Identification of Intermetallic Compounds and Its Formation Mechanism in Boron Steel Hot-Dipped in Al-7 wt.% Mn Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
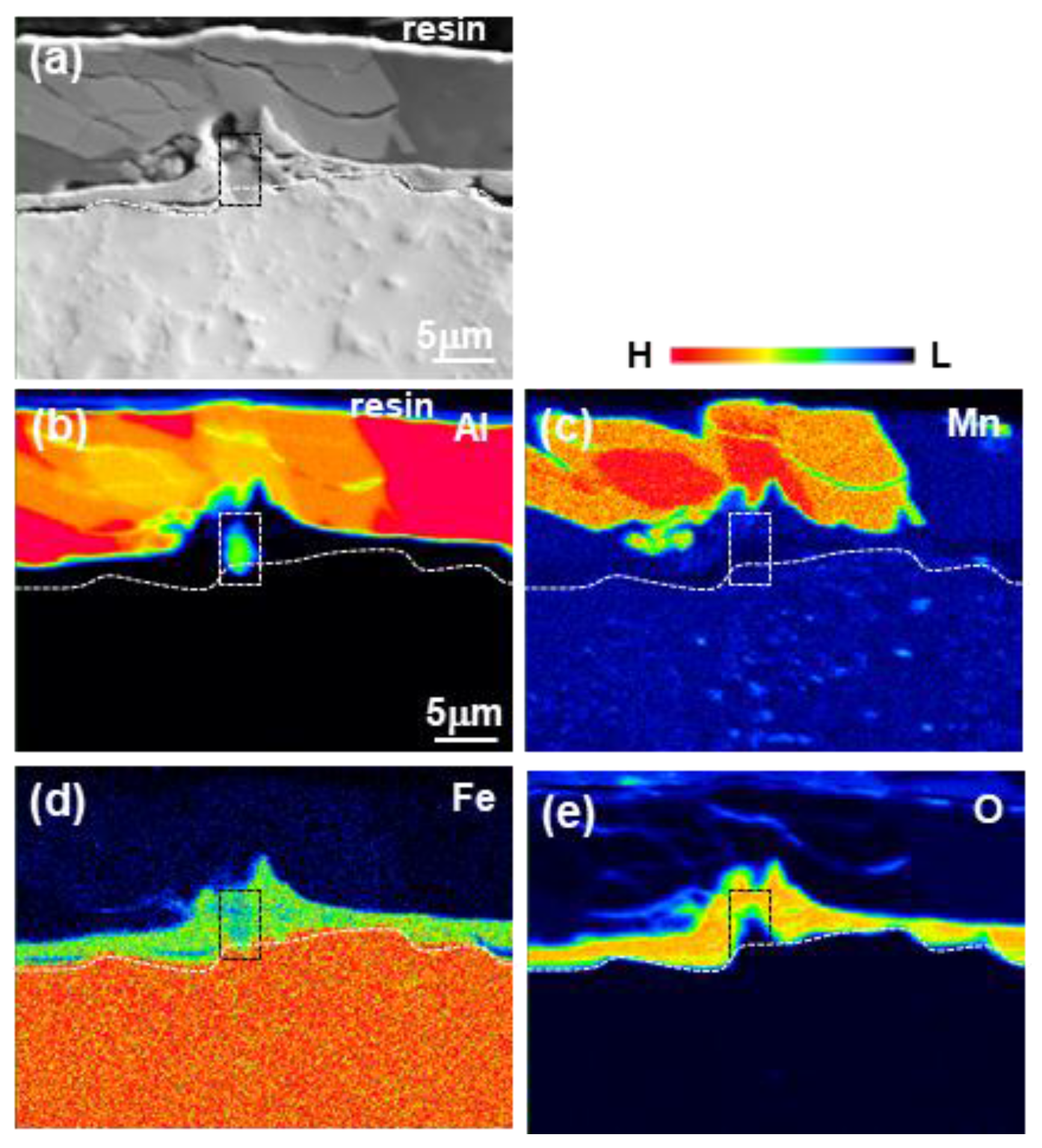
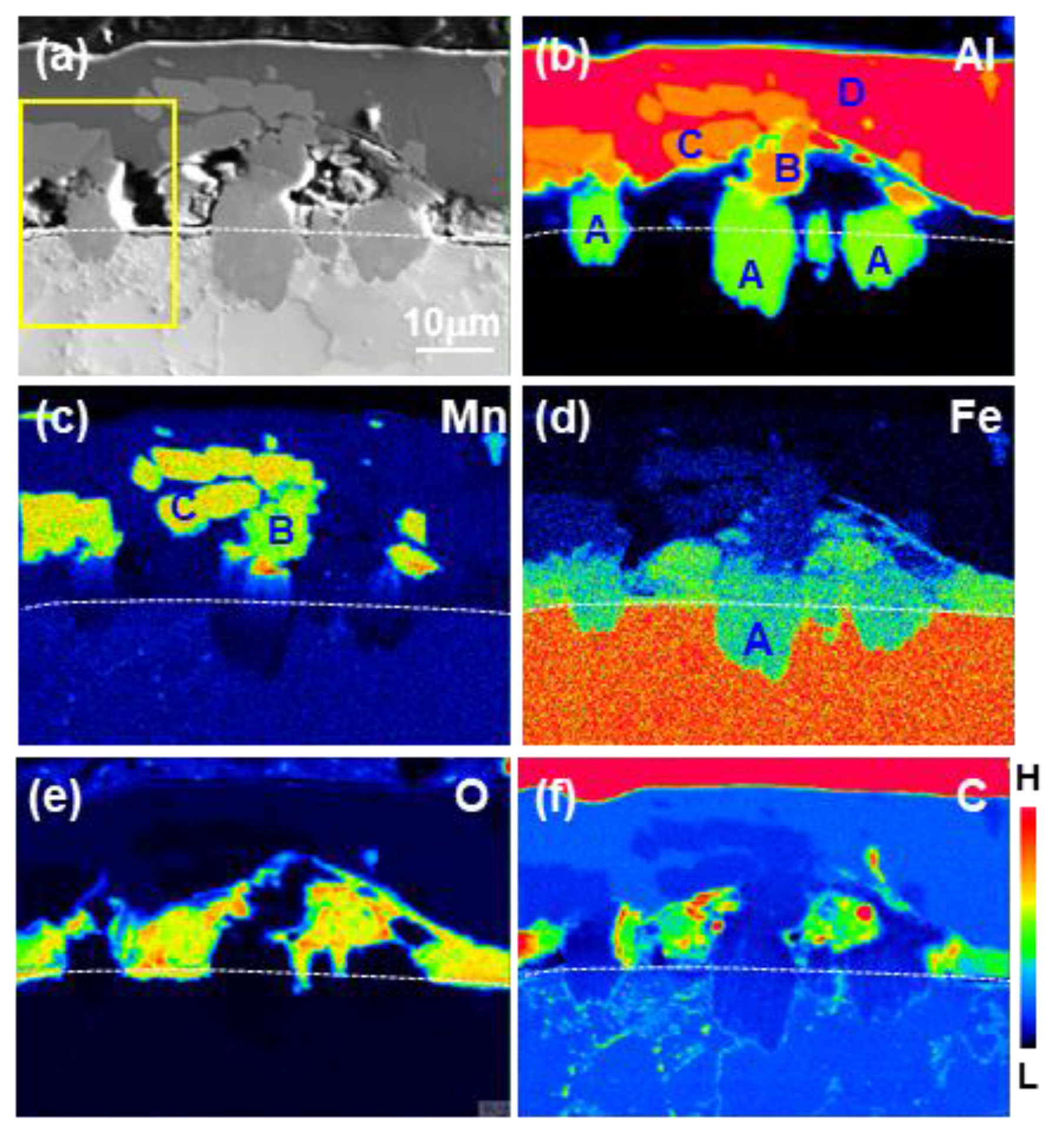
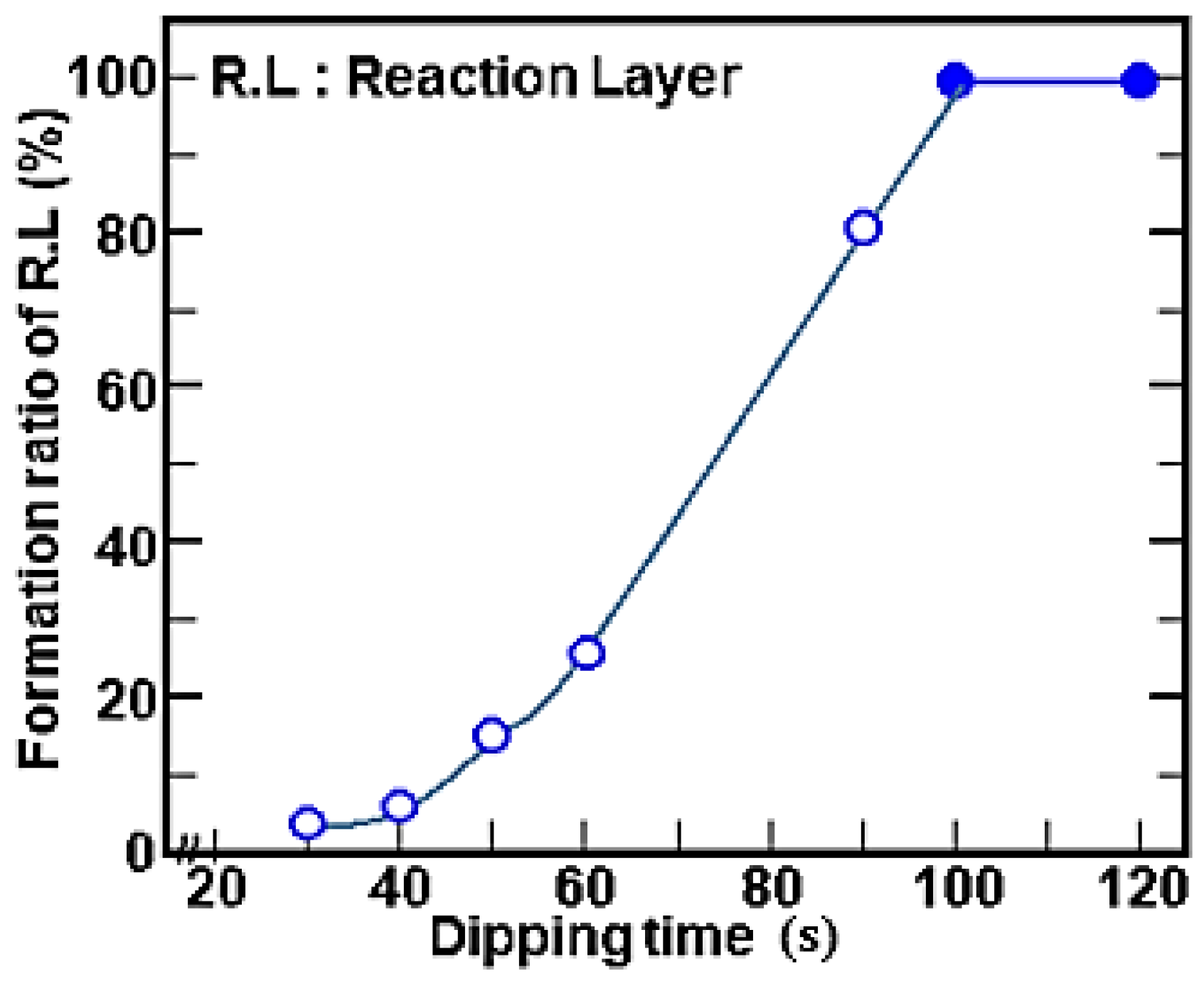
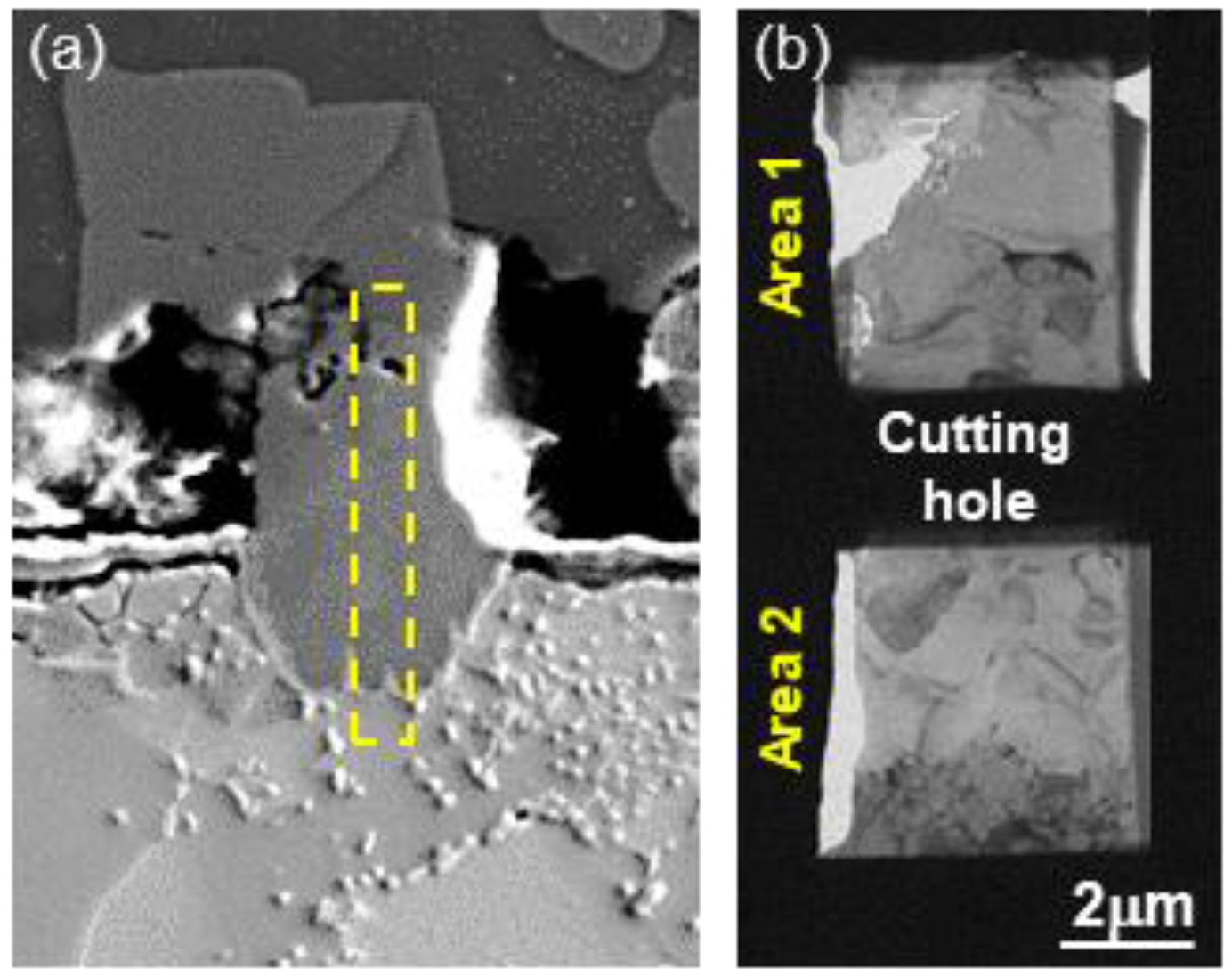
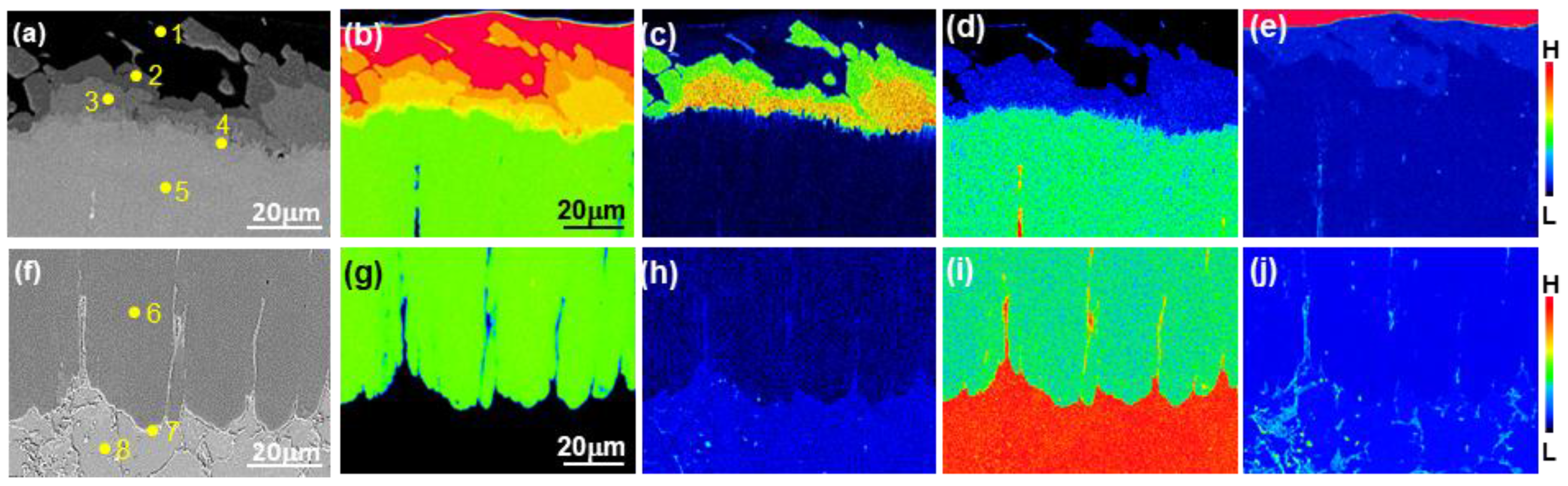
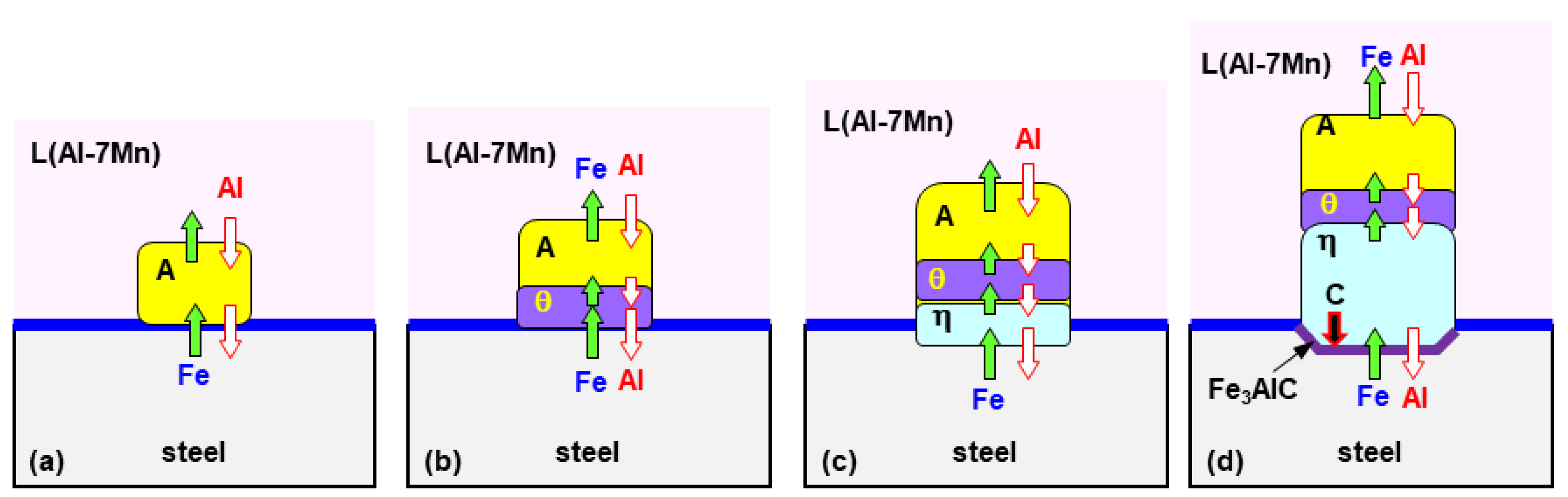
3.1. Formation of the Reaction Layer at Initial Hot Dipping
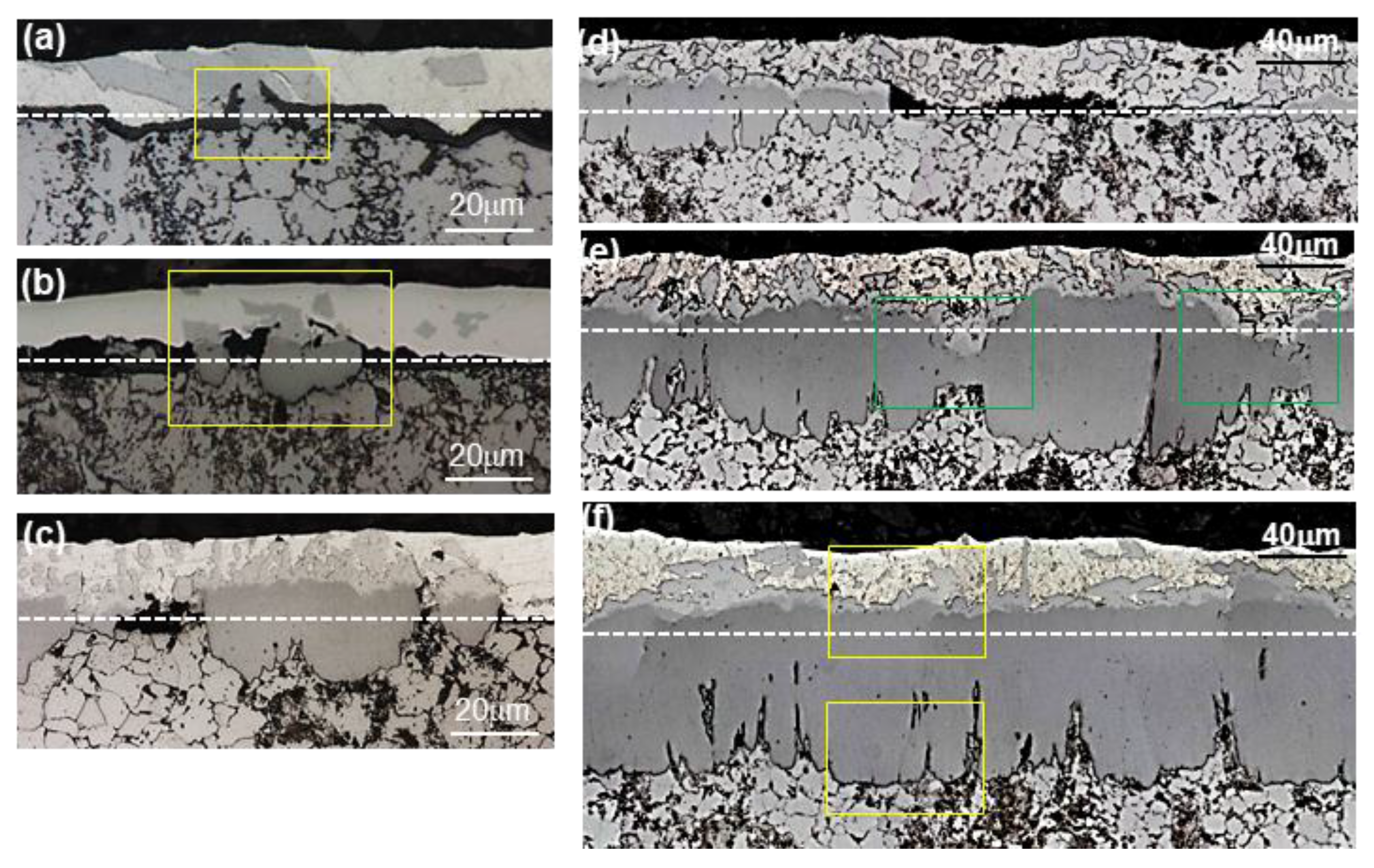
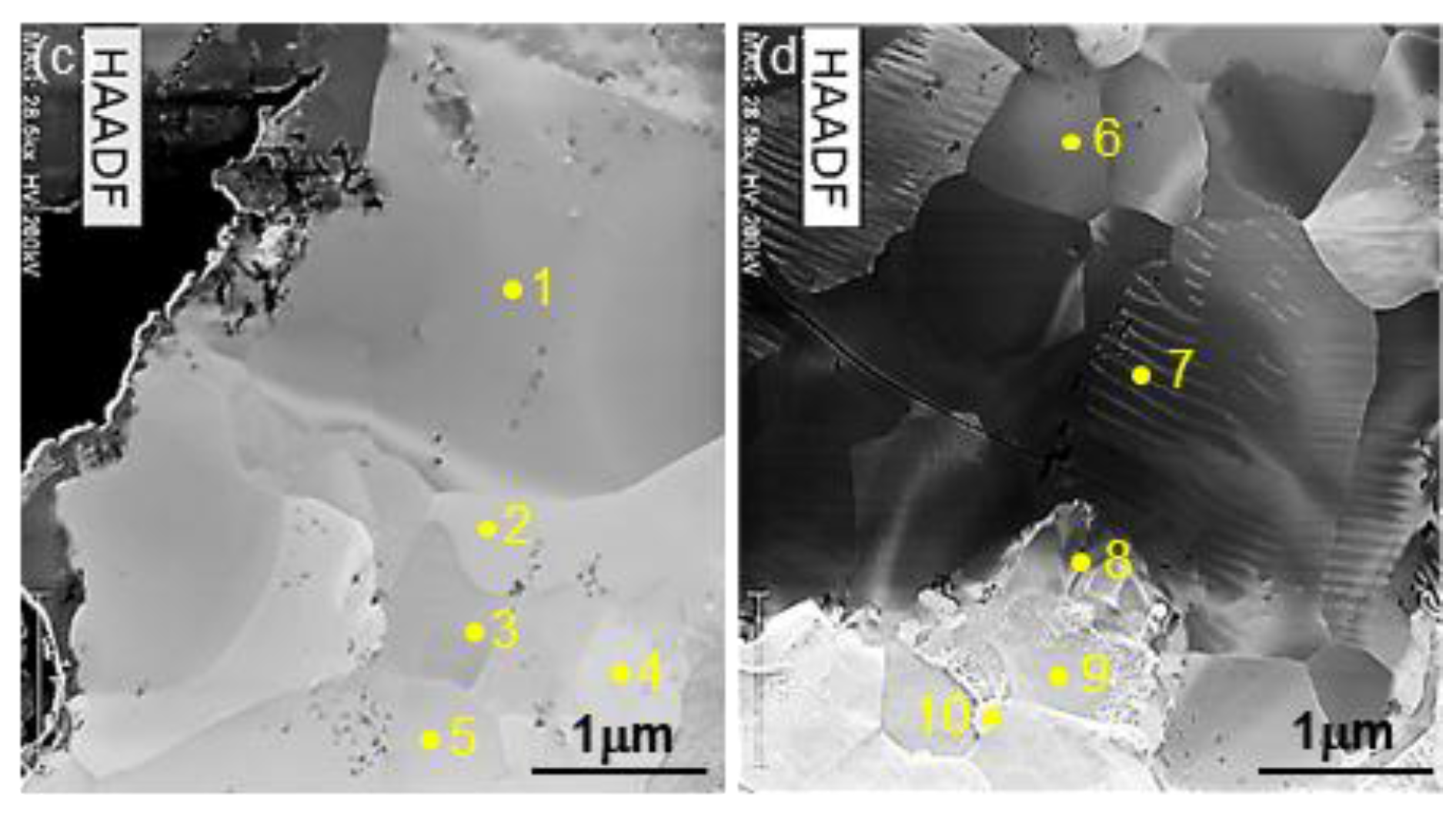
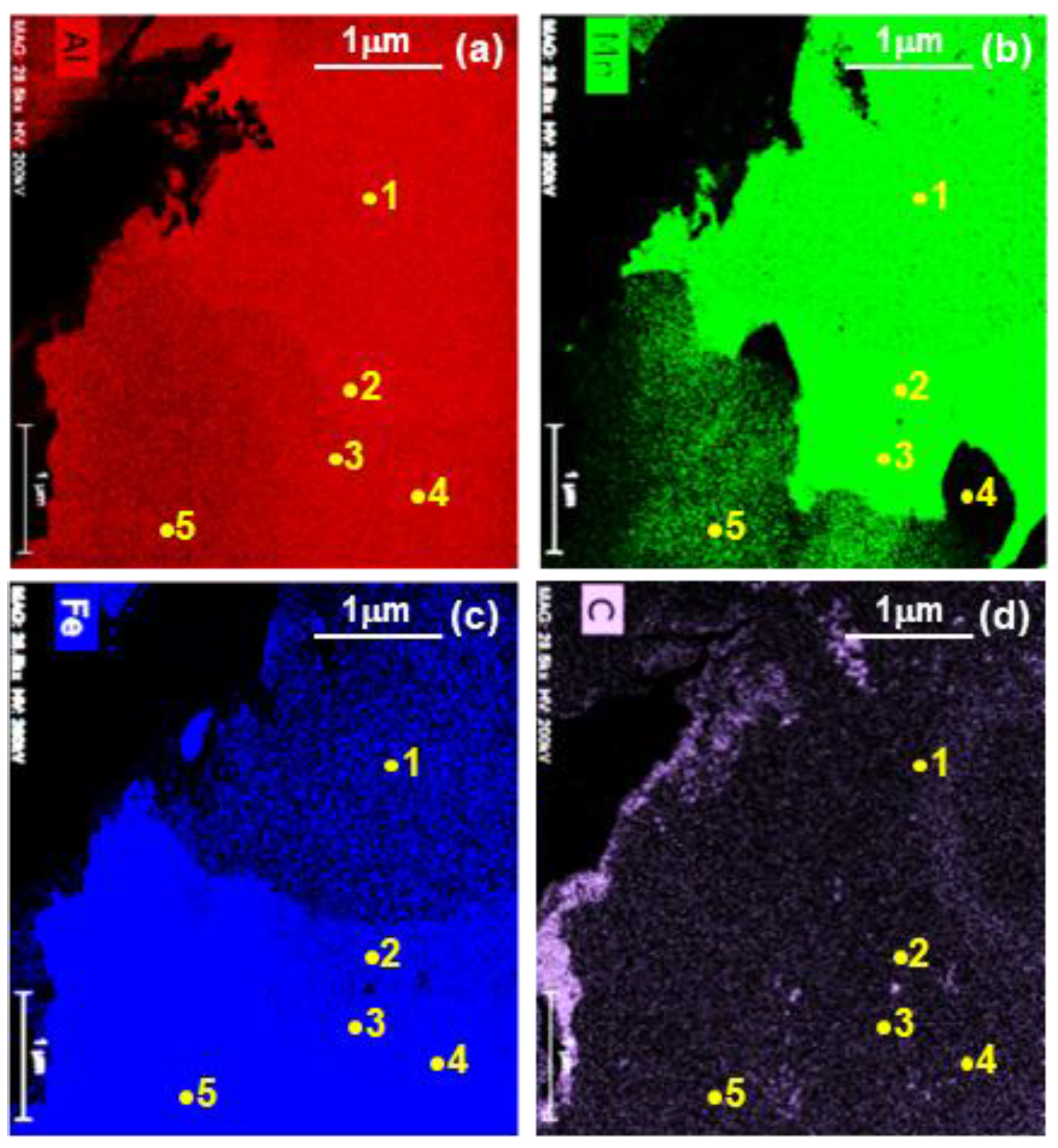
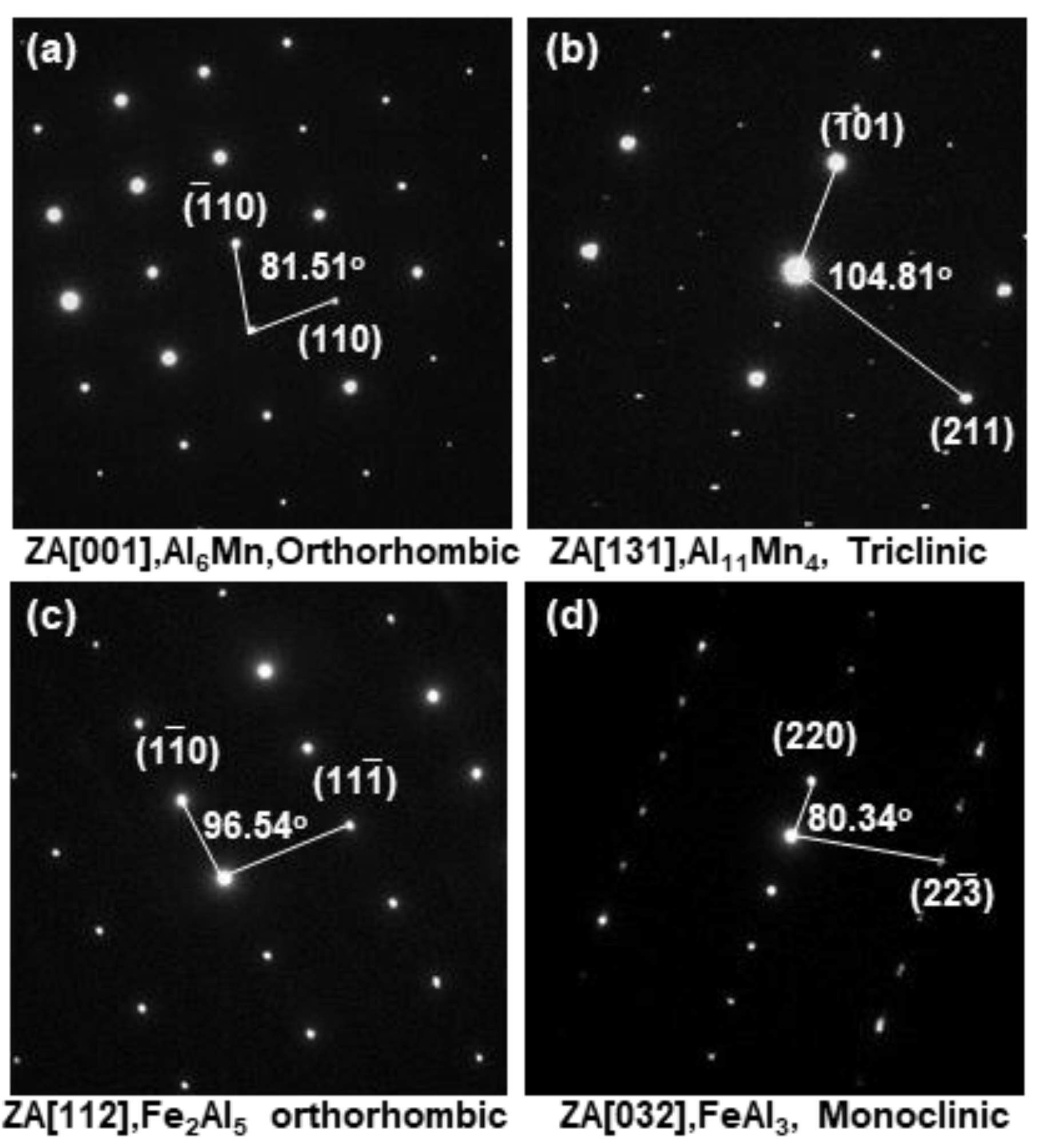
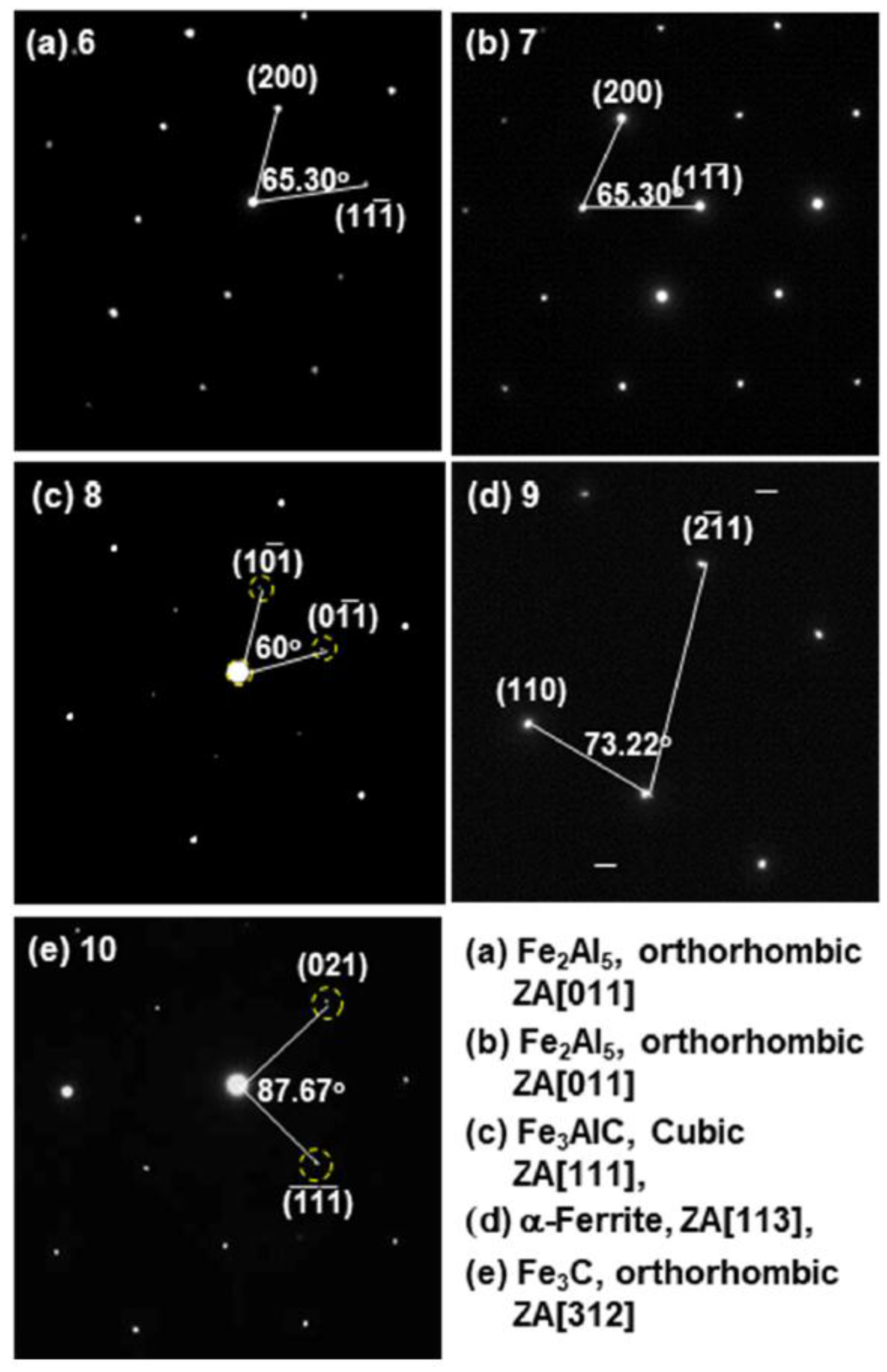
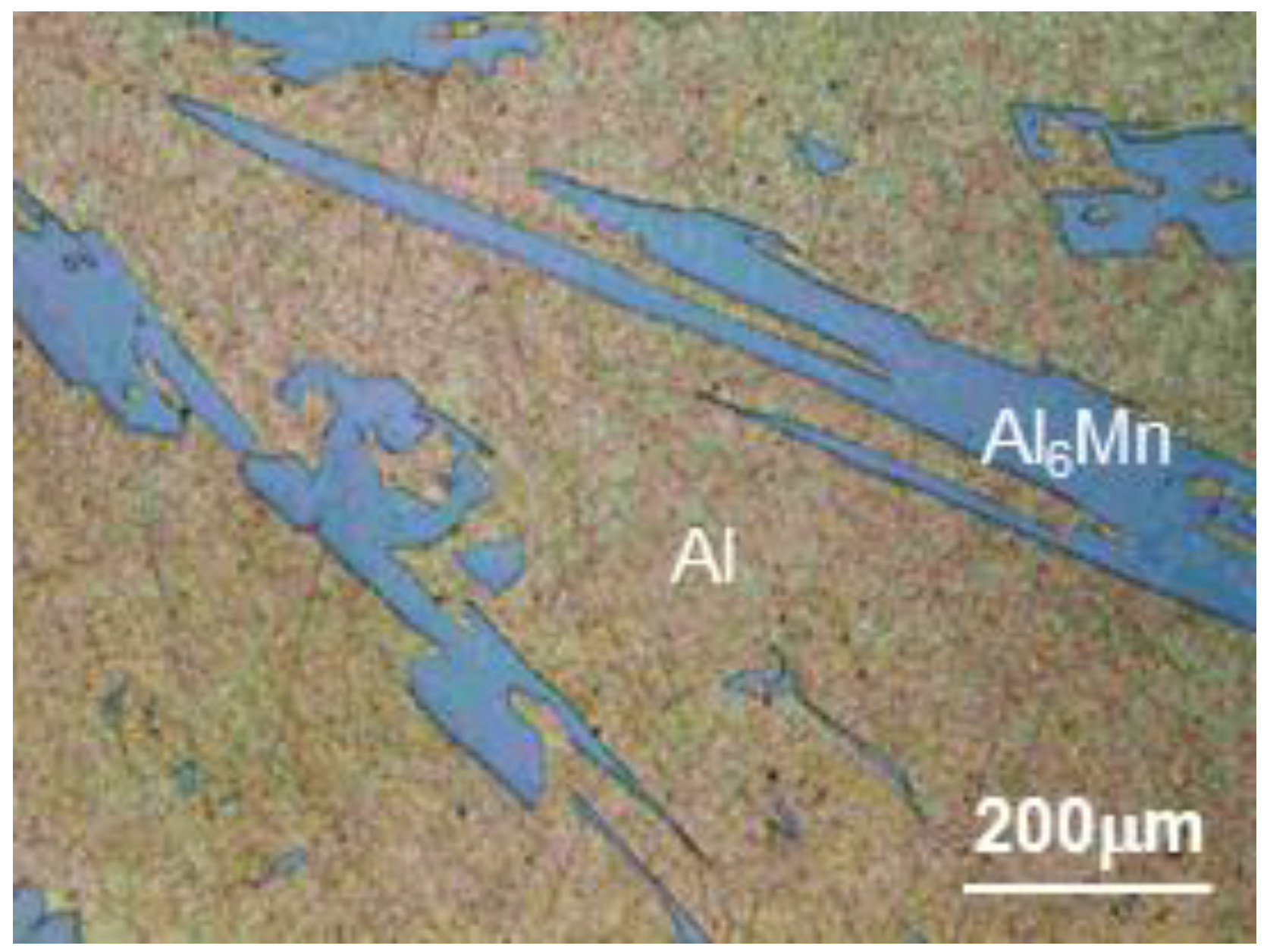
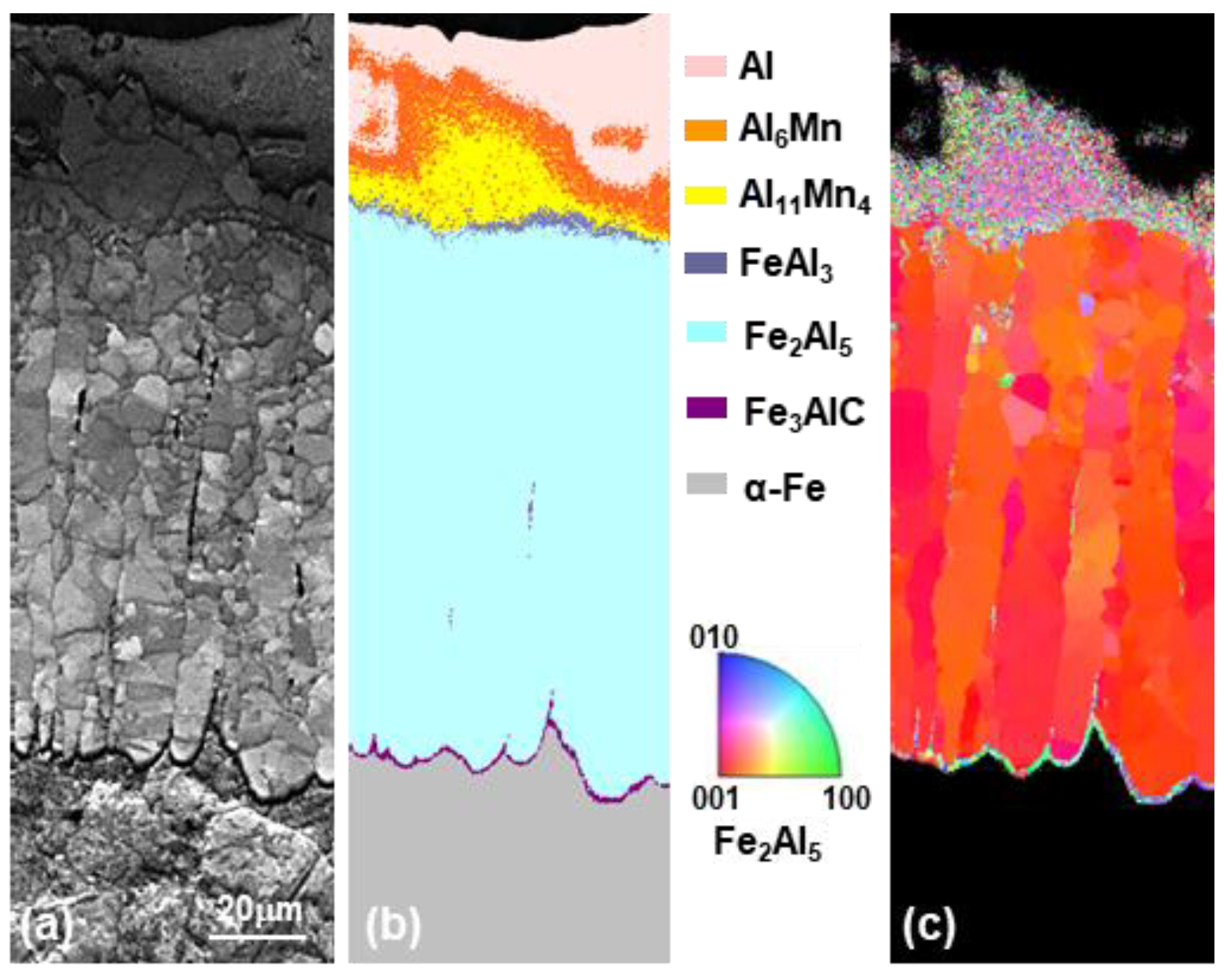
3.2. Evolution of Microstructure in the Coating Layer
4. Discussion
5. Conclusions
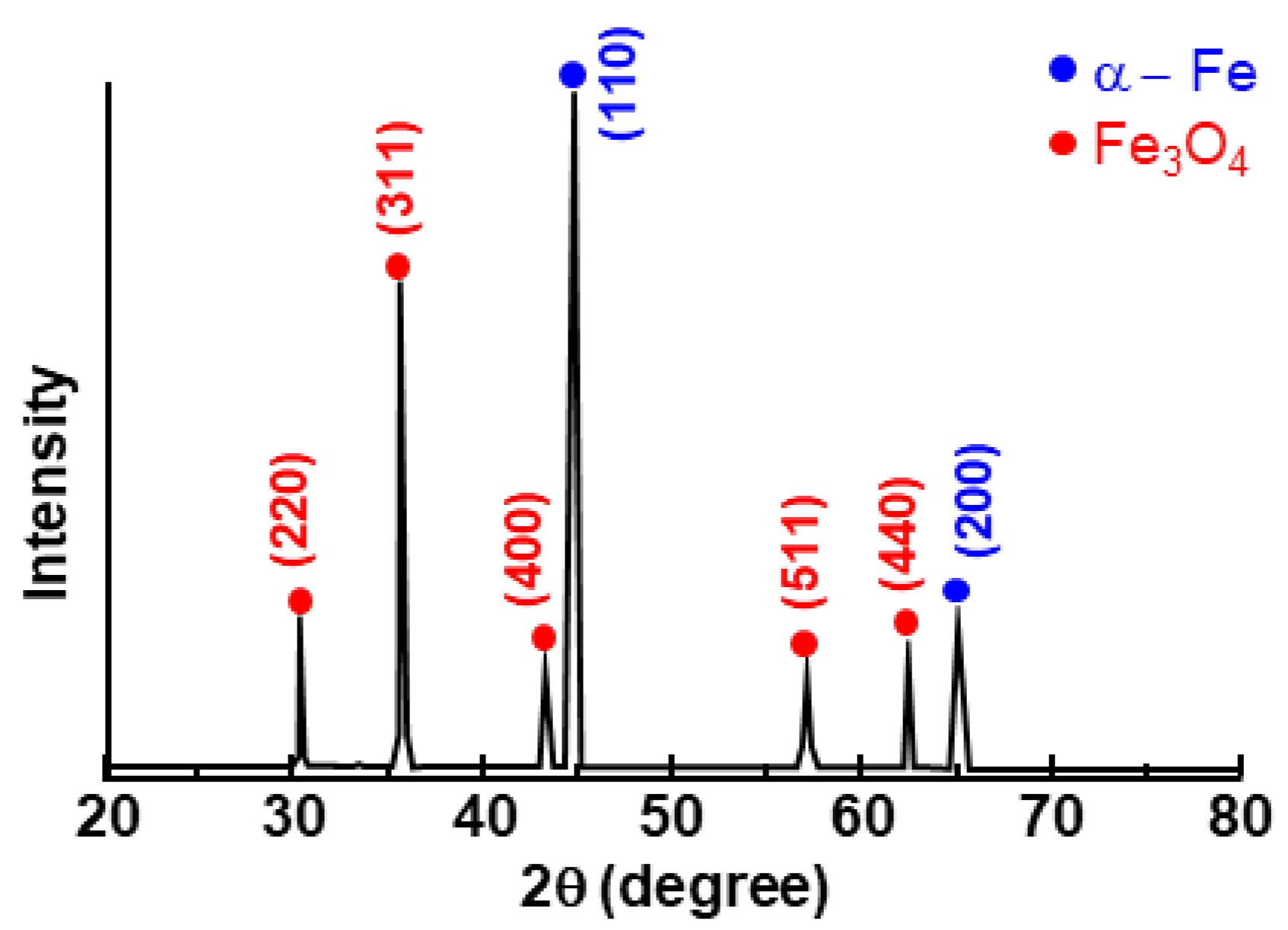
- The layer formed on the surface of the steel was classified into a reaction layer formed at the coating temperature and a solidification layer in which the liquid phase solidified during cooling. The solidification layer was composed of Al and Al6Mn phases, and the reaction layer was composed of Al11Mn4, FeAl3 (θ) and Fe2Al5 (η) phases. In particular, the η phase grew long inside the steel. The Fe3AlC (κ) phase was formed at the interface between the η phase and the steel in a very thin band of several nanometers.
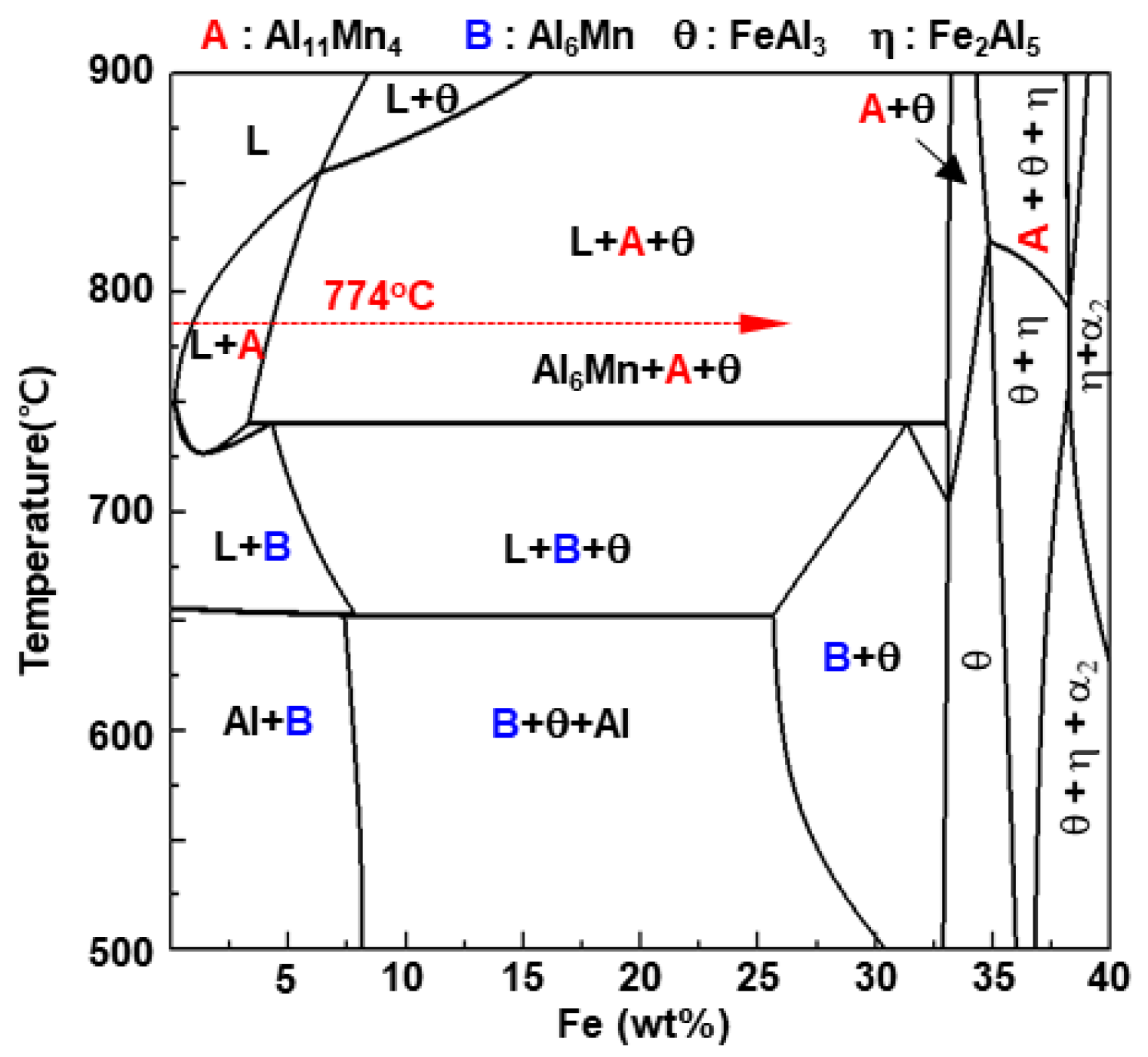
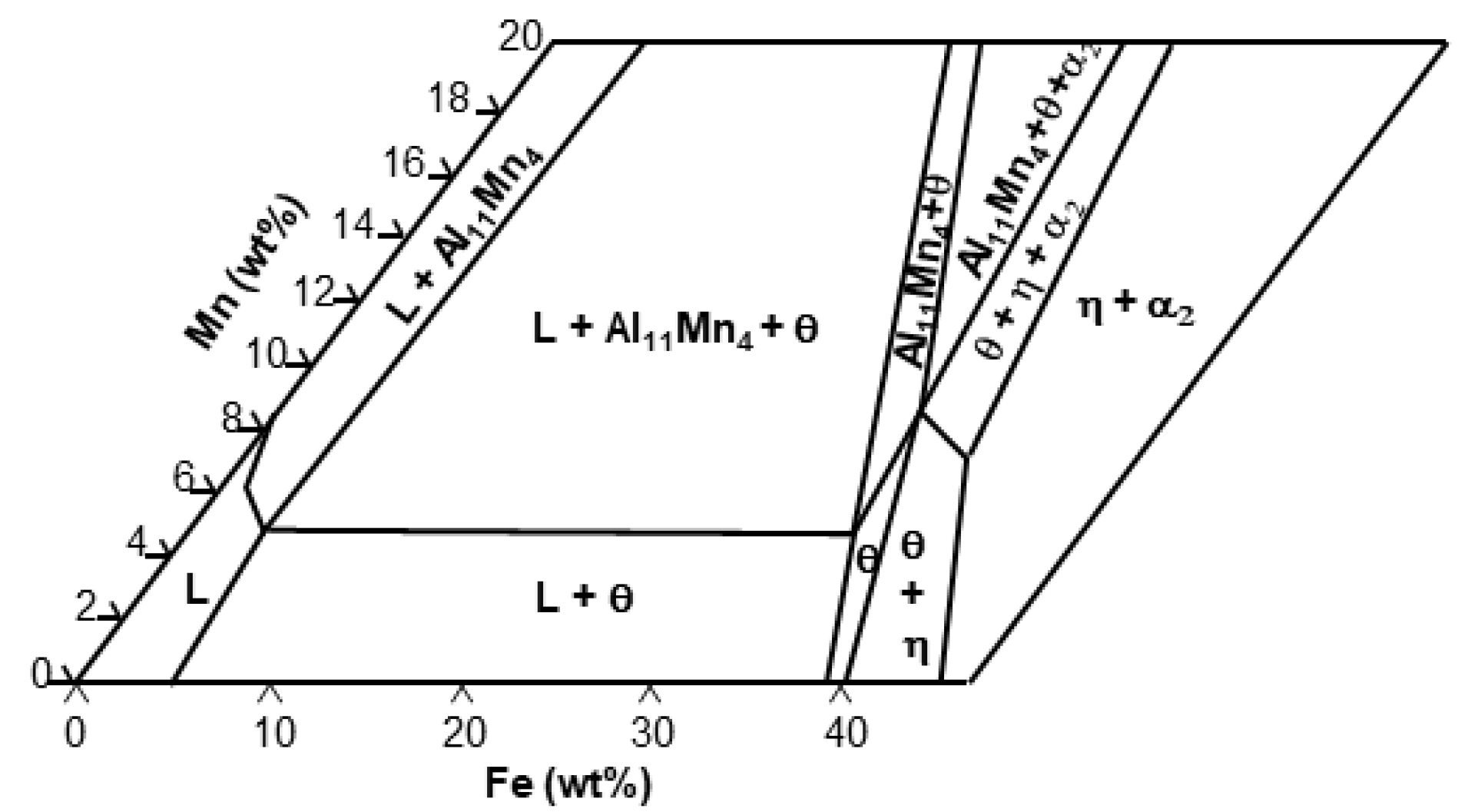
- The solid phase Al11Mn4 was eluted when Fe was dissolved in Al-Mn molten alloy at 773 °C at 0.6 wt.% or more from the (Al-7 wt.% Mn)-x wt.% Fe quasi phase diagram (Thermo-CalcTM). At the interface between Al11Mn4 phase and the steel, as Fe diffused toward the Al11Mn4 and Al diffused toward the steel, at the interface, θ phase was formed as the Fe concentration increased and the Al concentration decreased. In addition, η phase was formed due to inter-diffusion of Fe, Al between the θ phase and the steel. In other words, Al11Mn4 → θ → η was formed in which the Fe content was higher toward the steel by inter-diffusion in the solid phase state.
- The η phase formed by the reaction with the liquid phase initially had a fine polygonal structure. However, it formed inside the steel, growing to the order of Al11Mn4 → θ → η in the liquid phase, and grew in a long columnar form because the Al diffusion was along the c-axis and the <100> direction was fast, as already known.
- Fe3AlC (κ) phase was formed as the concentration of Al and C is increasing at the steel interface because the η phase diffused C toward steel due to the fact that η hardly contains C.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Senuma, T. Physical metallurgy of modern high strength steel sheets. Iron Steel Inst. Jpn. 2001, 41, 520–532. [Google Scholar] [CrossRef]
- Tetsuya, M.; Kohei, H.; Hidetaka, K. Ultra high-strength steel sheets for bodies, reinforcement parts, and seat frame parts of automobile—Ultra high-strength steel sheets leading to great improvement in crash-worthiness. JFE Tech. Rep. 2004, 4, 38–43. [Google Scholar]
- Bariani, P.F.; Bruschi, S. Testing formability in the hot stamping of HSS. CIRP Ann. Manuf. Technol. 2008, 57, 265–268. [Google Scholar] [CrossRef]
- Karbasian, H.; Tekkaya, A.E. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Naderi, M.; Ketabchi, M. Analysis of microstructure and mechanical properties of different high strength carbon steels after hot stamping. J. Mater. Process. Technol. 2011, 211, 1117–1125. [Google Scholar] [CrossRef]
- Wang, C.-J.; Chen, S.-M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601–6605. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Effect of silicon on the formation of intermetallic phases in aluminide coating on mild steel. Intermetallics 2011, 19, 1455–1460. [Google Scholar] [CrossRef]
- Saha, D.C.; Biro, E.; Gerlich, A.P.; Zhou, Y.N. Fiber laser welding of Al-Si-coated press-hardened steel. Weld. J. 2016, 95, 147–156. [Google Scholar]
- Jung, B.H.; Kong, J.P.; Kang, C.Y. Effect of hot-stamping heat treatment on the microstructure of Al-segregated zone in TWB laser joints of Al-Si-coated boron steel and Zn-coated DP steel. Korean J. Met. Mater. 2012, 50, 455–462. [Google Scholar]
- Yoon, T.-J.; Oh, M.-H.; Shin, H.-J.; Kang, C.-Y. Comparison of microstructure and phase transformation of laser-welded joints in Al-10wt.%Si-coated boron steel before and after hot stamping. Mater. Charact. 2017, 128, 195–202. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Chen, S.; Yang, D.; Zhang, M.; Huang, J.; Zhao, X. Interaction between the growth and dissolution of intermetallic compounds in the interfacial reaction between solid iron and liquid aluminum. Metall. Mater. Trans. A 2016, 47, 5088–5100. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Study of microstructure and phase evolution of hot-dipped aluminide mild steel during high-temperature diffusion using electron backscatter diffraction. Appl. Surf. Sci. 2011, 257, 4663–4668. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Naoi, D.; Kajihara, M. Growth behavior of Fe2Al5 during reactive diffusion between Fe and Al at solid-state temperatures. Mater. Sci. Eng. A 2007, 459, 375–382. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M. Kinetics of isothermal reactive diffusion between solid Fe and liquid Al. J. Mater. Sci. 2010, 45, 5676–5684. [Google Scholar] [CrossRef]
- Drewett, R. A review of some aspects concerning the formation of metallic diffusion coatings on ferrous metals. Corros. Sci. 1969, 9, 823–847. [Google Scholar] [CrossRef]
- Yeremenko, V.N.; Natanzon, Y.V.; Dybkov, V.I. The effect of dissolution on the growth of the Fe2Al5, interlayer in the solid iron—Liquid aluminium system. J. Mater. Sci. 1981, 16, 1748–1756. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Dong, X.; Yu, Q.; Xu, Q. Prepartion of Al-Mn alloy coatings on low carbon steel by hot-dip-aluminizing. Appl. Mech. Mater. 2012, 117–119, 1121–1124. [Google Scholar] [CrossRef]
- Pradhan, D.; Manna, M.; Dutta, M. Al–Mg–Mn alloy coating on steel with superior corrosion behavior. Surf. Coat. Technol. 2014, 258, 405–414. [Google Scholar] [CrossRef]
- ASM Handbook Volume 9: Metallography and Microstructures; Vander Voort, G.F. (Ed.) ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Bouche, K.; Barbier, F.; Coulet, A. Intermetallic compound layer grwoth between solid iron and molten aluminum. Mater. Sci. Eng. A 1998, 249, 167–175. [Google Scholar] [CrossRef]
- Shahverdi, H.R.; Ghomashchi, M.R.; Shabestari, S.; Hejazi, J. Microstructural analysis of interfacial reaction between molten aluminum and solid iron. J. Mater. Process. Technol. 2002, 124, 345–352. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Morphology and formation of Fe-Al intermetallic compoundss on iron hot-dipped in Al-Mg-Si alloy melt. Intermetallics 2014, 54, 136–142. [Google Scholar] [CrossRef]
- Yin, F.-C.; Zhao, M.-X.; Liu, Y.-X.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferrous Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Growth of intermetallic compounds in the aluminide mild steel during hot-dipped. Surf. Coat. Technol. 2009, 204, 824–828. [Google Scholar] [CrossRef]
- Awan, G.H.; Hasan, F.U. The morphology of coating/substrate interface in hot-dip-aluminized steels. Mat. Sci. Eng. A 2008, 472, 157–165. [Google Scholar] [CrossRef]
- Sasaki, T.; Yakou, T. Features of intermetallic compounds in aluminized steels formed using aluminum foil. Surf. Coat. Technol. 2006, 201, 2131–2139. [Google Scholar] [CrossRef]
- Heumann, T.; Dittrich, N. Structure character of the Fe2Al5 intermetallics compound in hot dip aluminizing process. Z Metallk 1959, 50, 617–625. [Google Scholar]
- Van Alboom, A.; Lemmens, B.; Breitbach, B.; de Grave, E.; Cottenier, S.; Verbeken, K. Multi-method identification and characterization of the intermetallic surface layers of hot-dip Al-coated steel: FeAl3 or Fe4Al13 and Fe2Al5 or Fe2Al5+x. Surf. Coat. Technol. 2017, 324, 419–428. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Khitouni, M.; Escoda, L.; Suñol, J.J. Recovery, grain growth and recrystallization of mechanically alloyed FeAl alloy. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2010; p. 012021. [Google Scholar]
- Burkhardt, U.; Grin, Y.; Ellner, M. Structure refinement of the iron-aluminum phase with the approximate composition Fe2Al5. Acta Crystallogr. Sect. B Struct. Sci. 1994, 50, 313–316. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. Microstructural evolution of intermetallic compounds in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Sakidja, R.; Perepezko, J.H.; Calhoun, P. Synthesis, thermodynamic stability and diffusion mechanism of Al5Fe2-based coatings. Oxid. Met. 2014, 81, 167–177. [Google Scholar] [CrossRef]


| Chemical Composition (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | S | Mn | Cr | Nb | Ti | B | P | S | Fe |
| 0.229 | 0.238 | 1.189 | 0.183 | 0.004 | 0.036 | 0.002 | 0.002 | 0.002 | Bal. |
| No. | Al | Mn | Fe | C | Rem. | Expected Phase |
|---|---|---|---|---|---|---|
| at.%/wt.% | at.%/wt.% | at.%/wt.% | at.%/wt.% | at.%/wt.% | ||
| 1 | 80.96/67.52 | 12.26/20.95 | 6.20/10.77 | 0.32/0.12 | Bal./Bal. | Al6Mn |
| 2 | 76.11/61.17 | 15.06/24.69 | 8.24/13.73 | 0.36/0.13 | Bal./Bal. | Al11Mn4 |
| 3 | 74.01/58.37 | 16.16/26.01 | 8.33/15.26 | 0.36/0.13 | Bal./Bal. | Al11Mn4 |
| 4 | 69.58/52.49 | 0.14/0.22 | 29.98/47.01 | 0.23/0.08 | Bal./Bal. | Fe2Al5 |
| 5 | 69.17/52.27 | 4.11/6.32 | 26.4/41.02 | 0.32/0.11 | Bal./Bal. | FeAl3 |
| No. | Al | Mn | Fe | C | Rem. | Expected Phase |
|---|---|---|---|---|---|---|
| at.%/wt.% | at.%/wt.% | at.%/wt.% | at.%/wt.% | at.%/wt.% | ||
| 6 | 69.84/51.95 | 0.17/0.25 | 29.62/47.02 | 0.22/0.07 | Bal./Bal. | Fe2Al5 |
| 7 | 68.67/51.60 | 0.15/0.23 | 30.9/48.06 | 0.26/0.09 | Bal./Bal. | Fe2Al5 |
| 8 | 18.24/11.52 | 1.37/1.76 | 62.5/81.69 | 17.89/5.03 | Bal./Bal. | Fe3AlC |
| 9 | 0.09/0.04 | 1.14/1.13 | 97.16/98.01 | 1.01/0.22 | Bal./Bal. | Fe |
| 10 | 0.03/0.02 | 7.07/8.15 | 74.37/87.16 | 18.53/4.67 | Bal./Bal. | Fe3C |
| No. | Al | Mn | Fe | C | Rem. | Expected Phase |
|---|---|---|---|---|---|---|
| 1 | 98.30 | 0.21 | 0.17 | 1.01 | Bal. | Al |
| 2 | 82.21 | 11.34 | 5.72 | 0.37 | Bal. | Al6Mn |
| 3 | 74.61 | 19.12 | 4.93 | 0.14 | Bal. | Al11Mn4 |
| 4 | 69.17 | 4.94 | 25.01 | 0.27 | Bal. | FeAl3 |
| 5 | 69.14 | 0.16 | 29.74 | 0.10 | Bal. | Fe2Al5 |
| 6 | 68.73 | 0.19 | 30.10 | 0.21 | Bal. | Fe2Al5 |
| 7 | 17.31 | 1.03 | 62.88 | 17.87 | Bal. | Fe3AlC |
| 8 | 0.02 | 1.21 | 95.94 | 1.81 | Bal. | Fe |
| Phase | Al | Mn | Fe | C |
|---|---|---|---|---|
| Al11Mn4 | 57.40 | 35.89 | 6.62 | 0.09 |
| FeAl3 (θ) | 57.05 | 7.67 | 35.14 | 0.14 |
| Fe2Al5 (η) | 54.71 | 0.32 | 44.9 | 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, S.-Y.; Yun, J.-G.; Lee, J.-H.; Shin, D.-I.; Kang, C.-Y. Identification of Intermetallic Compounds and Its Formation Mechanism in Boron Steel Hot-Dipped in Al-7 wt.% Mn Alloy. Coatings 2017, 7, 222. https://doi.org/10.3390/coatings7120222
Kwak S-Y, Yun J-G, Lee J-H, Shin D-I, Kang C-Y. Identification of Intermetallic Compounds and Its Formation Mechanism in Boron Steel Hot-Dipped in Al-7 wt.% Mn Alloy. Coatings. 2017; 7(12):222. https://doi.org/10.3390/coatings7120222
Chicago/Turabian StyleKwak, Sung-Yun, Jung-Gil Yun, Jae-Hyeong Lee, Dong-Ik Shin, and Chung-Yun Kang. 2017. "Identification of Intermetallic Compounds and Its Formation Mechanism in Boron Steel Hot-Dipped in Al-7 wt.% Mn Alloy" Coatings 7, no. 12: 222. https://doi.org/10.3390/coatings7120222





