Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dripping Test
2.2. Submersion Test
2.3. Saturation Test
3. Results
3.1. Preliminary Results
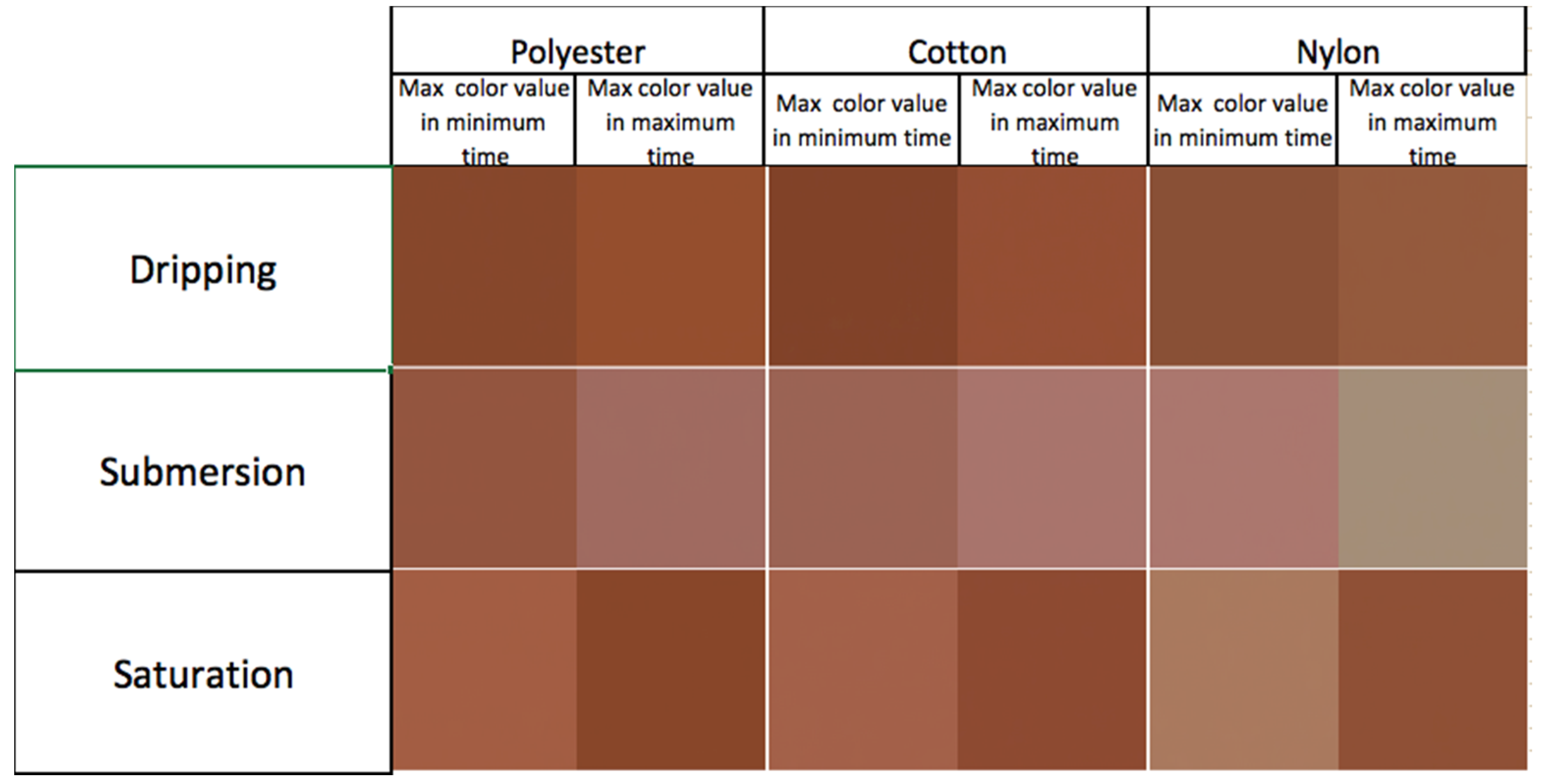
3.2. Final Testing—Dripping
3.3. Final Testing—Submersion
3.4. Final Testing—Saturation
4. Discussions
4.1. Preliminary Results
4.2. Final Testing—Dripping
4.3. Final Testing—Submersion
4.4. Final Testing—Saturation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The Fiber Year 2016: World Survey on Textiles & Nonwovens; The Fiber Year Consulting; The Fiber Year GmbH: Speicher, Switzerland, 2016; Issue 16.
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Zaffalon, V. Climate change, carbon mitigation and textiles. Text. World 2010, 160, 34–35. [Google Scholar]
- Montero, G.A.; Smith, C.B.; Hendrix, W.A.; Butcher, D.L. Supercritical fluid technology in textile processing: An overview. Ind. Eng. Chem. Res. 2000, 39, 4806–4812. [Google Scholar] [CrossRef]
- Saus, W.; Knittel, D.; Schollmeyer, E. Dyeing of textiles in supercritical carbon dioxide. Text. Res. J. 1993, 63, 135–142. [Google Scholar] [CrossRef]
- Perrut, M. Supercritical fluid applications: Industrial developments and economic issues. Ind. Eng. Chem. Res. 2000, 39, 4531–4535. [Google Scholar] [CrossRef]
- Gupta, S. Inkjet printing—A revolutionary ecofriendly technique for textile printing. Indian J. Fibre Text. Res. 2001, 26, 156–161. [Google Scholar]
- Xue, C.-H.; Shi, M.-M.; Chen, H.-Z.; Wu, G.; Wang, M. Preparation and application of nanoscale microemulsion as binder for fabric inkjet printing. Colloids Surf. A Physicochem. Eng. Asp. 2006, 287, 147–152. [Google Scholar] [CrossRef]
- Fan, Q.; Kim, Y.K.; Perruzzi, M.K.; Lewis, A.F. Fabric pretreatment and digital textile print quality. J. Imaging Sci. Technol. 2003, 47, 400–407. [Google Scholar]
- Samanta, A.K.; Konar, A. Dyeing of textiles with natural dyes. In Natural Dyes; Kumbasar, E.P.A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Samanta, A.K.; Agarwal, P. Application of natural dyes on textiles. Indian J. Fibre Text. Res. 2009, 3, 384–399. [Google Scholar]
- Sharma, D.; Gupta, C.; Aggarwal, S.; Nagpal, N. Pigment extraction from fungus for textile dyeing. Indian J. Fibre Text. Res. 2012, 37, 68–73. [Google Scholar]
- Hamlyn, P.F. Fungal biotechnology. British Mycological Society Newsletter, May 1998. [Google Scholar]
- Atalla, M.; El-Khrisy, E.A.M.; Youssef, Y.A.; Mohamed, A. Production of textile reddish brown dyes by fungi. Malays. J. Microbiol. 2011, 7, 33–40. [Google Scholar]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Feasibility of using red pigment producing fungi to stain wood for decorative applications. Can. J. Forest Res. 2011, 41, 1722–1728. [Google Scholar] [CrossRef]
- Saikawa, Y.; Watanabe, T.; Hashimoto, K.; Nakata, M. Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of Chlorociboria species. Phytochemistry 2000, 55, 237–240. [Google Scholar] [CrossRef]
- Golinksi, P.; Krick, T.P.; Blanchette, R.A.; Mirocha, C.J. Chemical characterization of a red pigment (5,8-dihydroxy-2,7-dimethoxy-1,4-naphthalenedione) produced by Arthrographis cuboidea in pink stained wood. Holzforschung 2009, 49, 407–410. [Google Scholar]
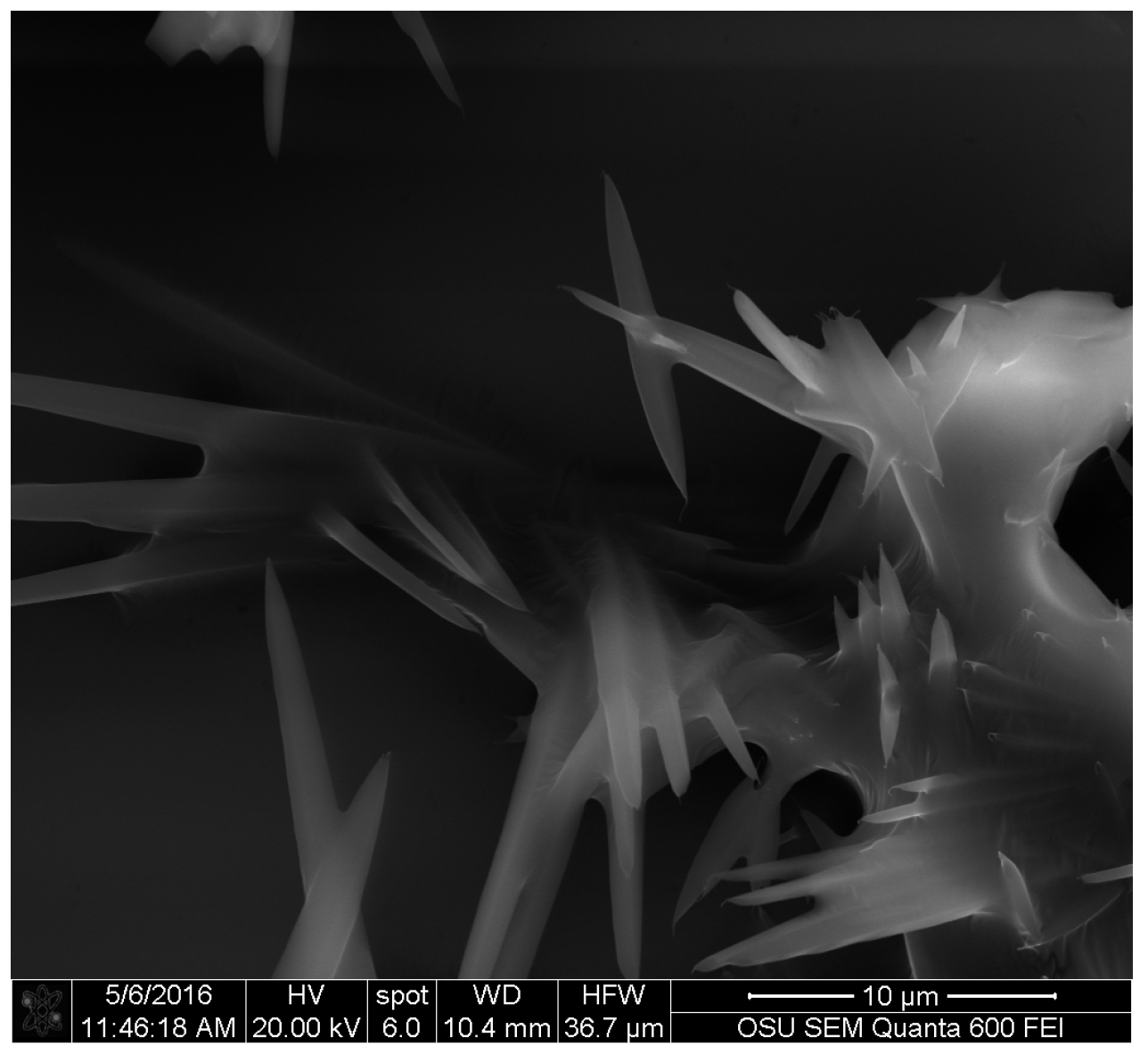
- Vega Gutierrez, S.; Robinson, S.C. Microscopic analysis of pigments extracted from spalting fungi. J. Fungi 2017, 3, 15. [Google Scholar] [CrossRef]
- Weber, G.; Chen, H.-L.; Hinsch, E.; Freitas, S.; Robinson, S. Pigments extracted from the wood-staining fungi Chlorociboria aeruginosa, Scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Color. Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.M.; Weber, G.; Chen, H.-L.; Robinson, S.C. Colorfastness of extracted wood-staining fungal pigments on fabrics: A new potential for textile dyes. J. Text. Apparel Technol. Manag. 2015, 9, 1–11. [Google Scholar]
- Robinson, S.C.; Gutierrez, S.M.V.; Garcia, R.A.C.; Iroume, N.; Vorland, N.R.; McClelland, A.; Huber, M.; Stanton, S. Potential for carrying dyes derived from spalting fungi in natural oils. J. Coat. Technol. Res. 2017, 14, 1107–1113. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Snider, H.; Cooper, P.A. Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems. AMB Express 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Adelantado, J.V.; Mateo-Castro, R.; Doménech-Carbó, M.T.; Bosch-Reig, F.; Doménech-Carbó, A.; Casas-Catalán, M.J.; Osete-Cortina, L. Identification of lipid binders in paintings by gas chromatography: Influence of the pigments. J. Chromatogr. A 2001, 922, 385–390. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Hinsch, E.M. A Comparative Analysis of Extracted Fungal Pigments and Commercially Available Dyes for Colorizing Textiles. Master’s Thesis, Oregon State University, Corvallis, OR, USA, June 2015. [Google Scholar]
- Vega Gutierrez, S.M. Spalting Fungi: Genetic Identification, Material Interactions and Microscopical Characteristics of Extracted Pigments. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, December 2017. [Google Scholar]
- Vega Gutierrez, S.M.; Vega Gutierrez, P.T.; Godinez, A.; Pittis, L.; Huber, M.; Stanton, S.; Robinson, S.C. Feasibility of coloring bamboo with the application of natural and extracted fungal pigments. Coatings 2016, 6, 37. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino Agurto, M.E.; Vega Gutierrez, S.M.; Chen, H.-L.; Robinson, S.C. Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes. Coatings 2017, 7, 152. https://doi.org/10.3390/coatings7100152
Palomino Agurto ME, Vega Gutierrez SM, Chen H-L, Robinson SC. Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes. Coatings. 2017; 7(10):152. https://doi.org/10.3390/coatings7100152
Chicago/Turabian StylePalomino Agurto, Mardonio E., Sarath M. Vega Gutierrez, Hsiou-Lien Chen, and Seri C. Robinson. 2017. "Wood-Rotting Fungal Pigments as Colorant Coatings on Oil-Based Textile Dyes" Coatings 7, no. 10: 152. https://doi.org/10.3390/coatings7100152




