S6 | 30 | 0.67 | 0.04 | 3.06 | 0.23 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzaal, M.; Yates, H.M.; Hodgkinson, J.L. Translation Effects in Fluorine Doped Tin Oxide Thin Film Properties by Atmospheric Pressure Chemical Vapour Deposition. Coatings 2016, 6, 43. https://doi.org/10.3390/coatings6040043
Afzaal M, Yates HM, Hodgkinson JL. Translation Effects in Fluorine Doped Tin Oxide Thin Film Properties by Atmospheric Pressure Chemical Vapour Deposition. Coatings. 2016; 6(4):43. https://doi.org/10.3390/coatings6040043
Chicago/Turabian StyleAfzaal, Mohammad, Heather M. Yates, and John L. Hodgkinson. 2016. "Translation Effects in Fluorine Doped Tin Oxide Thin Film Properties by Atmospheric Pressure Chemical Vapour Deposition" Coatings 6, no. 4: 43. https://doi.org/10.3390/coatings6040043






