Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films
Abstract
:1. Introduction
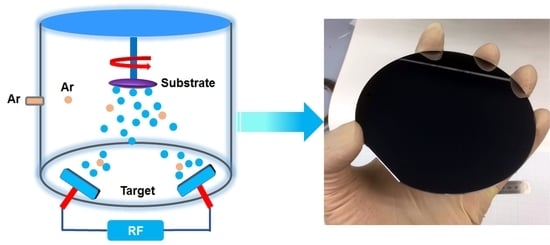
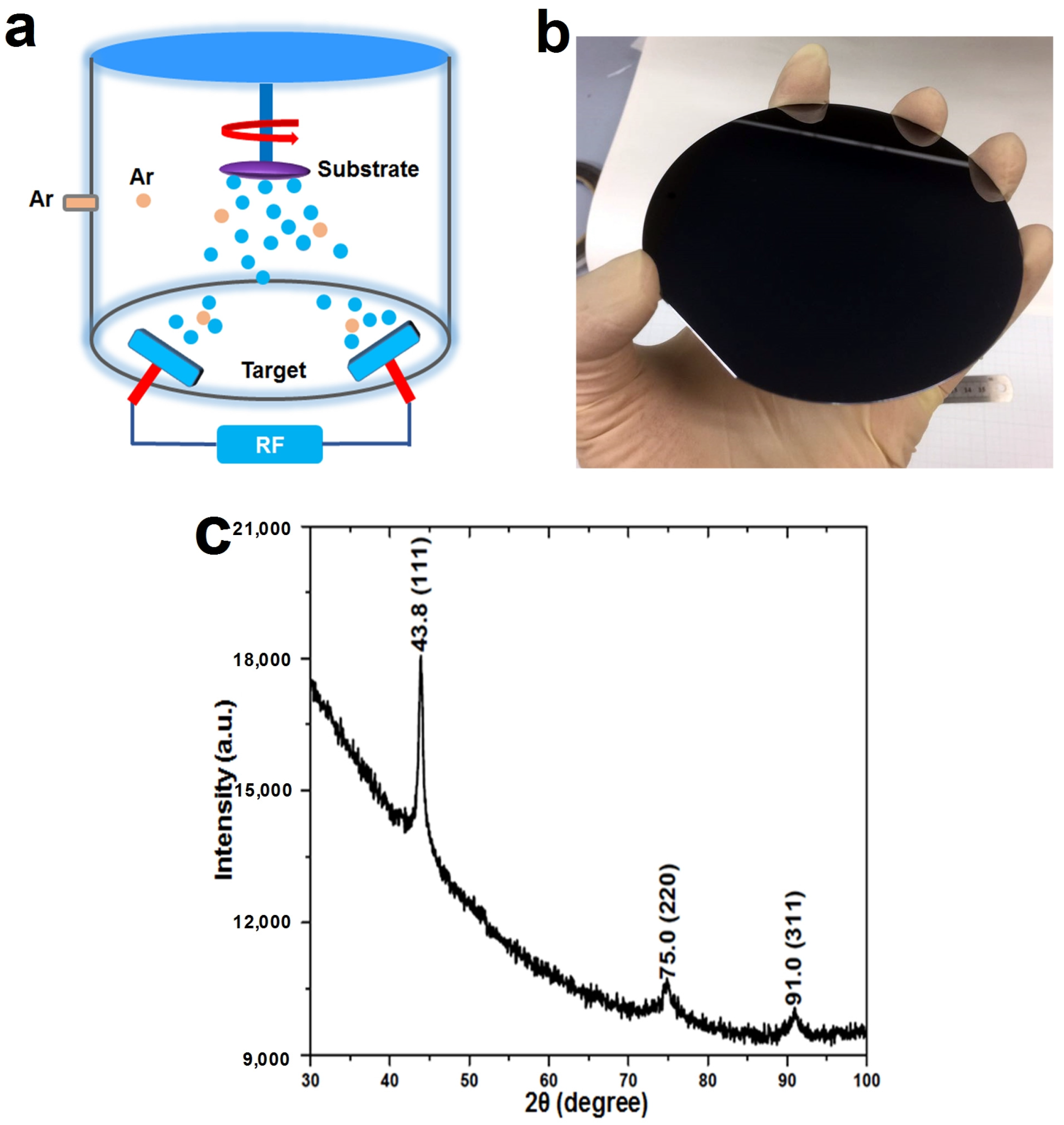
2. Materials and Methods
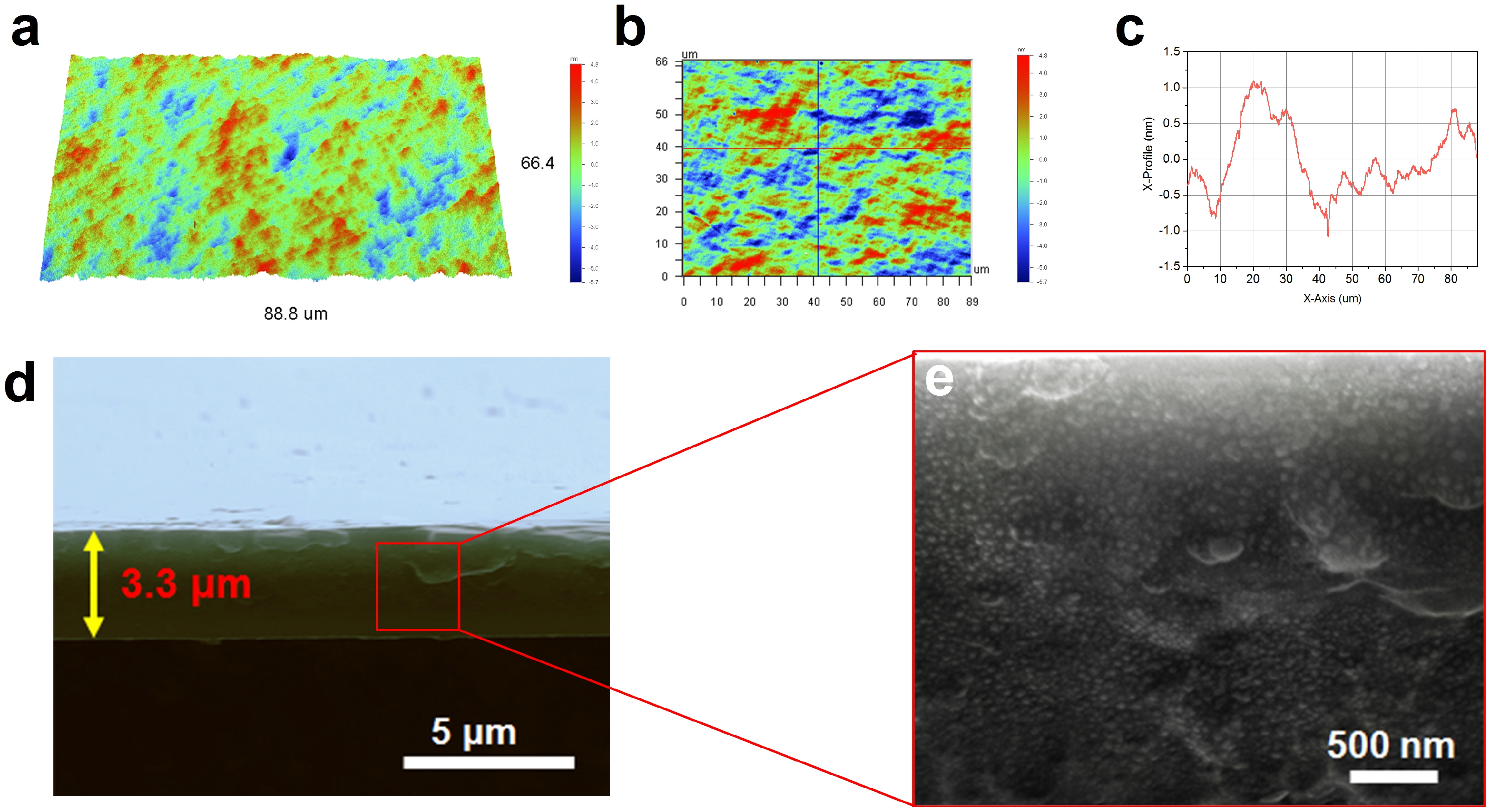
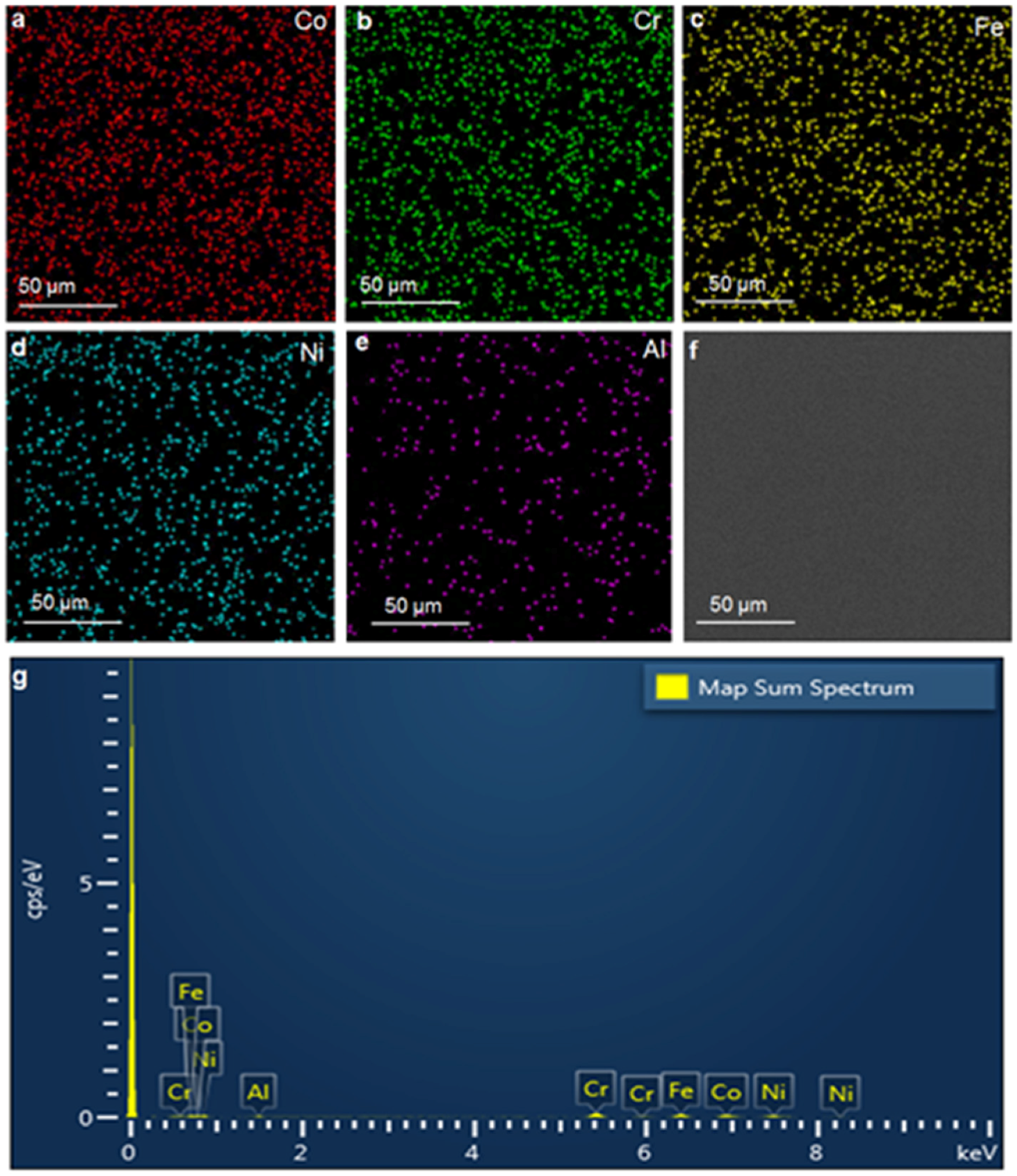
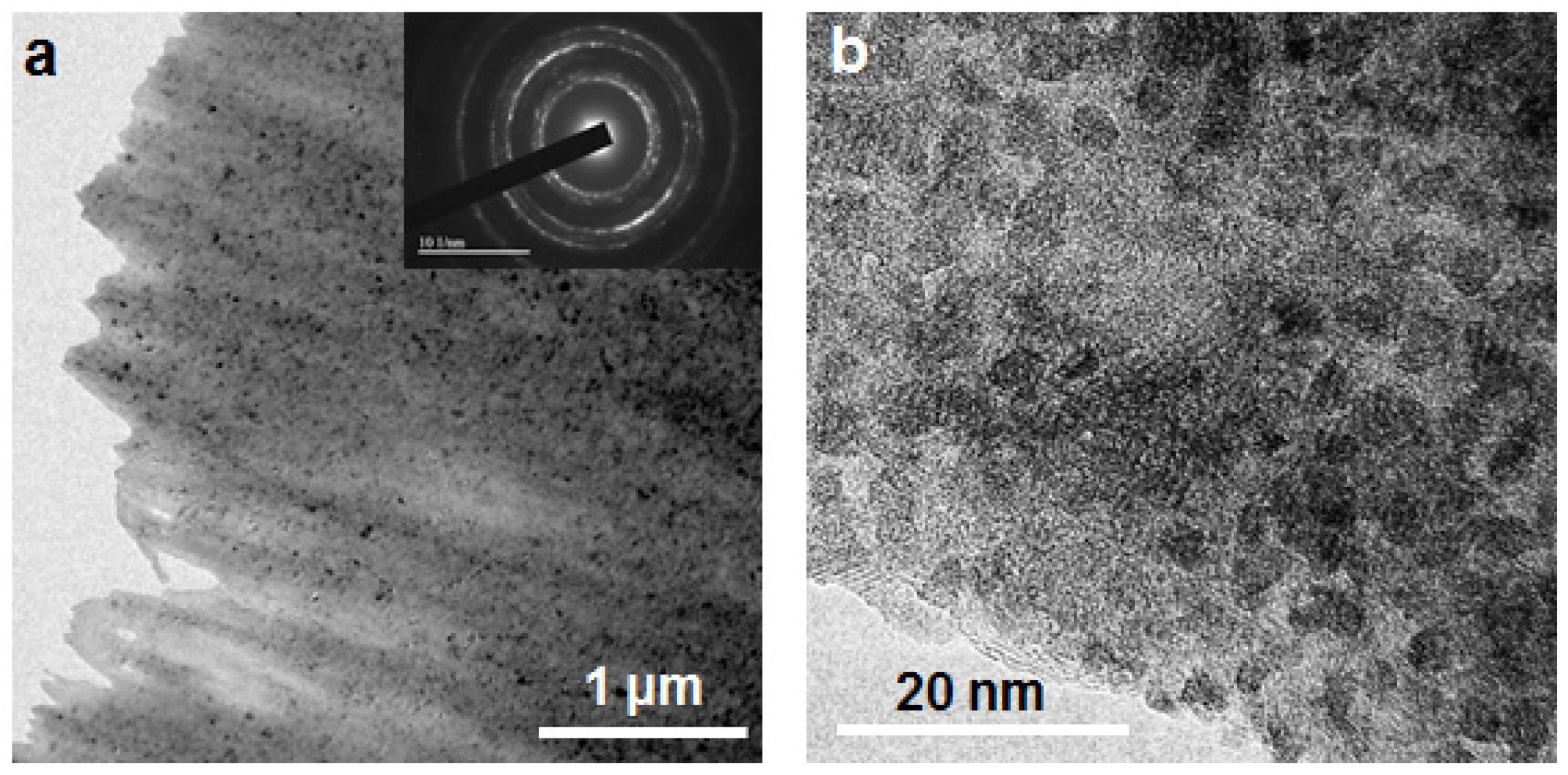
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Solid solution alloys of properties Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature. Appl. Phys. Lett. 2014, 90, 181904. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.; Tsai, M.; Wang, W.; Lin, S.; Yeh, J. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Cocrfeni, A.; Lin, C.; Tsai, H. Intermetallics evolution of microstructure, hardness, and corrosion properties of high-entropy. Intermetallics 2011, 19, 288–294. [Google Scholar]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.J.; Liaw, P.K.; Zhang, Y. High-entropy Al0.3CoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, S.; Gao, M.C.; Liaw, P.K.; Zhang, Y. A successful synthesis of the CoCrFeNiAl0.3 single-crystal, high-entropy alloy by Bridgman solidification. JOM 2013, 65, 1751–1758. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Sun, Y.F.; Goh, M.H.; Ng, F.L.; Nguyen, Q.B.; Fujii, H.; Nai, S.M.L.; Wei, J.; Shek, C.H. Friction stir welding of a CoCrFeNiAl0.3 high entropy alloy. Mater. Lett. 2017, 205, 142–144. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Ren, M.; Yang, C.; Fu, H. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Kao, Y.; Chen, S.; Chen, T.; Chu, P.; Yeh, J.; Lin, S. Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 2011, 509, 1607–1614. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, S.; Qiao, J.; Wang, Z.; Gao, M.C.; Jiao, Z.; Yang, H.; Zhang, Y. Superior high tensile elongation of a single-crystal CoCrFeNiAl0.3 high-entropy alloy by Bridgman solidification. Intermetallics 2014, 54, 104–109. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Jia, H.; Liu, F.; An, Z.; Li, W.; Wang, G.; Chu, J.P.; Jang, J.S.C.; Gao, Y.; Liaw, P.K. Thin-film metallic glasses for substrate fatigue-property improvements. Thin Solid Films 2014, 561, 2–27. [Google Scholar] [CrossRef]
- Chu, J.P.; Greene, J.E.; Jang, J.S.C.; Huang, J.C.; Shen, Y.L.; Liaw, P.K.; Yokoyama, Y.; Inoue, A.; Nieh, T.G. Bendable bulk metallic glass: Effects of a thin, adhesive, strong, and ductile coating. Acta Mater. 2012, 60, 3226–3238. [Google Scholar] [CrossRef]
- Chu, J.P.; Liu, T.Y.; Li, C.L.; Wang, C.H.; Jang, J.S.C.; Chen, M.J.; Chang, S.H.; Huang, W.C. Fabrication and characterizations of thin film metallic glasses: Antibacterial property and durability study for medical application. Thin Solid Films 2014, 561, 102–107. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Luo, S.; Lee, J.H.; Liu, C.W.; Shieh, J.M.; Shen, C.H.; Wu, T.T.; Jang, D.; Greer, J.R. Strength, stiffness, and microstructure of Cu(In,Ga)Se2 thin films deposited via sputtering and co-evaporation. Appl. Phys. Lett. 2014, 105, 011907. [Google Scholar] [CrossRef]
- Dou, D.; Li, X.C.; Zheng, Z.Y.; Li, J.C. Coatings of FeAlCoCuNiV high entropy alloy. Surf. Eng. 2016, 32, 766–770. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Dou, D.; Li, J.; Centre, E.T. Microstructure and properties of coating of FeAlCuCrCoMn high entropy alloy deposited by direct current magnetron sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, L.; Lui, H.W.; Cai, M.H.; Yeh, J.W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Braeckman, B.R.; Boydens, F.; Hidalgo, H.; Dutheil, P.; Jullien, M.; Thomann, A.-L.; Depla, D. High entropy alloy thin films deposited by magnetron sputtering of powder targets. Thin Solid Films 2015, 580, 71–76. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, G.J.; Wen, D.H.; Zhang, T.H. Nanoindentation creep behavior in a CoCrFeCuNi high-entropy alloy film with two different structure states. Mater. Sci. Eng. A 2015, 621, 111–117. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys-a comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, L.; He, W.; Liaw, P.K. Alloying and processing effects on the aqueous corrosion behavior of high-entropy alloys. Entropy 2014, 16, 895–911. [Google Scholar] [CrossRef]
- Qiu, Y.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 2015, 31, 1235–1243. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-solution CrCoCuFeNi high-entropy alloy thin films synthesized by sputter deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef]
- Blanco, A.; Chomski, E.; Grabtchak, S.; Marta, I.; John, S.; Leonard, S.W.; Cefe, L.; Francisco, M.; Hernan, M.; Mondia, J.P.; et al. Large-scale synthesis of a silicon photonic crystal with a complete three-dimensional bandgap. Nature 2000, 405, 437–440. [Google Scholar] [PubMed]
- Zhang, P.; Li, S.; Zhang, Z. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, W.; Guan, Z.; Cui, Z. Multi-layer laser solid forming of Zr65Al7.5Ni10Cu17.5 amorphous coating: Microstructure and corrosion resistance. Opt. Laser Technol. 2015, 69, 17–22. [Google Scholar] [CrossRef]

| Sample | Ecorr (VSCE) | Icorr (μA cm−2) | Ep (VSCE) |
|---|---|---|---|
| HEA | −0.451 | 0.103 | 1.07 |
| Steel | −0.482 | 0.125 | 0.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Liao, W.; Zhang, H.; Surjadi, J.U.; Sun, D.; Lu, Y. Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films. Coatings 2017, 7, 156. https://doi.org/10.3390/coatings7100156
Gao L, Liao W, Zhang H, Surjadi JU, Sun D, Lu Y. Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films. Coatings. 2017; 7(10):156. https://doi.org/10.3390/coatings7100156
Chicago/Turabian StyleGao, Libo, Weibing Liao, Hongti Zhang, James Utama Surjadi, Dong Sun, and Yang Lu. 2017. "Microstructure, Mechanical and Corrosion Behaviors of CoCrFeNiAl0.3 High Entropy Alloy (HEA) Films" Coatings 7, no. 10: 156. https://doi.org/10.3390/coatings7100156