Fabrication of an Anisotropic Superhydrophobic Polymer Surface Using Compression Molding and Dip Coating
Abstract
:1. Introduction
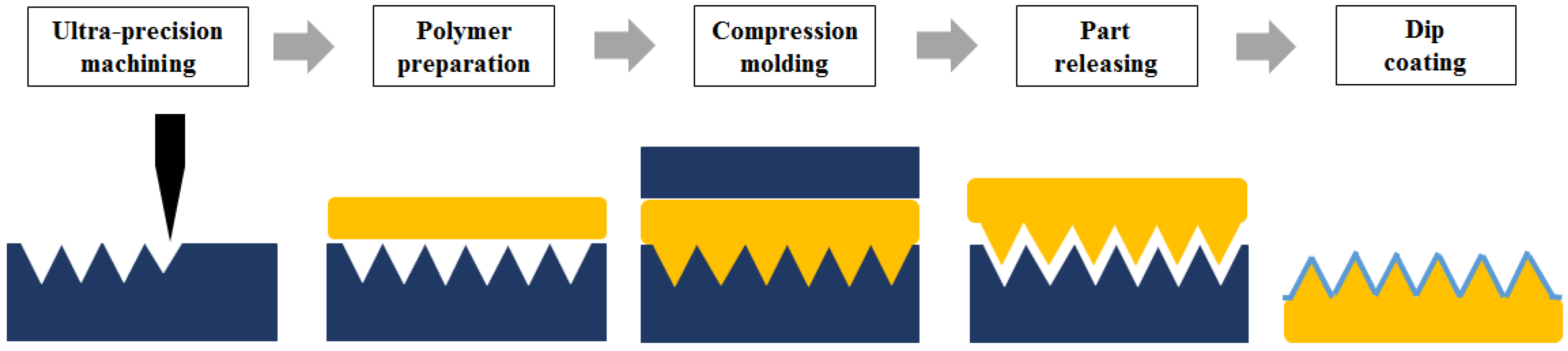
2. Materials and Methods
3. Results
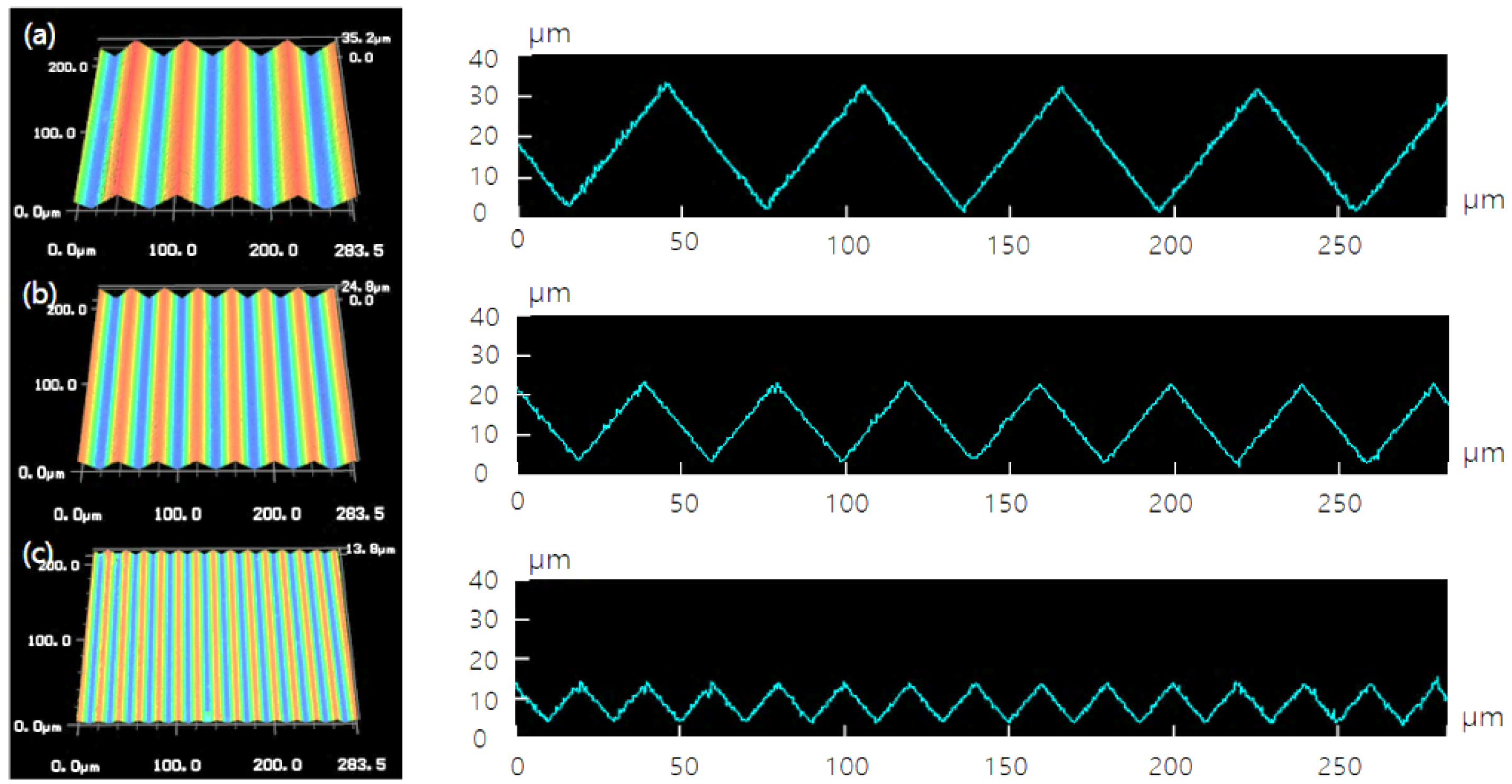
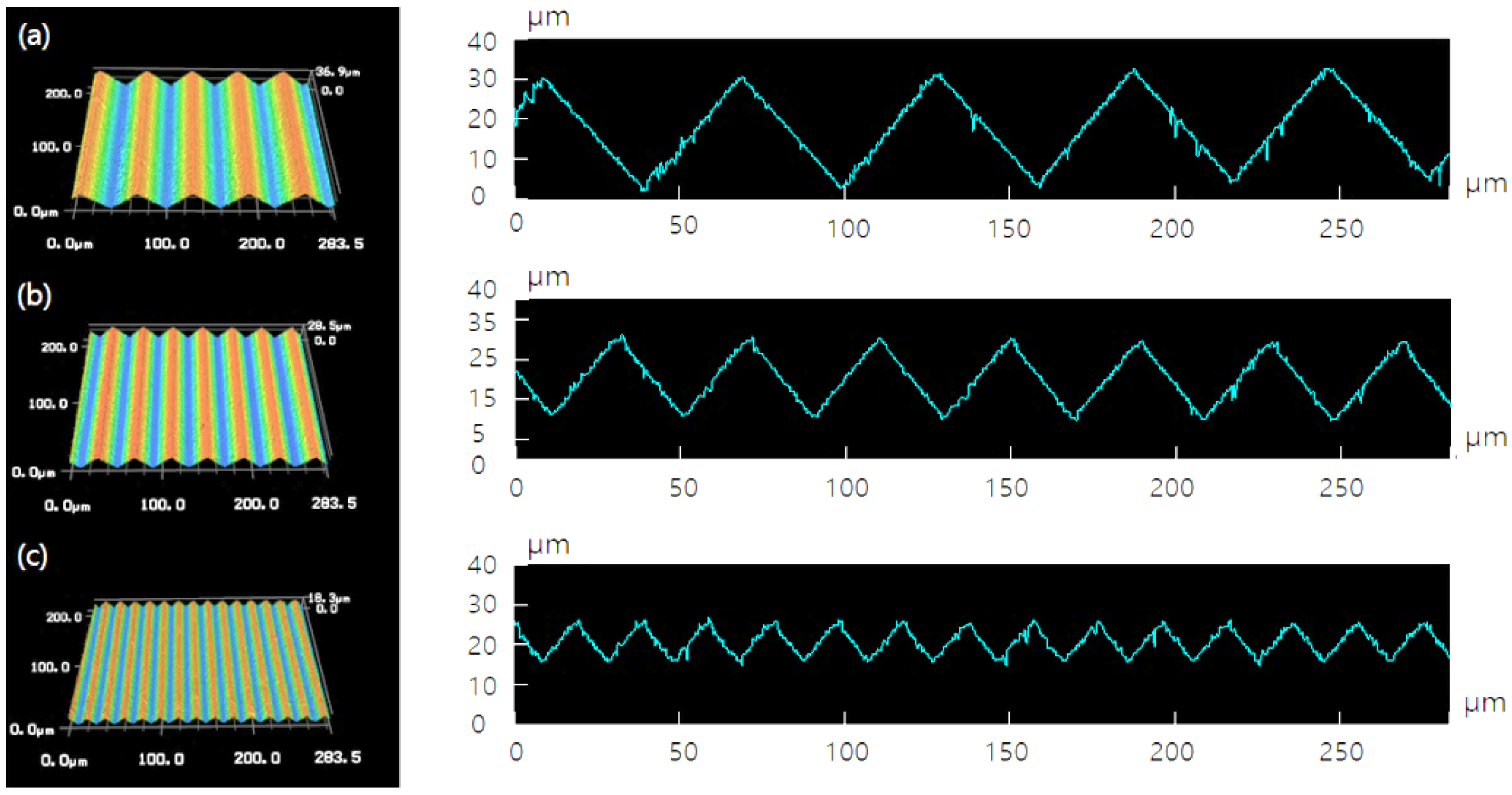
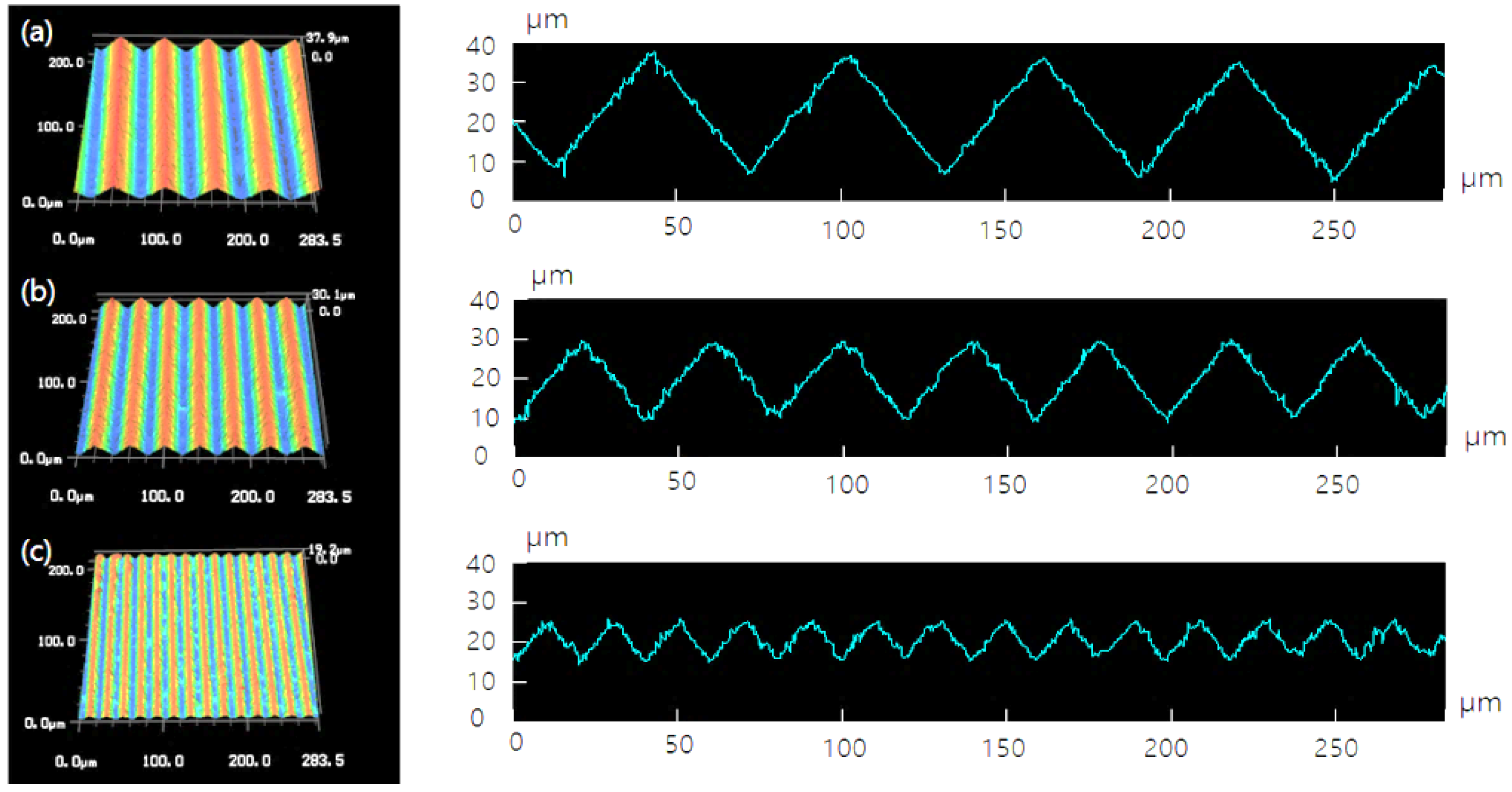
3.1. Surface Morphology
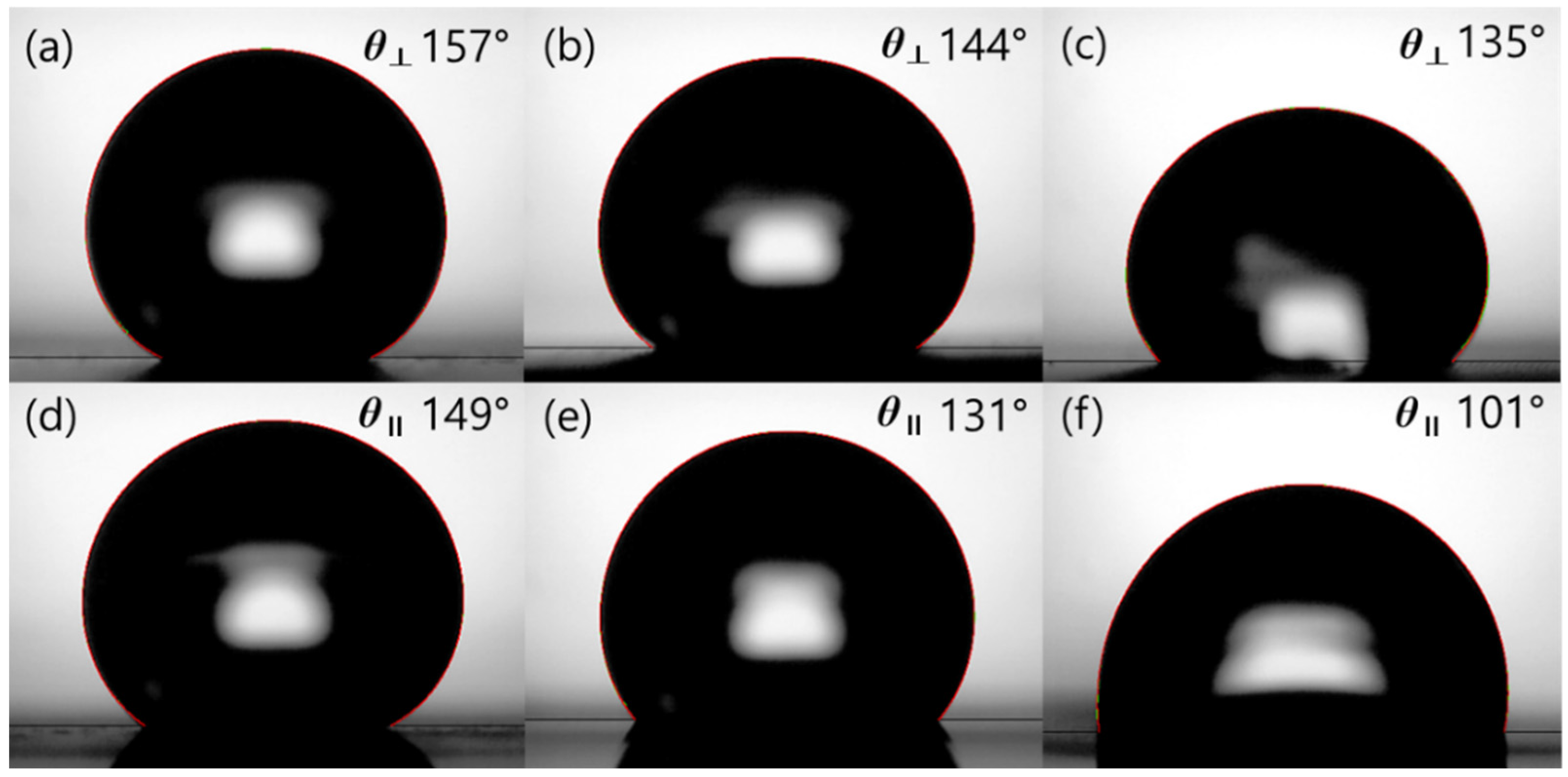
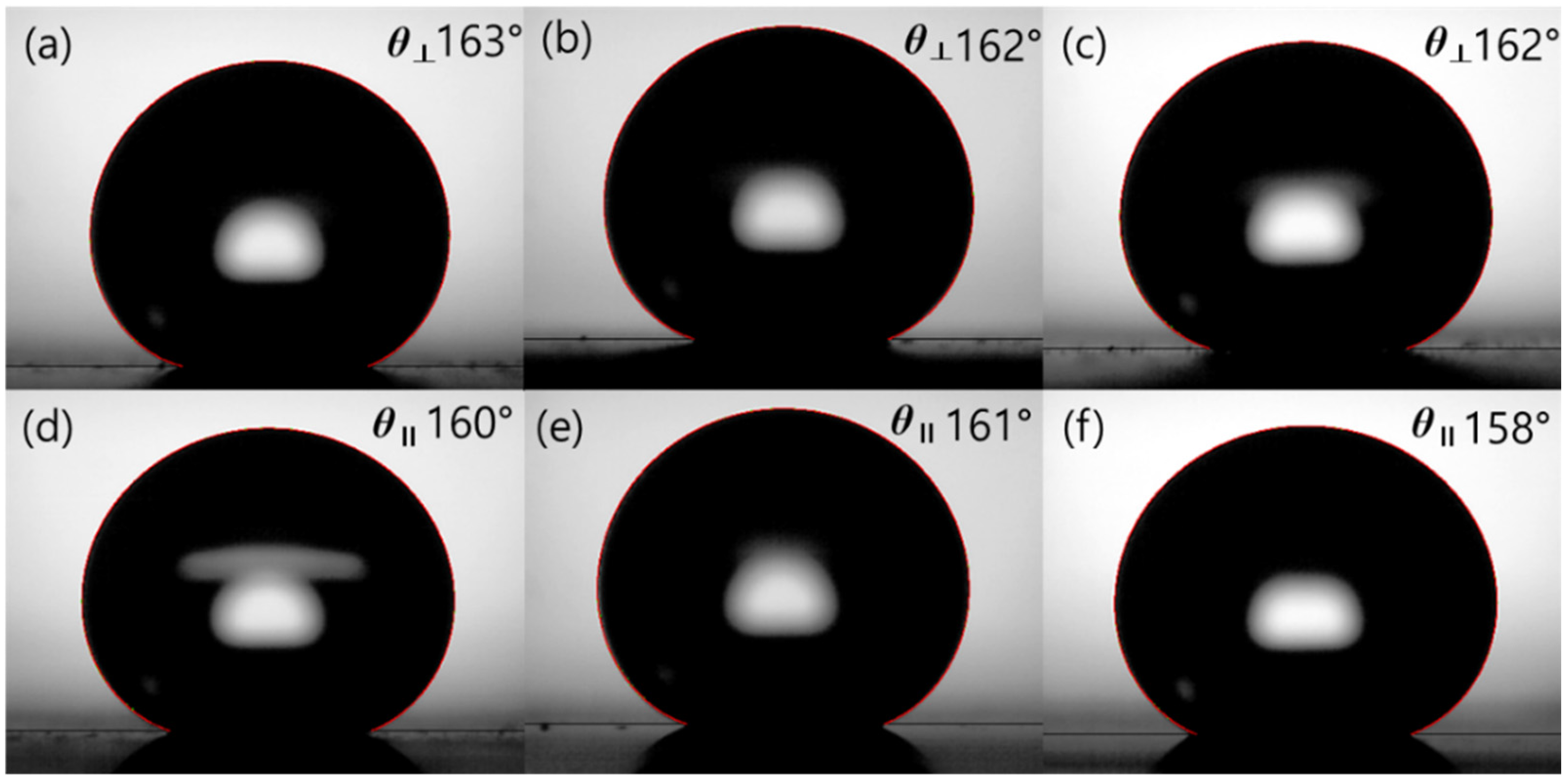
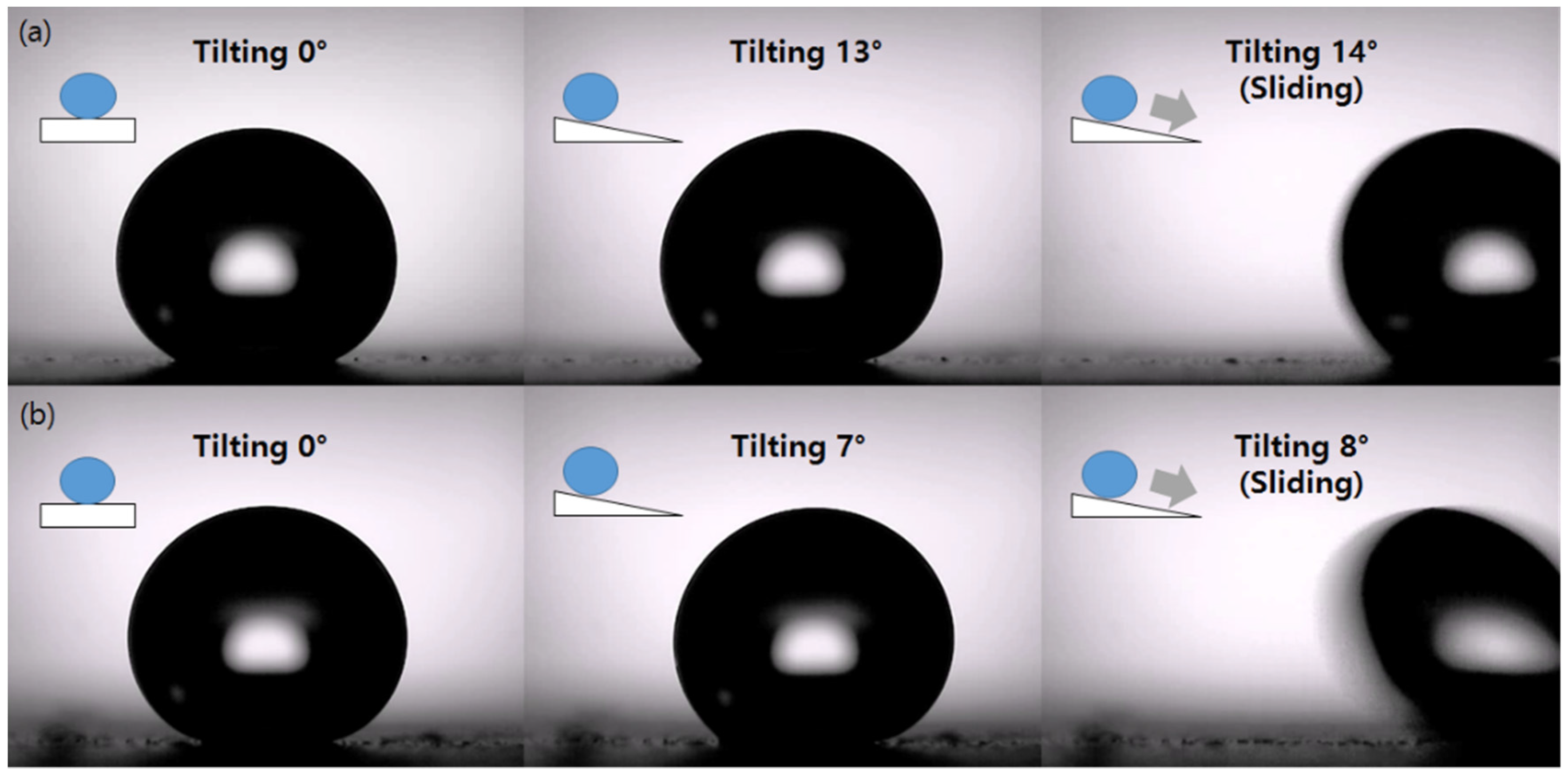
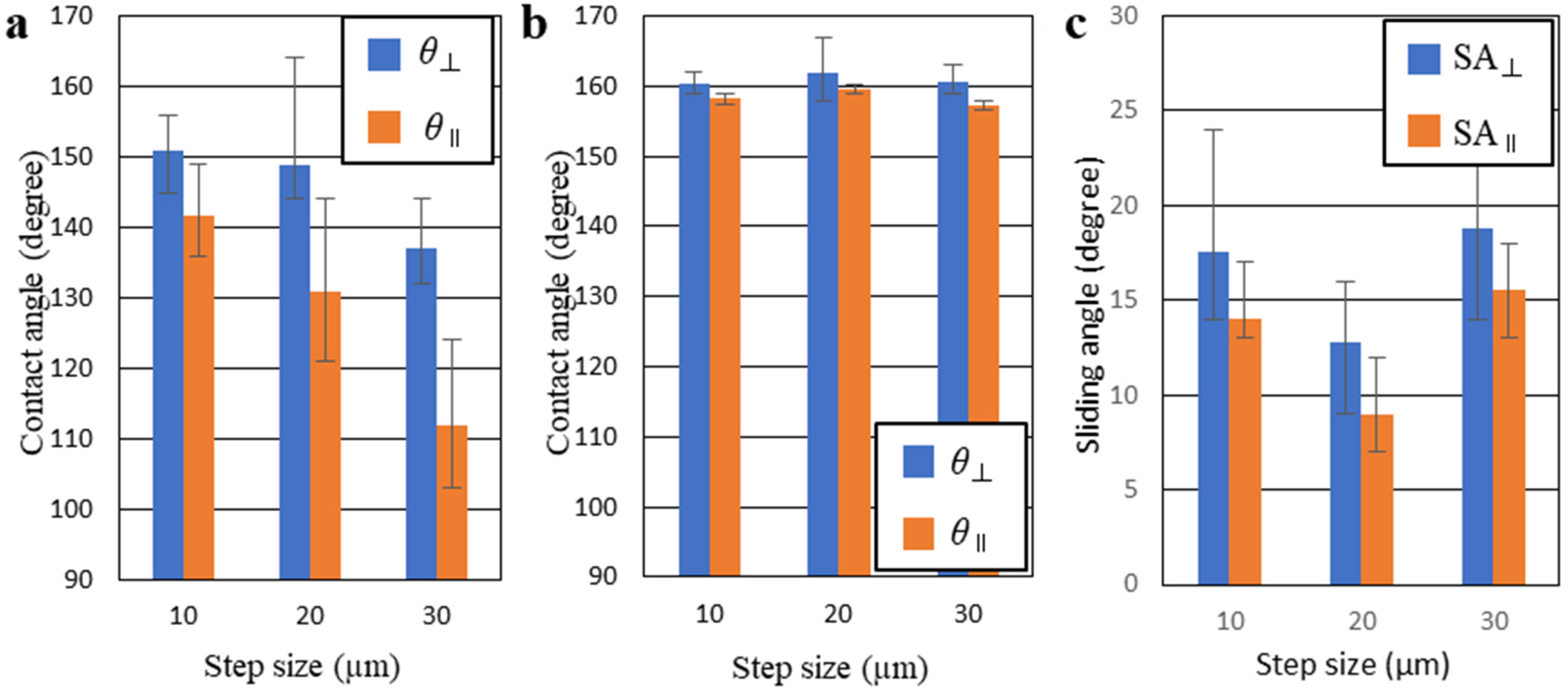
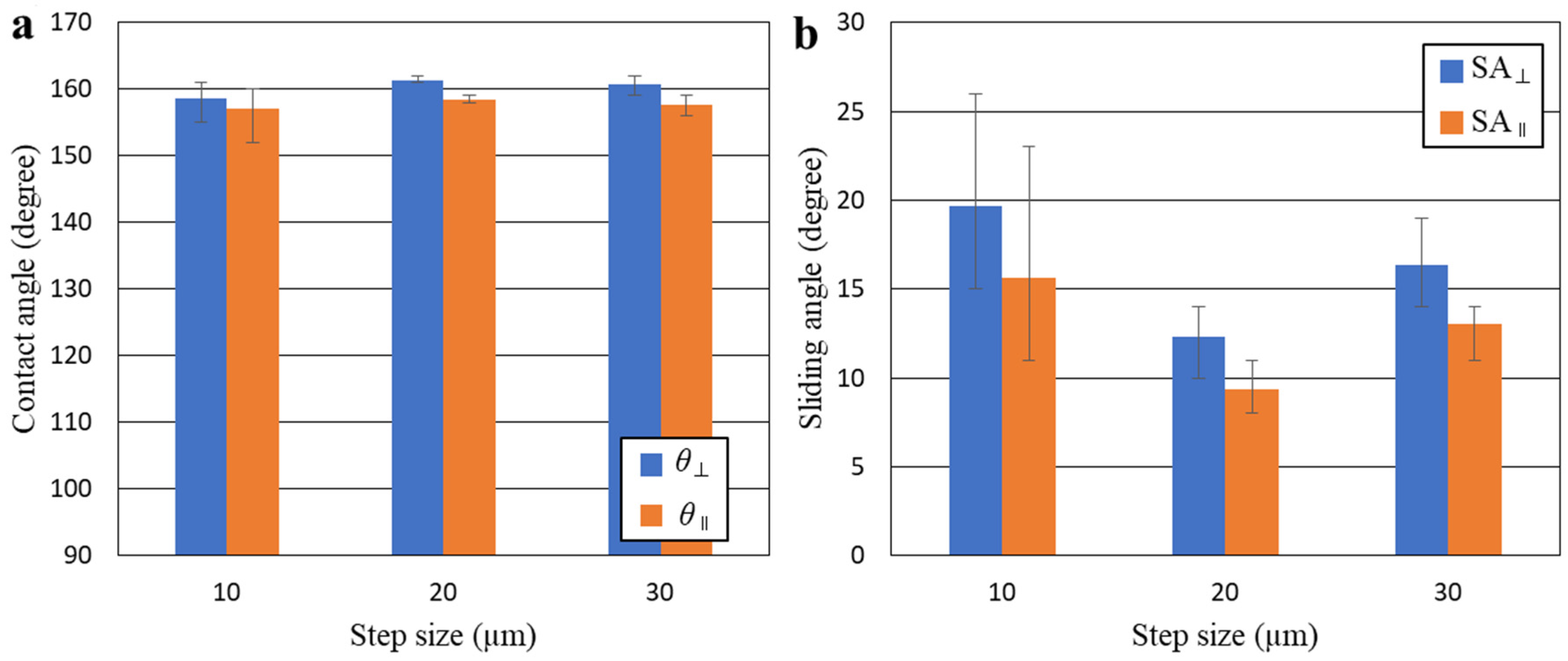
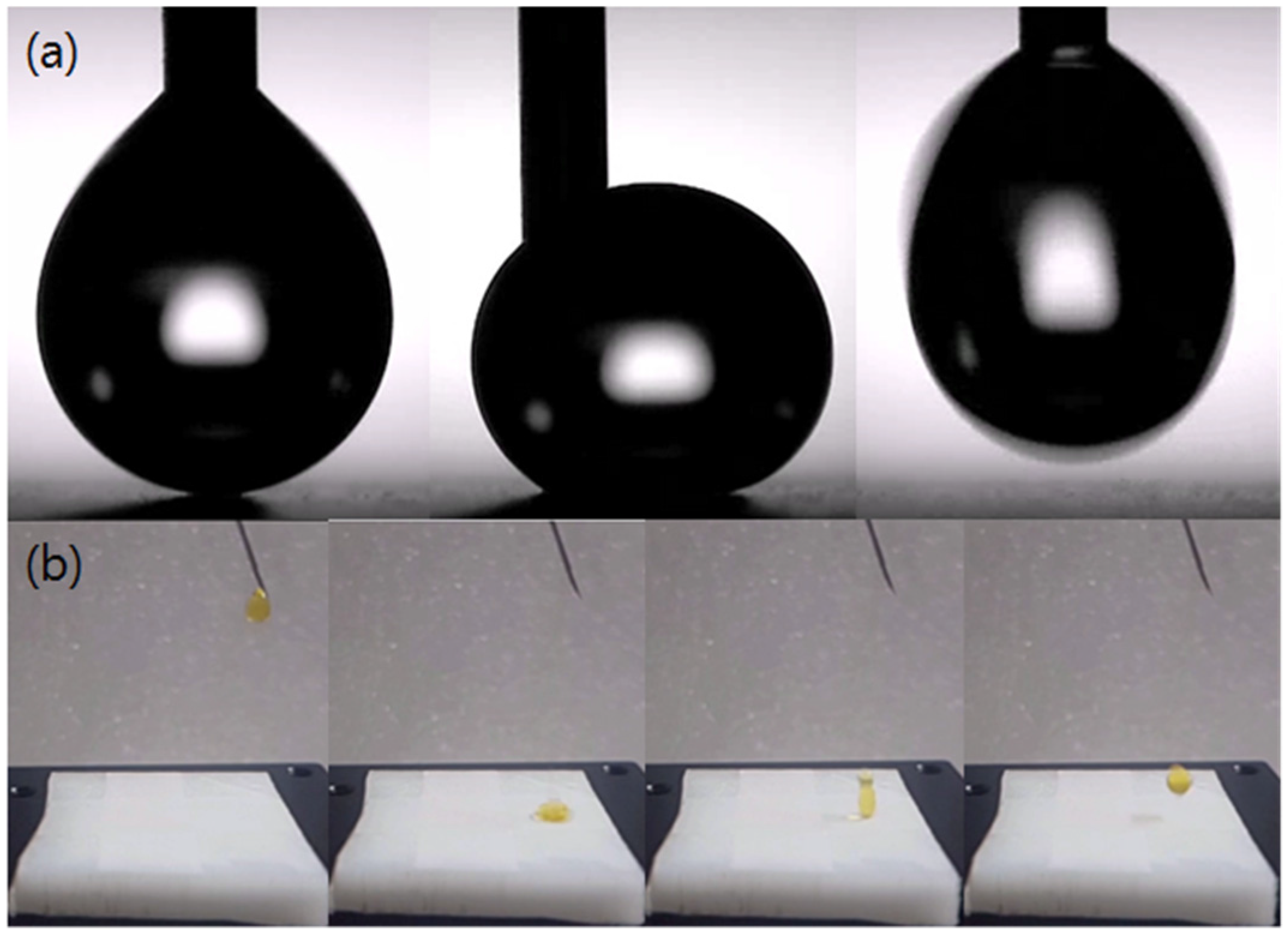
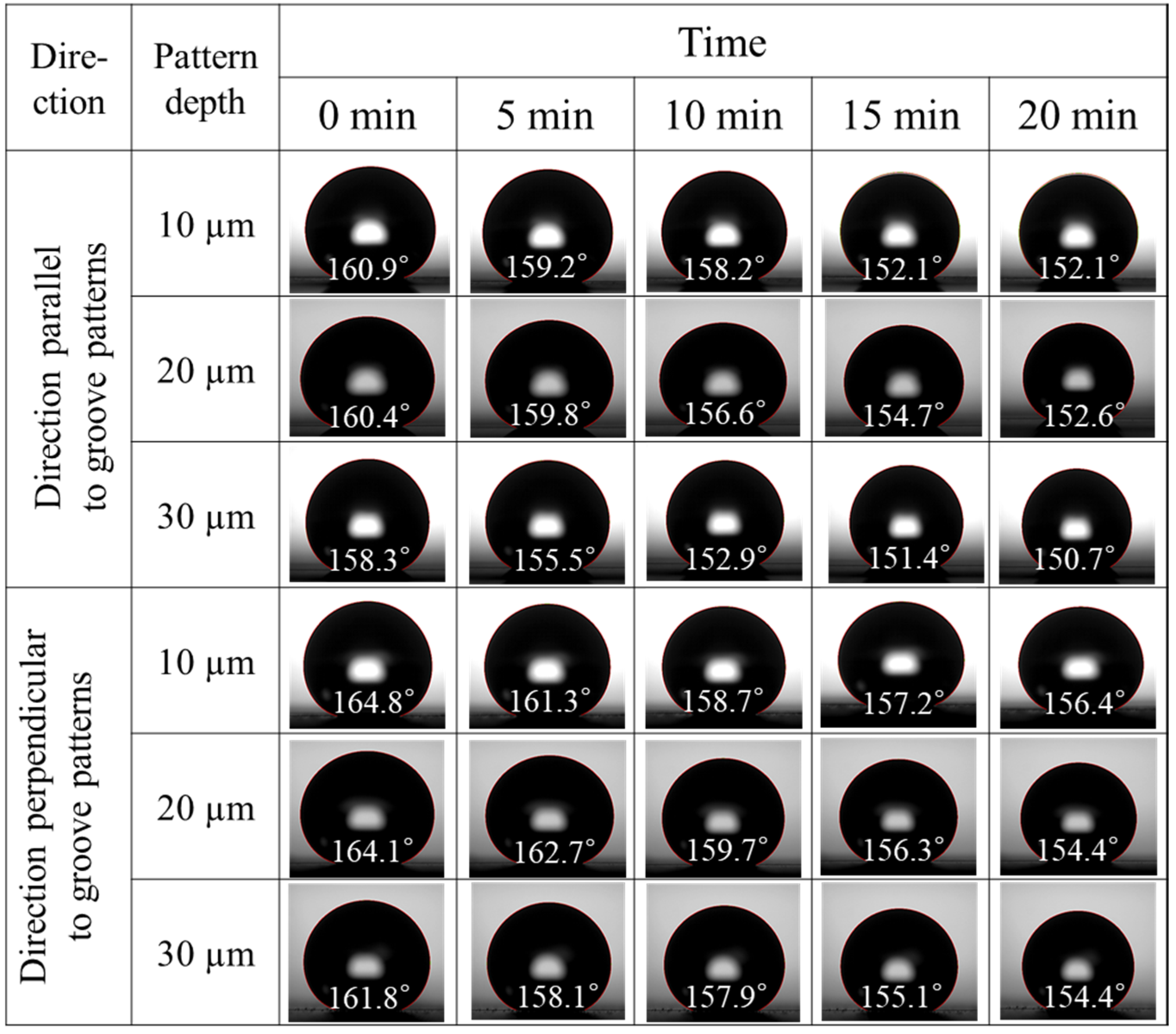
3.2. Wettability
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Sas, I.; Gorga, R.E.; Joines, J.A.; Thoney, K.A. Literature review on superhydrophobic self-cleaning surfaces produced by electrospinning. J. Polym. Sci. Polym. Phys. 2012, 50, 824–845. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Malshe, A.; Rajurkar, K.; Samant, A.; Hansen, H.N.; Bapat, S.; Jiang, W. Bio-inspired functional surfaces for advanced applications. CIRP Ann. Manuf. Technol. 2013, 62, 607–628. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Davaasuren, G.; Ngo, C.; Oh, H.; Chun, D.M. Geometric study of transparent superhydrophobic surfaces of molded and grid patterned polydimethylsiloxane (PDMS). Appl. Surf. Sci. 2014, 314, 530–536. [Google Scholar] [CrossRef]
- Chun, D.M.; Davaasuren, G.; Ngo, C.; Kim, C.; Lee, G.; Ahn, S. Fabrication of transparent superhydrophobic surface on thermoplastic polymer using laser beam machining and compression molding for mass production. CIRP Ann. Manuf. Technol. 2014, 63, 525–528. [Google Scholar] [CrossRef]
- Ngo, C.; Davaasuren, G.; Oh, H.; Chun, D.M. Transparency and superhydrophobicity of cone-shaped micropillar array textured polydimethylsiloxane. Int. J. Precis. Eng. Manuf. 2015, 16, 1347–1353. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.M.; Ngo, C.; Lee, K. Fast fabrication of superhydrophobic metallic surface using nanosecond laser texturing and low-temperature annealing. CIRP Ann. Manuf. Technol. 2016, 65, 519–522. [Google Scholar] [CrossRef]
- Ngo, C.V.; Chun, D.M. Laser Printing of Superhydrophobic Patterns from Mixtures of Hydrophobic Silica Nanoparticles and Toner Powder. Sci. Rep. 2016, 6, 36735. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Johnson, L.M.; López, G.P. Anisotropic wetting surfaces with one-dimesional and directional structures: Fabrication approaches, wetting properties and potential applications. Adv. Mater. 2012, 24, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Dubov, A.L.; Mourran, A.; Möller, M.; Vinogradova, O.I. Contact angle hysteresis on superhydrophobic stripes. J. Chem. Phys. 2014, 141, 074710. [Google Scholar] [CrossRef] [PubMed]
- Dubov, A.L.; Mourran, A.; Möller, M.; Vinogradova, O.I. Regimes of wetting transitions on superhydrophobic textures conditioned by energy of receding contact lines. Appl. Phys. Lett. 2015, 106, 241601. [Google Scholar] [CrossRef]
- Kavousanakis, M.E.; Colosqui, C.E.; Papathanasiou, A.G. Engineering the geometry of stripe-patterned surfaces towards efficient wettability switching. Colloid Surf. A 2013, 436, 309–317. [Google Scholar] [CrossRef]
- Bormashenko, E. Comment on water droplet motion control on superhydrophobic surfaces: Exploiting the Wenzel-to-Cassie transition. Langmuir 2011, 27, 12769–12770. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J.; Wu, S.; Chen, Q.; Zhao, S.; Zhang, H.; Sun, H.; Jiang, L. Three-level biomimetic rice-leaf surfaces with controllable anisotropic sliding. Adv. Funct. Mater. 2011, 21, 2927–2932. [Google Scholar] [CrossRef]
- Kang, S.M.; Lee, C.; Kim, H.N.; Lee, B.J.; Lee, J.E.; Kwak, M.K.; Suh, K. Directional oil sliding surfaces with hierarchical anisotropic groove microstructures. Adv. Mater. 2013, 25, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lim, H.S.; Lee, D.Y.; Kwak, D.; Cho, K. Tunable anisotropic wettability of rice leaf-like wavy surfaces. Adv. Funct. Mater. 2013, 23, 547–553. [Google Scholar] [CrossRef]
- Wang, S.; Yu, N.; Wang, T.; Ge, P.; Ye, S.; Xue, P.; Liu, W.; Shen, H.; Zhang, J.; Yang, B. Morphology-patterned anisotropic wetting surface for fluid control and gas–liquid separation in microfluidics. ACS Appl. Mater. Interface 2016, 8, 13094–13103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Q.; Li, M.; Li, X. Anisotropic wetting characteristics on submicrometer-scale periodic grooved surface. Langmuir 2007, 23, 6212–6217. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fang, G.; Li, Y.; Qiao, G. Anisotropic wetting behavior arising from superhydrophobic surfaces: Parallel grooved structure. J. Phys. Chem. B 2008, 112, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, J.; Cheng, J.; Wu, K. Anisotropic wetting properties on a precision-ground micro-V-grooved Si surface related to their micro-characterized variables. J. Micromech. Microeng. 2014, 24, 075004. [Google Scholar] [CrossRef]
- Liang, Y.; Shu, L.; Natsu, W.; He, F. Anisotropic wetting characteristics versus roughness on machined surfaces of hydrophilic and hydrophobic materials. Appl. Surf. Sci. 2015, 331, 41–49. [Google Scholar] [CrossRef]
- Tie, L.; Guo, Z.; Liu, W. Anisotropic wetting properties on various shape of parallel grooved microstructure. J. Colloid Interface Sci. 2015, 453, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, X.; Hou, G.; Huazhen, C.; Guoqu, Z. Facile approach to prepare a quasi-one-dimensional anisotropic wetting surface on copper substrate and its wetting properties. RSC Adv. 2015, 5, 64749–64755. [Google Scholar] [CrossRef]
- Asakura, K.; Yan, J. Water repellency control of oxygen-free copper surface by diamond-cut micro grooves. Int. J. Automot. Technol. 2015, 9, 396–402. [Google Scholar] [CrossRef]
- Balu, B.; Berry, A.D.; Patel, K.T.; Breedveld, V.; Hess, D.W. Directional Mobility and Adhesion of Water Drops on Patterned Superhydrophobic Surfaces. J. Adhes. Sci. Technol. 2011, 25, 627–642. [Google Scholar] [CrossRef]
- Contreras, C.B.; Chagas, G.; Strumia, M.C.; Weibel, D.E. Permanent superhydrophobic polypropylene nanocomposite coatings by a simple one-step dipping process. Appl. Surf. Sci. 2014, 307, 234–240. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; Wei, S.; Liu, Y.; Lian, J.; Jiang, Q. Robust superhydrophobic surface on Al substrate with durability, corrosion resistance and ice-phobicity. Sci. Rep. 2016, 6, 20933. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lu, Y.; Zhan, X.; Li, J.; Sun, Q. A robust, anti-acid, and high-temperature–humidity-resistant superhydrophobic surface of wood based on a modified TiO2 film by fluoroalkyl silane. Surf. Coat. Technol. 2015, 262, 33–39. [Google Scholar] [CrossRef]
- Li, S.; Jin, M.; Yu, C.; Liao, M. Wetting behavior of superhydrophobic surface in the liquid influenced by the existing of air layer. Colloids Surf. Physicochem. Eng. Asp. 2013, 430, 46–50. [Google Scholar] [CrossRef]
- Ramanathan, R.; Weibel, D.E. Novel liquid–solid adhesion superhydrophobic surface fabricated using titanium dioxide and trimethoxypropyl silane. Appl. Surf. Sci. 2012, 258, 7950–7955. [Google Scholar] [CrossRef]
- Bernagozzi, I.; Antonini, C.; Villa, F.; Marengo, M. Fabricating superhydrophobic aluminum: An optimized one-step wet synthesis using fluoroalkyl silane. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 919–924. [Google Scholar] [CrossRef]
- Chen, Y.; He, B.; Lee, J.; Patankar, N.A. Anisotropy in the wetting of rough surfaces. J. Colloid Interface Sci. 2005, 281, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Rose, F.R.; Gadegaard, N.; Alexander, M.R. Effect of sessile drop volume on the wetting anisotropy observed on grooved surfaces. Langmuir 2009, 25, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Kong, L.; Cheung, C.; To, S.; Cheng, C. Modeling and characterization of generation of 3D micro-structured surfaces with self-cleaning and optical functions. Optik Int. J. Light Electron Opt. 2013, 124, 2848–2853. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86, 144101. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Yao, X.; Chen, Q.; Xu, L.; Li, Q.; Song, Y.; Gao, X.; Quéré, D.; Jiang, L. Bioinspired ribbed nanoneedles with robust superhydrophobicity. Adv. Funct. Mater. 2010, 20, 656–662. [Google Scholar] [CrossRef]
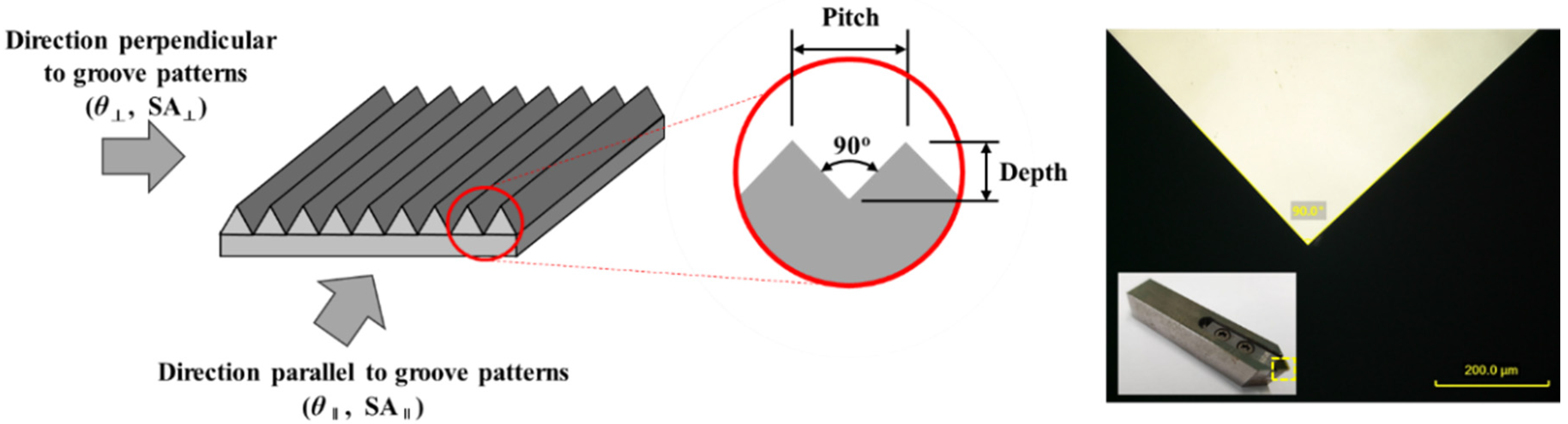

| Items | Conditions |
|---|---|
| Cutting tool | 90° Single crystal diamond tool |
| Workpiece | Electroplated copper mold |
| Cutting depth | 10 µm, 20 µm, 30 µm (Rough machining 5 µm × N, Semi-finishing 3 µm, and finishing 2 µm) |
| Pitch | 20 µm, 40 µm, 60 µm |
| Feed rate | 12,000 mm/min |
| Cutting oil | Isoparaffinic hydrocarbon solvent (ISOPAR-H) |
| Groove Depth | Direction Parallel to Groove Patterns | Direction Perpendicular to the Groove Patterns | ||||
|---|---|---|---|---|---|---|
| Advancing Contact Angle | Receding Contact Angle | Contact Angle Hysteresis | Advancing Contact Angle | Receding Contact Angle | Contact Angle Hysteresis | |
| 10 µm | 159.9° | 132.6° | 27.2° | 164.0° | 127.8° | 36.1° |
| 20 µm | 168.3° | 154.1° | 14.2° | 174.2° | 149.8° | 24.4° |
| 30 µm | 167.2° | 146.5° | 20.7° | 174.8° | 140.8° | 34.1° |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-M.; Ngo, C.-V.; Jeong, J.-Y.; Jeon, E.-c.; Je, T.-J.; Chun, D.-M. Fabrication of an Anisotropic Superhydrophobic Polymer Surface Using Compression Molding and Dip Coating. Coatings 2017, 7, 194. https://doi.org/10.3390/coatings7110194
Lee K-M, Ngo C-V, Jeong J-Y, Jeon E-c, Je T-J, Chun D-M. Fabrication of an Anisotropic Superhydrophobic Polymer Surface Using Compression Molding and Dip Coating. Coatings. 2017; 7(11):194. https://doi.org/10.3390/coatings7110194
Chicago/Turabian StyleLee, Kyong-Min, Chi-Vinh Ngo, Ji-Young Jeong, Eun-chae Jeon, Tae-Jin Je, and Doo-Man Chun. 2017. "Fabrication of an Anisotropic Superhydrophobic Polymer Surface Using Compression Molding and Dip Coating" Coatings 7, no. 11: 194. https://doi.org/10.3390/coatings7110194






