In-Situ Growth and Characterization of Indium Tin Oxide Nanocrystal Rods
Abstract
:1. Introduction
2. Experimental
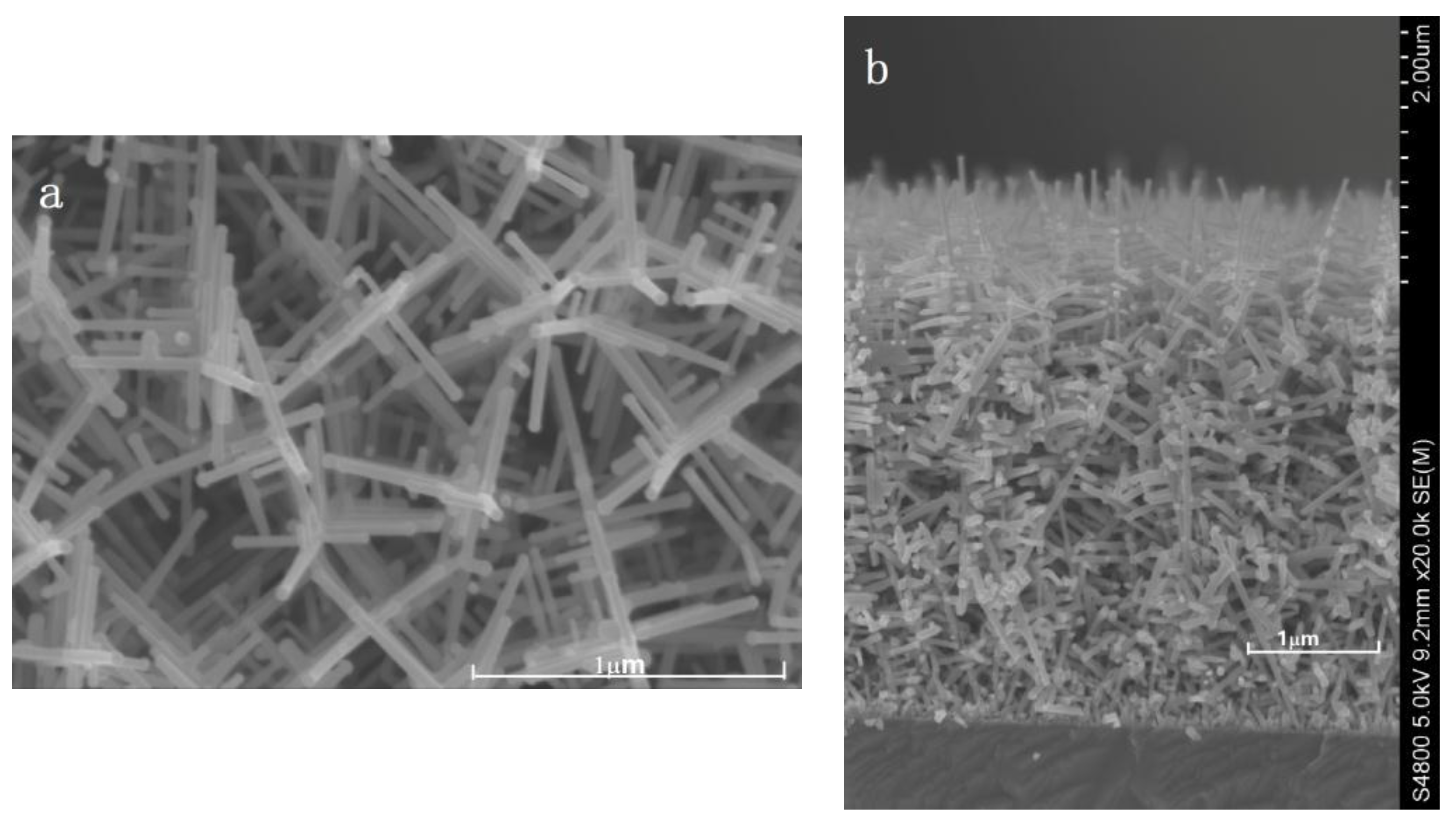
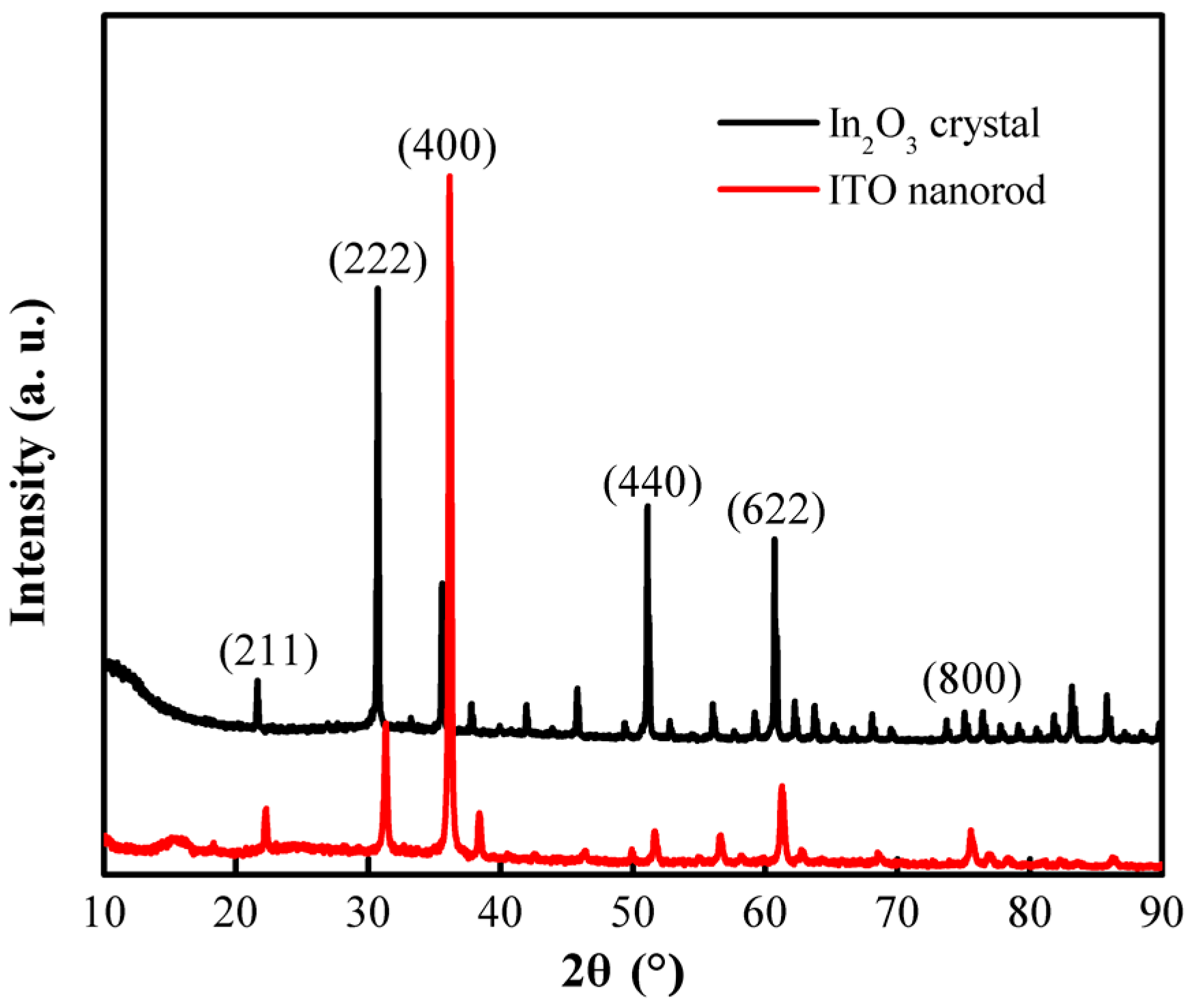
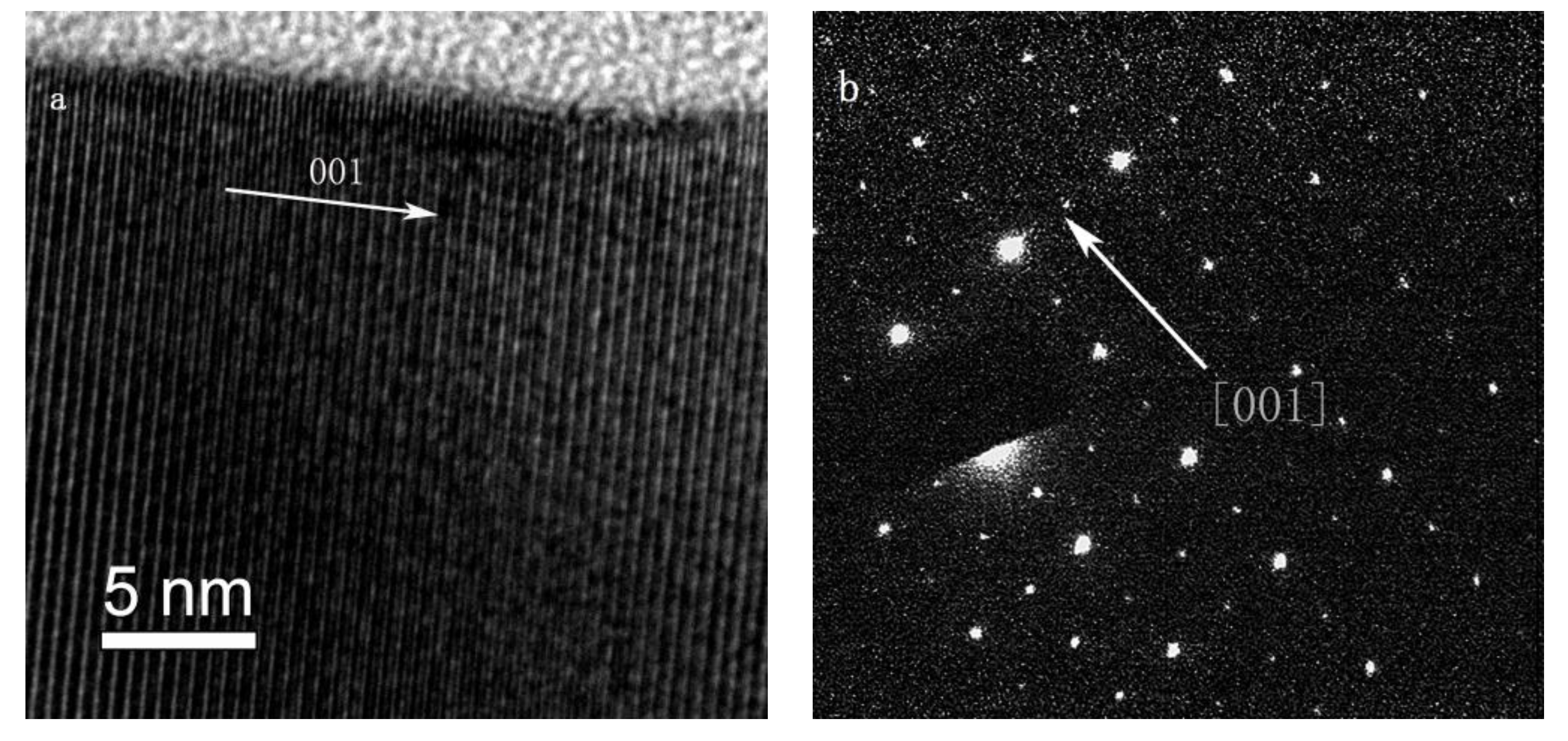
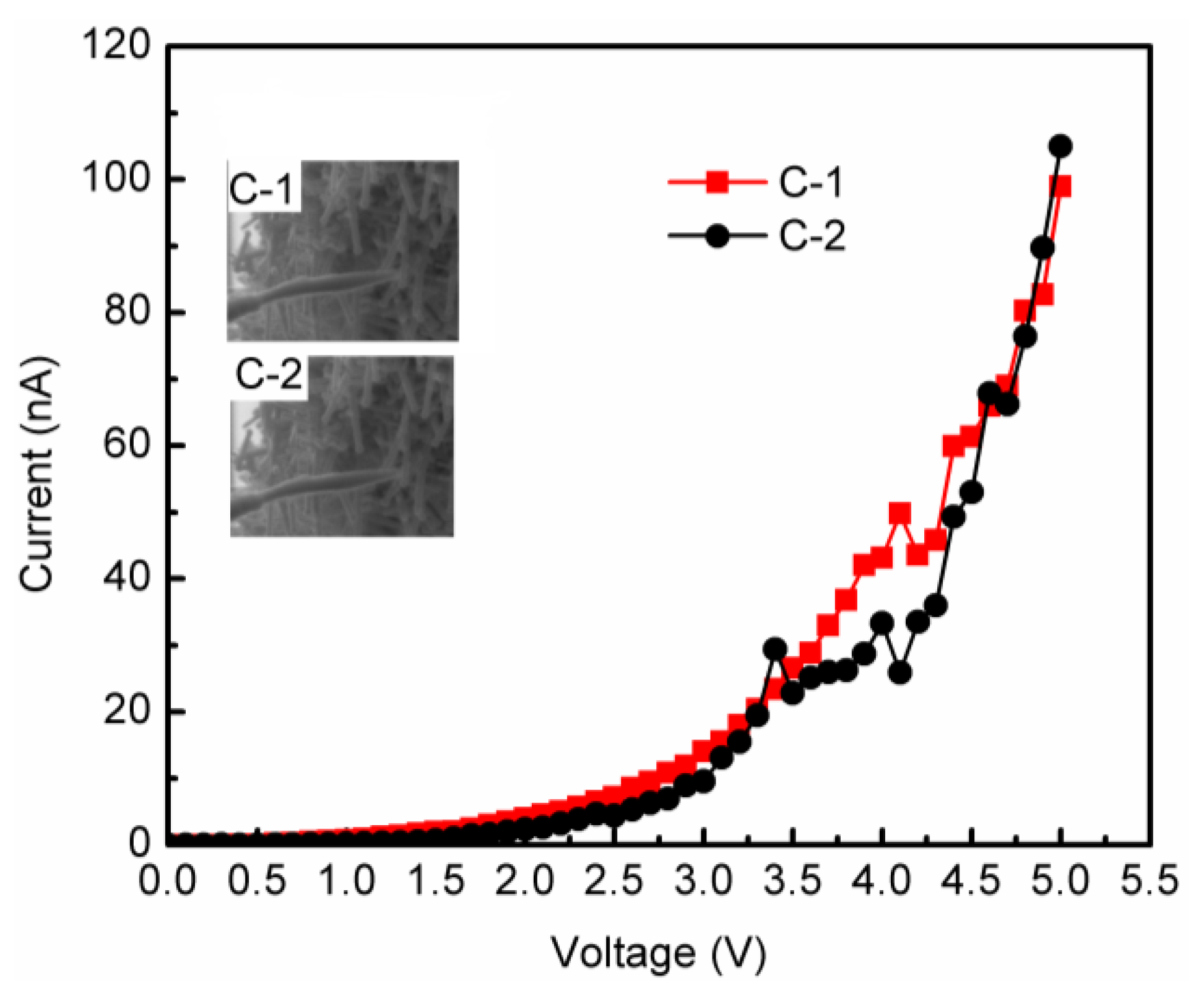
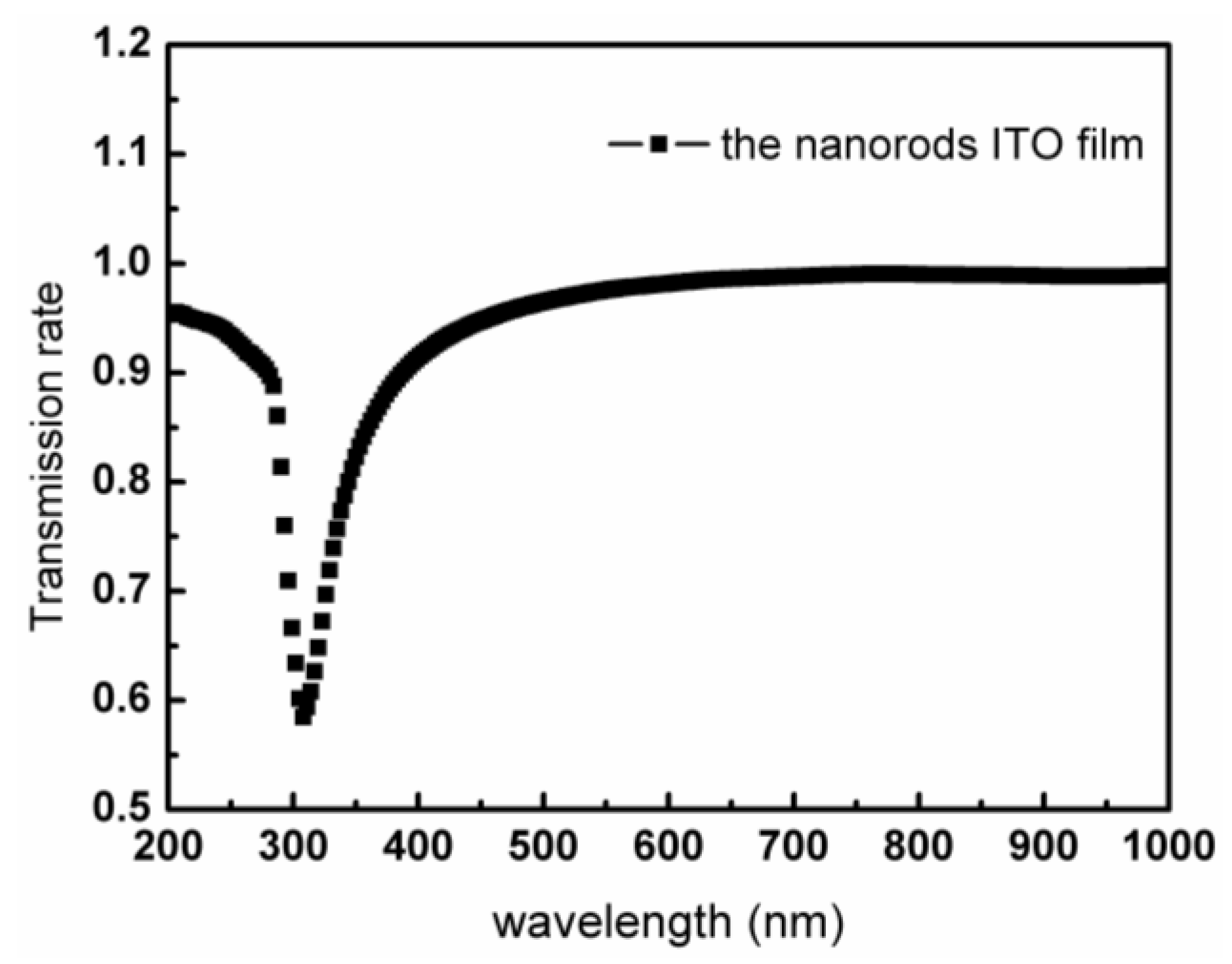
3. Results and Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Lin, Y.C.; Chang, S.J.; Su, Y.K.; Tsai, T.Y.; Chang, C.S.; Shei, S.C.; Hsu, S.J.; Liu, C.H.; Liaw, U.H.; Chen, S.C.; et al. Nitride-based light-emitting diodes with Ni/ITO p-type ohmic contacts. IEEE Photonics Technol. Lett. 2002, 14, 1668–1670. [Google Scholar] [CrossRef]
- Phillips, J.M.; Cava, R.J.; Thomas, G.A.; Carter, S.A.; Kwo, J.; Siegrist, T.; Krajewski, J.J.; Marshall, J.H.; Peck, W.F., Jr.; Rapkine, D.H. Zinc-indium-oxide: A high conductivity transparent conducting oxide. Appl. Phys. Lett. 1995, 67, 2246–2248. [Google Scholar] [CrossRef]
- Alam, M.Z.; De Leon, I.; Boyd, R.W. Large optical nonlinearity of indium tin oxide in its epsilon-near-zero region. Science 2016, 352, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, X.; Liu, Q.; Zuo, Z.; Liu, H.; Zhang, M.; Hu, X. Effects of indium–tin oxide film morphologies on light-output characteristics of GaN-based light-emitting diodes. J. Nanoelectron. Optoelectron. 2014, 9, 549–553. [Google Scholar] [CrossRef]
- Jin, S.X.; Li, J.; Lin, J.Y.; Jiang, H.X. InGaN/GaN quantum well interconnected microdisk light emitting diodes. Appl. Phys. Lett. 2000, 77, 3236–3238. [Google Scholar] [CrossRef]
- Das, S.R.; Sadeque, S.; Jeong, C.; Chen, R.; Alam, M.A.; Janes, D.B. Copercolating networks: An approach for realizing high-performance transparent conductors using multicomponent nanostructured networks. Nanophotonics 2016, 5, 180–195. [Google Scholar] [CrossRef]
- Chiu, C.J.; Shih, S.S.; Weng, W.Y.; Chang, S.J.; Hung, Z.D.; Tsai, T.Y. Deep UV Ta2O5/zinc-indium-tin-oxide thin film photo-transistor. IEEE Photonics Technol. Lett. 2012, 24, 1018–1020. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, H.J.; Lee, C.L.; Ahn, C.W. Fabrication of three-dimensional hybrid nanostructure-embedded ITO and its application as a transparent electrode for high-efficiency solution processable organic photovoltaic devices. Nanoscale 2017, 9, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Nuchuay, P.; Chaikeeree, T.; Horprathum, M.; Mungkung, N.; Kasayapanand, N.; Oros, C.; Limwichean, S.; Nuntawong, N.; Chananonnawathorn, C.; Patthanasettakul, V.; et al. Engineered omnidirectional antireflection ITO nanorod films with super hydrophobic surface via glancing-angle ion-assisted electron-beam evaporation deposition. Curr. Appl. Phys. 2017, 17, 222–229. [Google Scholar] [CrossRef]
- Pattathil, P.; Giannuzzi, R.; Qualtieri, A.; Barawi, M.; Manca, M. Widely tunable localized surface plasmon scattering in mesoporous ITO electrodes. MRS Adv. 2016, 1, 3903–3908. [Google Scholar] [CrossRef]
- Richardson, B.J.; Zhu, L.; Yu, Q. Design and development of plasmonic nanostructured electrodes for ITO-free organic photovoltaic cells on rigid and highly flexible substrates. Nanotechnology 2017, 28, 165401. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.M.; Zhao, J.T.; Yin, X.T.; Huang, S.L. Sensitivity and selectivity of SnO2-based sensor for CO and H2 detections: A novel method to detect simultaneously the CO and H2 concentrations. Adv. Sci. Technol. 2017, 99, 40–47. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Kim, J.-S.; Kim, T.-H.; Hong, Y.J.; Kang, Y.C.; Lee, J.-H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, J.; Zhu, Z.Q.; Gong, H.; O’Shea, S.J. Quartz crystal microbalance coated with sol–gel-derived indium–tin oxide thin films as gas sensor for NO detection. Colloids Surf. A Physicochem. Eng. Aspects 2004, 236, 23–30. [Google Scholar] [CrossRef]
- Liu, P.T.; Chou, Y.T.; Teng, L.F. Environment-dependent metastability of passivation-free indium zinc oxide thin film transistor after gate bias stress. Appl. Phys. Lett. 2009, 95, 233504. [Google Scholar] [CrossRef]
- Minami, T. Present status of transparent conducting oxide thin-film development for Indium-Tin-Oxide (ITO) substitutes. Thin Solid Films 2008, 516, 5822–5828. [Google Scholar] [CrossRef]
- Lyubchyk, A.; Vicente, A.; Soule, B.; Alves, P.U.; Mateus, T.; Mendes, M.J.; Águas, H.; Fortunato, E.; Martins, R. Mapping the electrical properties of ZnO-based transparent conductive oxides grown at room temperature and improved by controlled postdeposition annealing. Adv. Electron. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.K.; Jeong, J.; Kim, W.; Min, B.K.; Do, Y.R. Wafer-scale growth of ITO nanorods by radio frequency magnetron sputtering deposition. J. Electrochem. Soc. 2011, 158, K131–K135. [Google Scholar] [CrossRef]
- Limmer, S.J.; Cruz, S.V.; Cao, G.Z. Films and nanorods of transparent conducting oxide ITO by a citric acid sol route. Appl. Phys. A 2004, 79, 421–424. [Google Scholar] [CrossRef]
- Fallah, H.R.; Vahid, M.J. Substrate temperature effect on transparent heat reflecting nanocrystalline ITO films prepared by electron beam evaporation. Renew. Energy 2010, 35, 1527–1530. [Google Scholar] [CrossRef]
- Canhola, P.; Martins, N.; Raniero, L.; Pereira, S.; Fortunato, E.; Ferreira, I.; Martins, R. Role of annealing environment on the performances of large area ITO films produced by RF magnetron sputtering. Thin Solid Films 2005, 487, 271–276. [Google Scholar] [CrossRef]
- Lee, H.Y.; Huang, H.L.; Pchelyakov, O.P.; Pakhanov, N.A. Performance improvement mechanisms of bias-assisted photoelectrochemical treated GaSb-based solar cells with ITO nanorod array. Prog. Photovolt. Res. Appl. 2016, 24, 195–199. [Google Scholar] [CrossRef]
- Chen, K.; Guo, P.; Dao, T.D.; Li, S.Q.; Ishiii, S.; Nagao, T.; Chang, R.P.H. Protein-functionalized indium-tin oxide nanoantenna arrays for selective infrared biosensing. Adv. Opt. Mater. 2017, 1700091. [Google Scholar] [CrossRef]
- He, L.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016, 109, 1–101. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Wagner, R.S.; Levitt, A.P. Whisker Technology; AP Levitt Wiley: New York, NY, USA, 1970; pp. 47–119. [Google Scholar]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399. [Google Scholar] [CrossRef]
- Detchprohm, T.; Hiramatsu, K.; Itoh, K.; Akasaki, I. Relaxation process of the thermal strain in the GaN/α-Al2O3 heterostructure and determination of the intrinsic lattice constants of GaN free from the strain. Jpn. J. Appl. Phys. 1992, 31, L1454. [Google Scholar] [CrossRef]
- Bel Hadj Tahar, R.; Ban, T.; Ohya, Y.; Takahashi, Y. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, W.; Ma, A.; Tang, J.; Lin, J.; Fang, J. Study of quasi-monodisperse In2O3 nanocrystals: Synthesis and optical determination. J. Am. Chem. Soc. 2005, 127, 5276–5277. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Lou, Y.; Wang, Z.; Xu, X. In-Situ Growth and Characterization of Indium Tin Oxide Nanocrystal Rods. Coatings 2017, 7, 212. https://doi.org/10.3390/coatings7120212
Shen Y, Lou Y, Wang Z, Xu X. In-Situ Growth and Characterization of Indium Tin Oxide Nanocrystal Rods. Coatings. 2017; 7(12):212. https://doi.org/10.3390/coatings7120212
Chicago/Turabian StyleShen, Yan, Youxin Lou, Zhihao Wang, and Xiangang Xu. 2017. "In-Situ Growth and Characterization of Indium Tin Oxide Nanocrystal Rods" Coatings 7, no. 12: 212. https://doi.org/10.3390/coatings7120212





