Flexible Thermoelectric Composite Films of Polypyrrole Nanotubes Coated Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
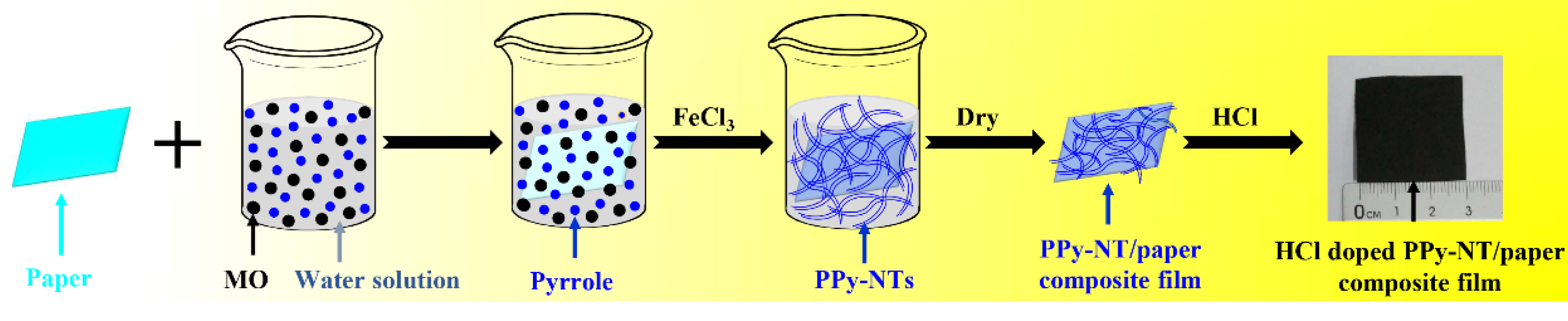
2.2. Preparation of the Flexible PPy–NT/Paper Composite Films
2.3. Characterizations
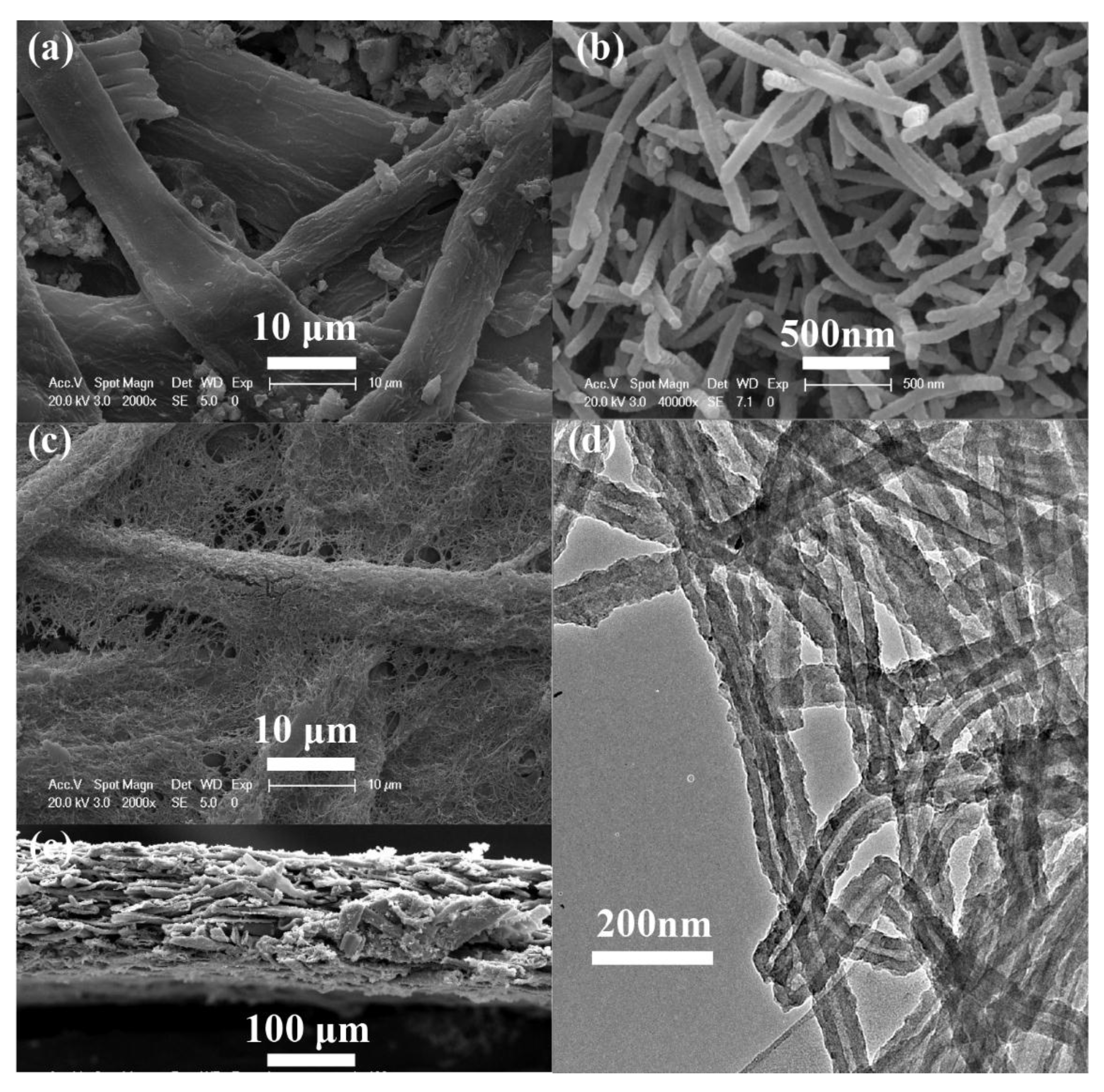
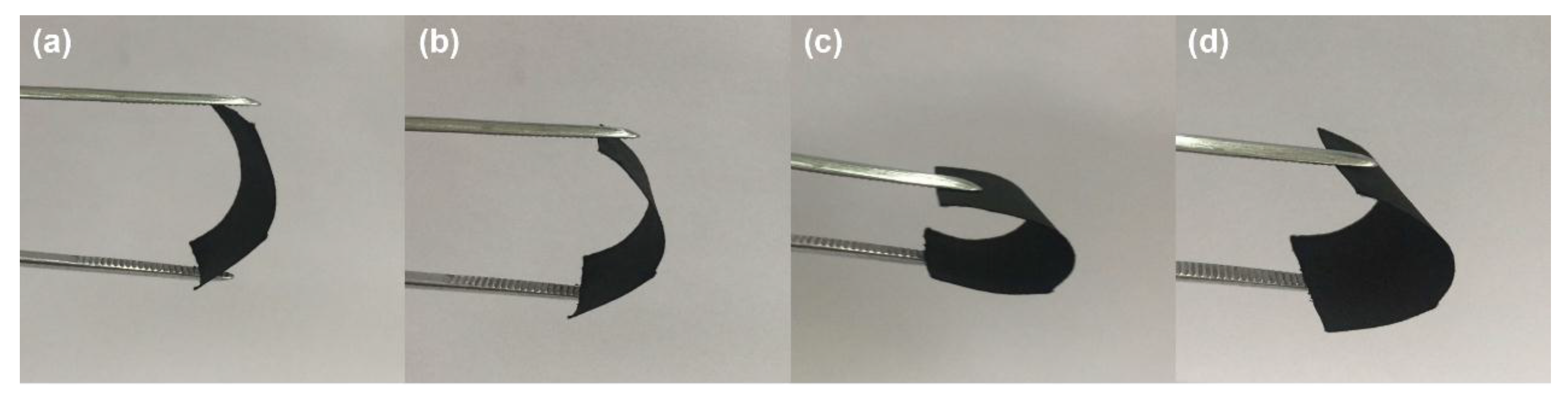
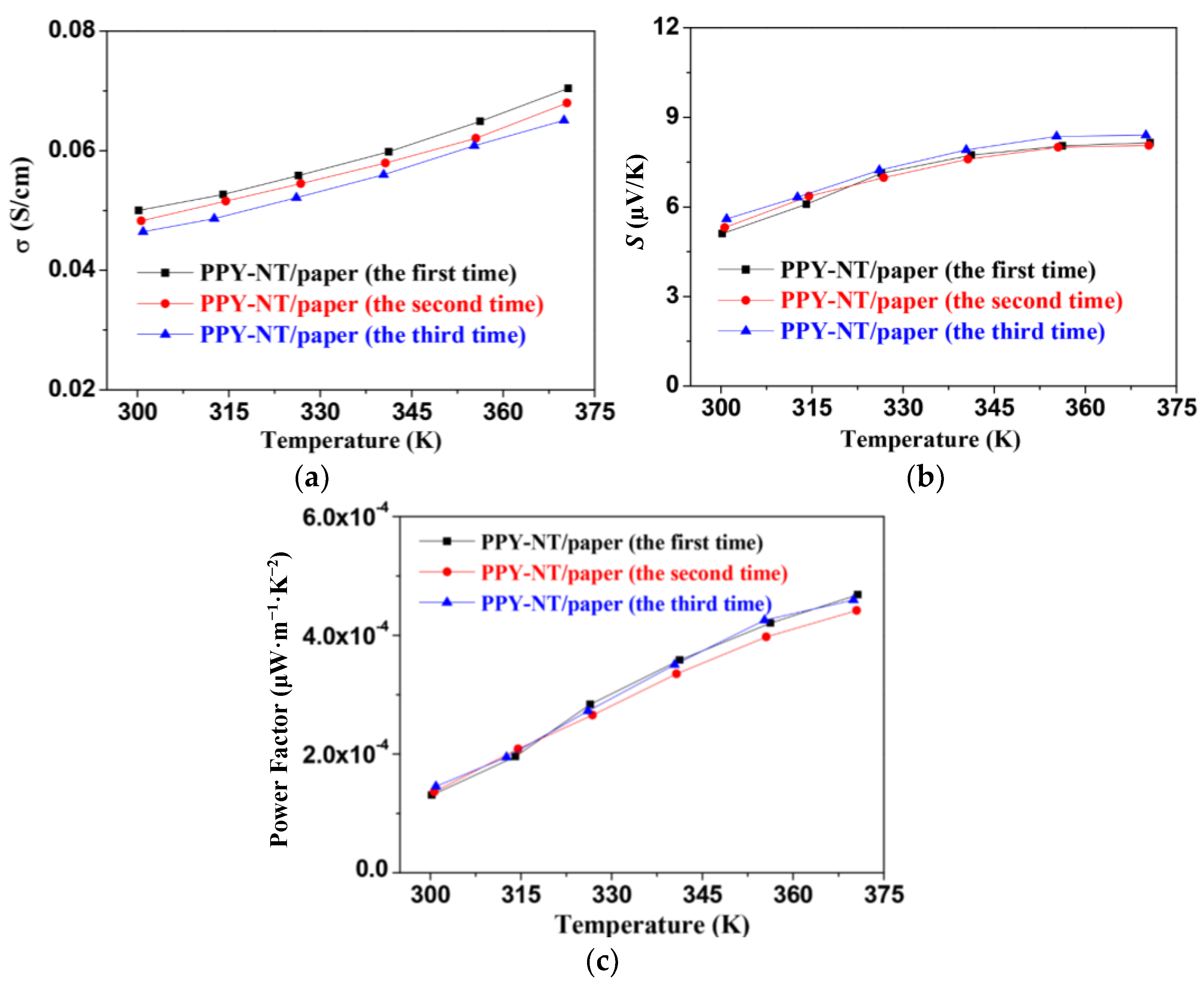
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, C.; Ma, H.; Tian, Z. Thermoelectric properties of crystalline and amorphous polypyrrole: A computational study. Appl. Therm. Eng. 2017, 111, 1441–1447. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jin, C.; Zhang, T.; Yin, Q. Preparation of polypyrrole/graphene nanosheets composites with enhanced thermoelectric properties. RSC Adv. 2014, 4, 46187–46193. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.; Shen, S.; Yin, J. Preparation and thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2014, 195, 132–136. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.-Y. Enhanced thermoelectric performance by self-assembled layered morphology of polypyrrole nanowire/single-walled carbon nanotube composites. Compos. Sci. Technol. 2016, 129, 130–136. [Google Scholar] [CrossRef]
- Han, S.; Zhai, W.; Chen, G.; Wang, X. Morphology and thermoelectric properties of graphene nanosheets enwrapped with polypyrrole. RSC Adv. 2014, 4, 29281–29285. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, L.; Du, K.; Yin, Q.; Yin, Q. Polypyrrole/Graphene/Polyaniline Ternary Nanocomposite with High Thermoelectric Power Factor. ACS Appl. Mater. Interfaces 2017, 9, 20124–20131. [Google Scholar] [CrossRef] [PubMed]
- Sapurina, I.; Li, Y.; Alekseeva, E.; Bober, P.; Trchová, M.; Morávková, Z.; Stejskal, J. Polypyrrole nanotubes: The tuning of morphology and conductivity. Polymer 2017, 113, 247–258. [Google Scholar] [CrossRef]
- Škodová, J.; Kopecký, D.; Vrňata, M.; Varga, M.; Prokeš, J.; Cieslar, M.; Bober, P.; Stejskal, J. Polypyrrole-silver composites prepared by the reduction of silver ions with polypyrrole nanotubes. Polym. Chem. 2013, 4, 3610–3616. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Pei, W.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Misra, S.; Bharti, M.; Singh, A.; Debnath, A.; Aswal, D.; Hayakawa, Y. Nanostructured polypyrrole: Enhancement in thermoelectric figure of merit through suppression of thermal conductivity. Mater. Res. Express 2017, 4, 085007. [Google Scholar] [CrossRef]
- Shaktawat, V.; Sharma, K.; Saxena, N. Structural and electrical characterization of protonic acid doped polypyrrole. J. Ovonic Res. 2010, 6, 239–245. [Google Scholar]
- Shaktawat, V.; Jain, N.; Saxena, R.; Saxena, N.; Sharma, T. Electrical conductivity and optical band gap studies of polypyrrole doped with different acids. J. Optoelectron. Adv. Mater. 2007, 9, 2130–2132. [Google Scholar]
- Jiang, Q.; Liu, C.; Xu, J.; Lu, B.; Song, H.; Shi, H.; Yao, Y.; Zhang, L. Paper: An effective substrate for the enhancement of thermoelectric properties in PEDOT:PSS. J. Polym. Sci. Part A Polym. Phys. 2014, 52, 737–742. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Polymer thermoelectric modules screen-printed on paper. RSC Adv. 2014, 4, 28802–28806. [Google Scholar] [CrossRef]
- Kopecká, J.; Kopecký, D.; Vrňata, M.; Fitl, P.; Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Prokeš, J.; Sapurina, I. Polypyrrole nanotubes: Mechanism of formation. RSC Adv. 2014, 4, 1551–1558. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Jia, R.; Xu, J.; Shen, S. Thermoelectric properties of flexible PEDOT:PSS/polypyrrole/paper nanocomposite films. Materials 2017, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lo, S.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.; Kanatzidis, M. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.; Heo, S.; Kim, H. Influence of powder morphology on thermoelectric anisotropy of spark-plasma-sintered Bi–Te-based thermoelectric materials. Acta Mater. 2011, 59, 405–411. [Google Scholar] [CrossRef]
- Singh, J.; Verma, S. A Comparison of figure of merit for some common thermocouples in the high temperature range. Glob. J. Res. Eng. Electr. Electron. Eng. 2013, 13, 7–12. [Google Scholar]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Matil, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Du, Y.; Jia, R.; Xu, J.; Shen, S.Z. Flexible Thermoelectric Composite Films of Polypyrrole Nanotubes Coated Paper. Coatings 2017, 7, 211. https://doi.org/10.3390/coatings7120211
Li J, Du Y, Jia R, Xu J, Shen SZ. Flexible Thermoelectric Composite Films of Polypyrrole Nanotubes Coated Paper. Coatings. 2017; 7(12):211. https://doi.org/10.3390/coatings7120211
Chicago/Turabian StyleLi, Jun, Yong Du, Runping Jia, Jiayue Xu, and Shirley Z. Shen. 2017. "Flexible Thermoelectric Composite Films of Polypyrrole Nanotubes Coated Paper" Coatings 7, no. 12: 211. https://doi.org/10.3390/coatings7120211





