Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics
Abstract
:1. Introduction
2. Materials and Experimental Methodology
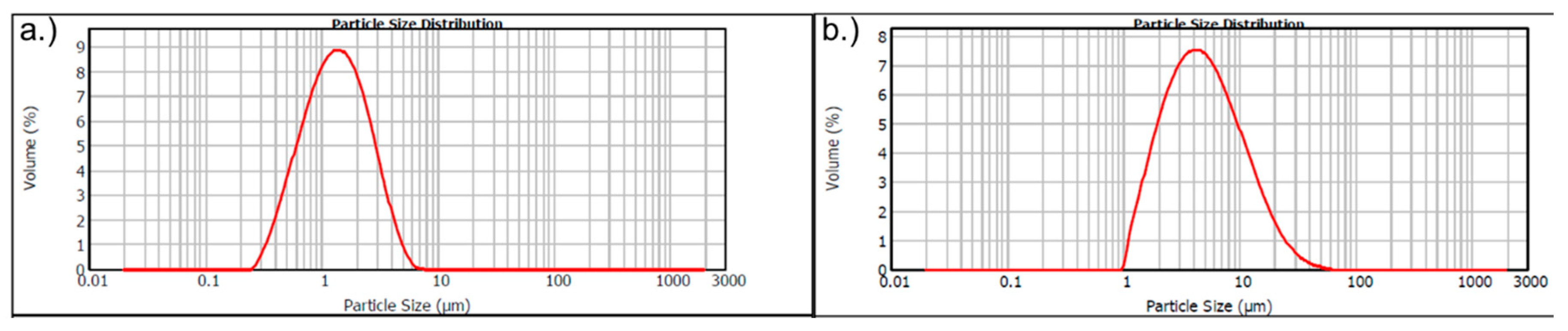
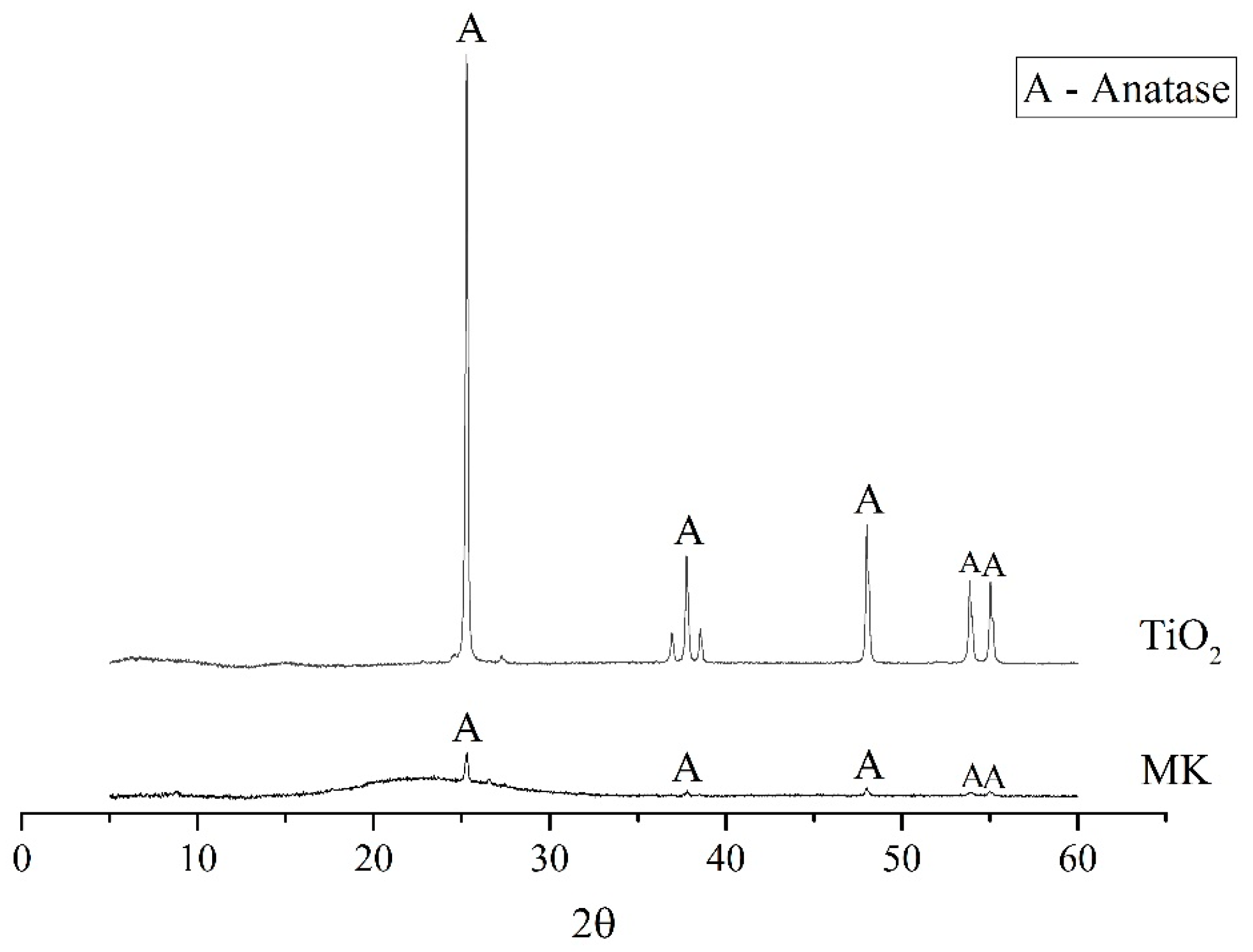
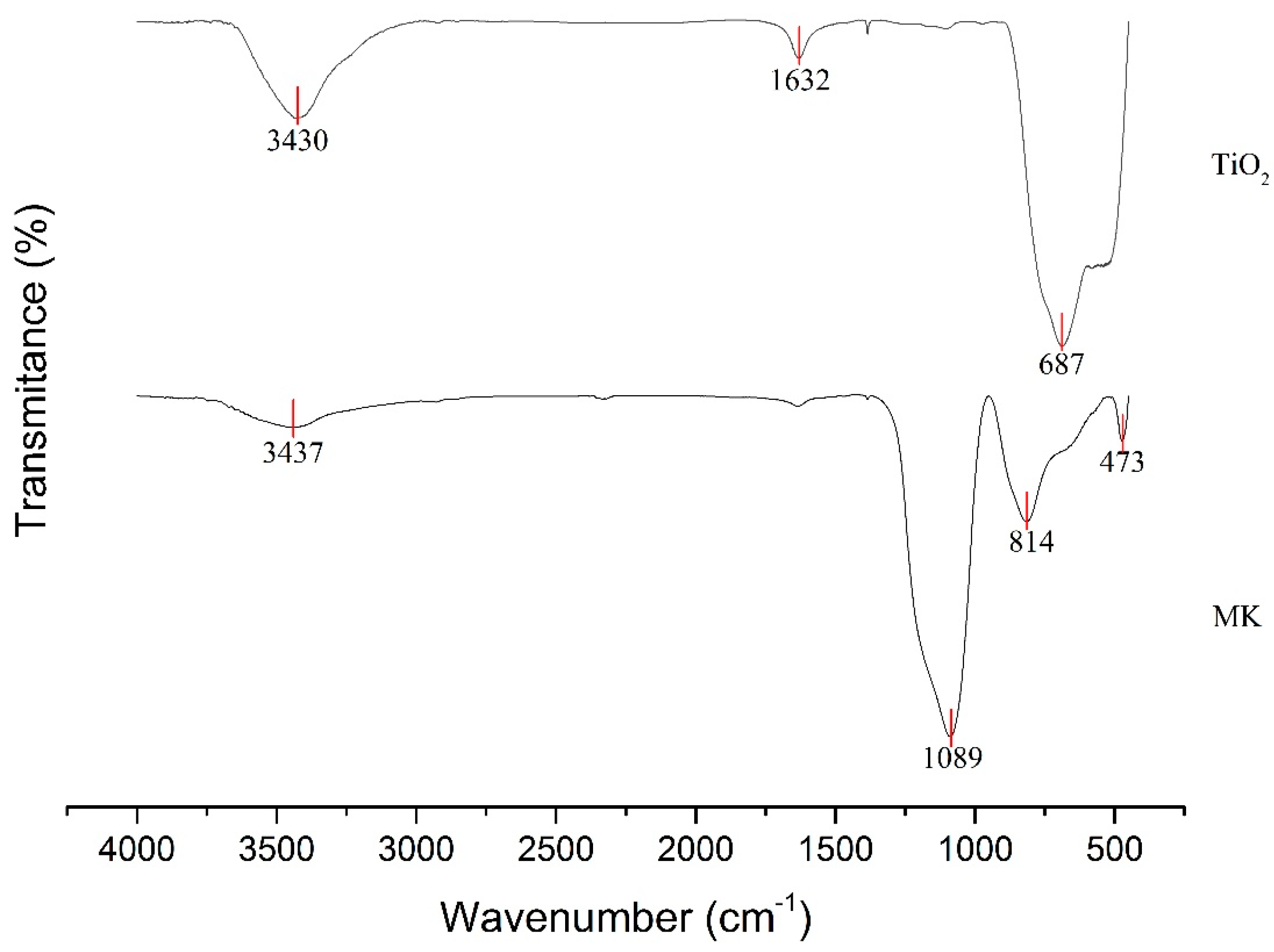
2.1. Materials
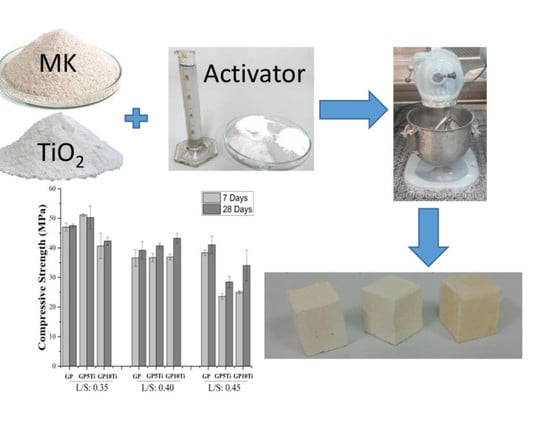
2.2. Preparation of Geopolymeric Mixtures
2.3. Physical and Mechanical Properties
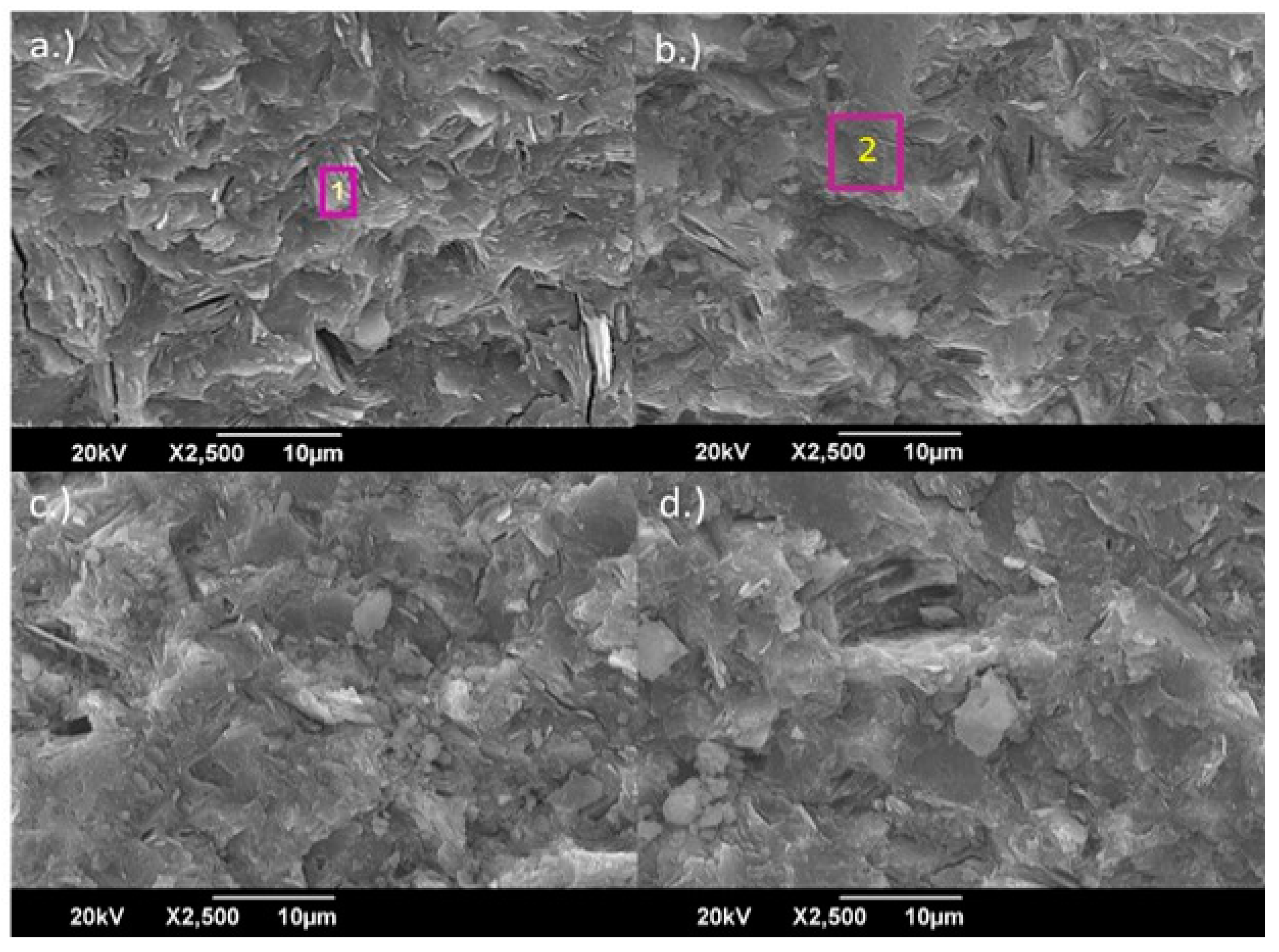
2.4. Microstructural Analysis
3. Results and Discussion
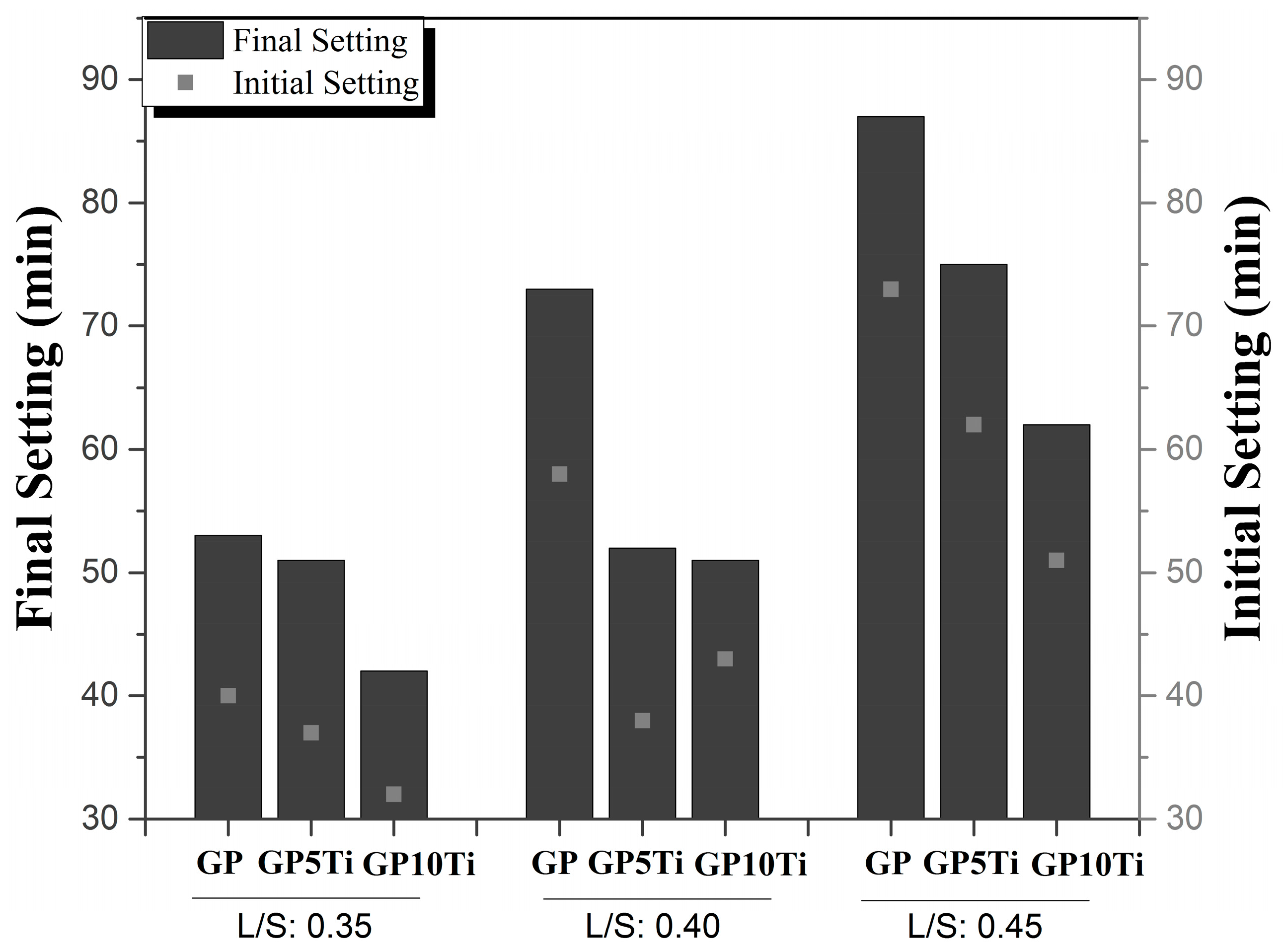
3.1. Fluidity and Setting Time
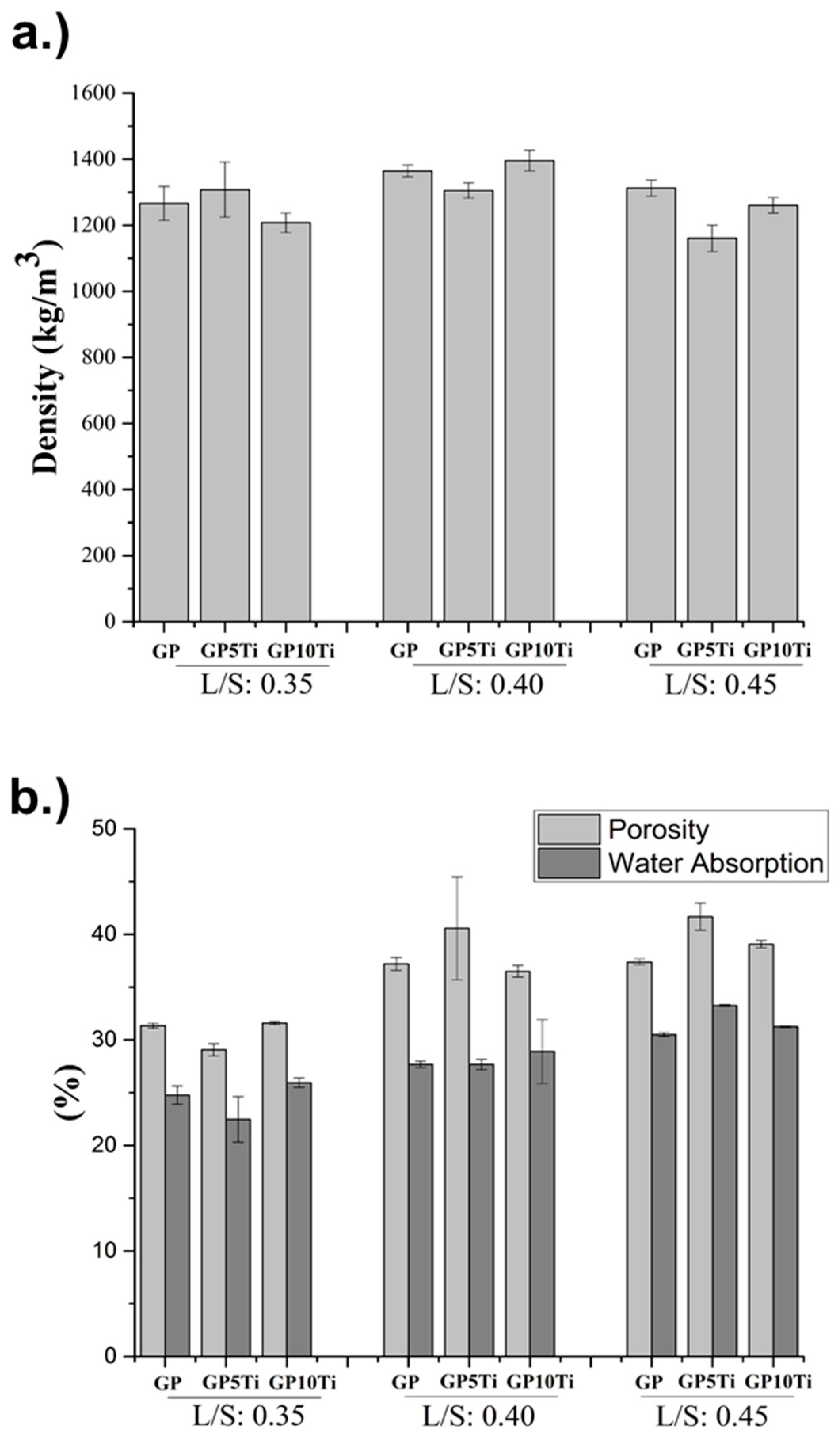
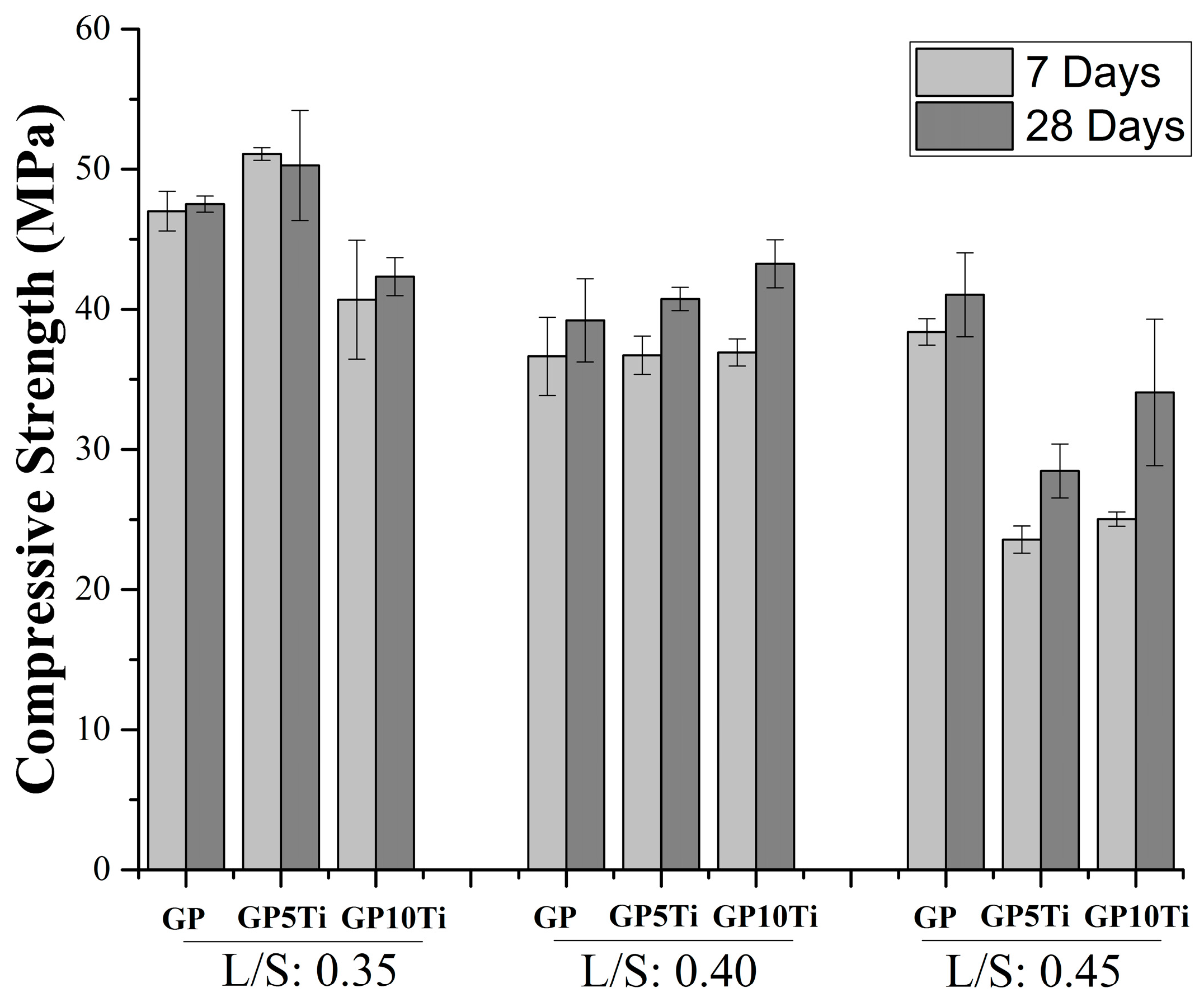
3.2. Physical and Mechanical Properties
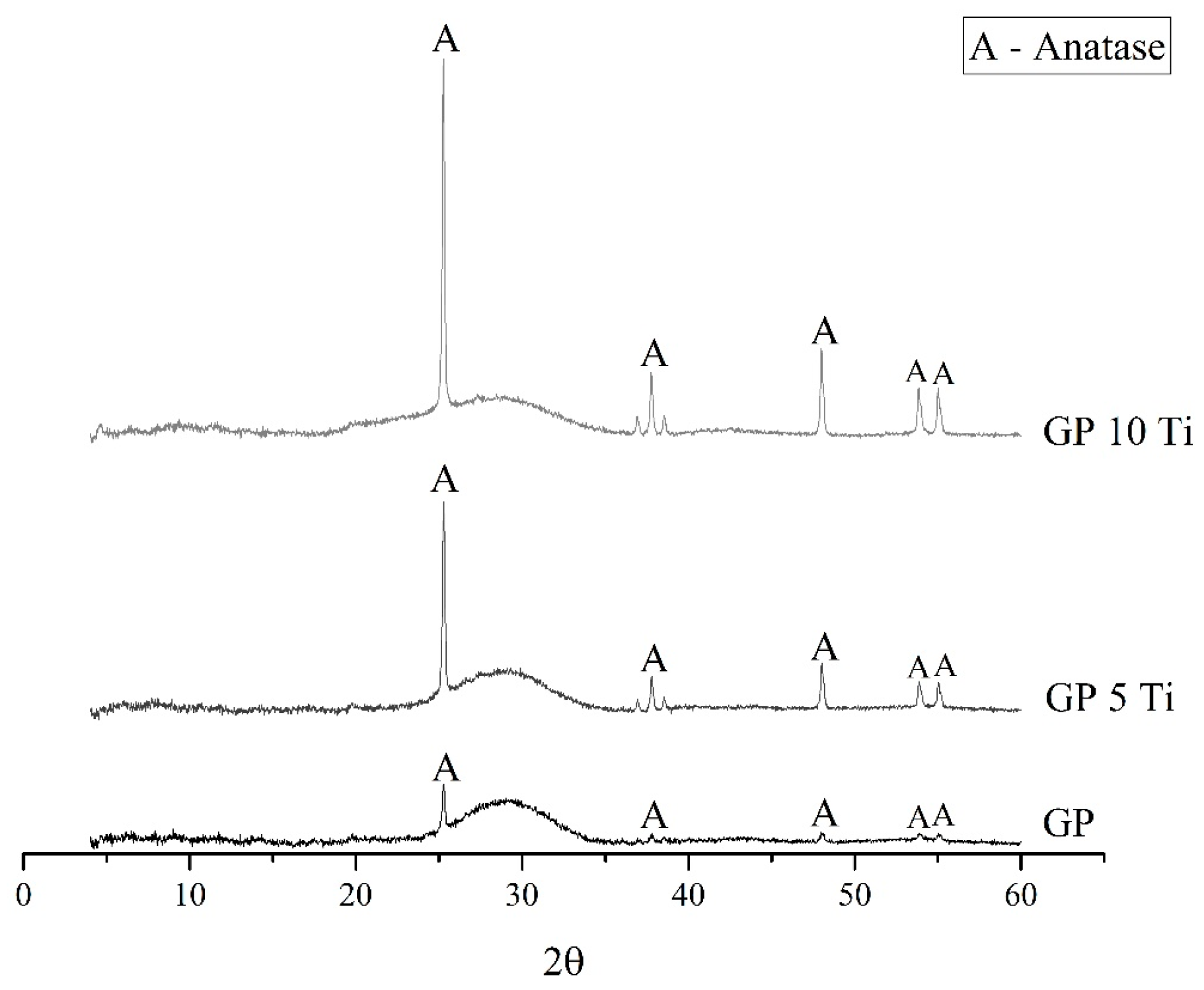
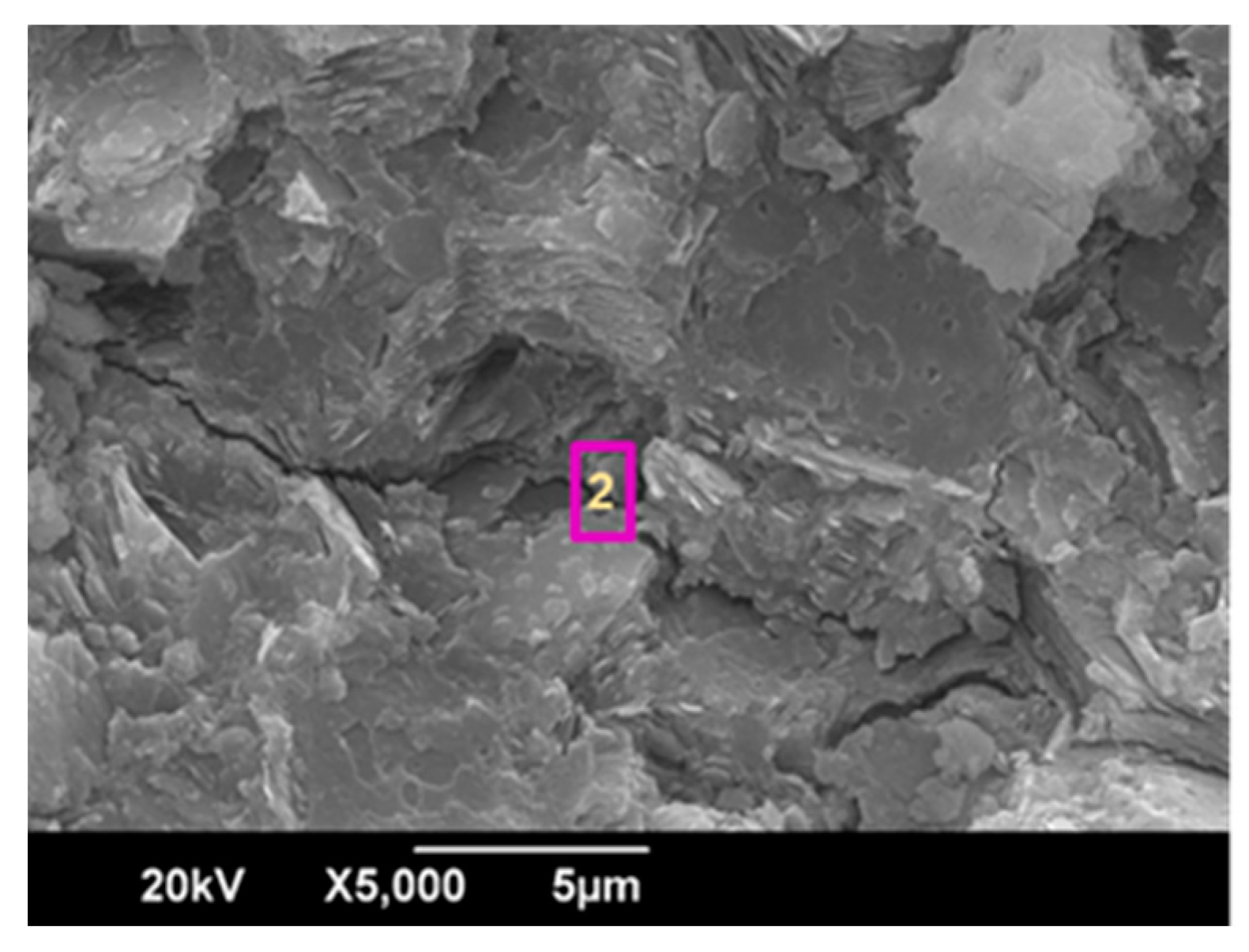
3.3. Characterization of the Geopolymer Microstructure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejia, J.; Mejia de Gutierrez, R. Eco-efficient alkali-activated cement based on red clay brick wastes suitable for the manufacturing of building materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]
- McLellan, C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Weil, M.; Dombrowski, K.; Buchwald, A. Life-cycle analysis of geopolymers. In Geopolymers: Structure Processing Properties and Industrial Applications, 1st ed.; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 194–210. [Google Scholar]
- Duxon, P.; Fernandez-Jimenez, A.; Provis, J.; Luckey, G.; Palomo, A.; van Deventer, J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.; Low, I. Influence of mixing methods of nano silica on the microstructural and mechanical properties of flax fabric reinforced geopolymer composites. Constr. Build. Mater. 2016, 123, 541–552. [Google Scholar] [CrossRef]
- Riahi, S.; Nazari, A. The effects of nanoparticles on early age compressive strength of ash-based geopolymers. Ceram Int. 2012, 38, 4467–4476. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Luo, W.; Zhou, W. Effects of adding nano-TiO2 on compressive strength, drying shrinkage, carbonation and microstructure of fluidized bed fly ash based geopolymer paste. Constr. Build. Mater. 2016, 106, 115–125. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Z.; Zhang, Y.; Dai, J. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, K.; Mo, B.; Li, X.; Cui, X. Preparation and characterization of a reflective and heat insulative coating based on geopolymers. Energy Build. 2015, 87, 220–225. [Google Scholar] [CrossRef]
- Ohama, Y.; Van Gemert, D. Introduction. In Application of Titanium Dioxide Photocatalysis to Construction Materials, 1st ed.; Ohama, Y., Van Gemert, D., Eds.; State of the Art Report of the RILEM Technical committee 194-TDP; Springer: Dordrecht, The Netherlands, 2011; Volume 5, pp. 1–4. ISBN 978-94-007-1296-6. [Google Scholar]
- Wan, Q.; Rao, F.; Song, S.; Garcia, R.; Estrella, R.; Patiño, C.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Gao, M.; Huang, Y.; Zhang, X.; Yu, H. Photocatalytic degradation of atrazine by boron-doped TiO2 with a turnable rutile/anatase ratio. Appl. Catal. B 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.; Mejía de Gutiérrez, R.; Sulekar, S.; Davis, C.; Nino, J. Thermal properties of novel binary geopolymers based on metakaolin and alternative silica sources. Appl. Clay Sci. 2015, 118, 276–282. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Liew, Y.; Kamarudin, H.; Mustafa, A.; Bnhussain, M.; Luqman, M.; Nizar, I.; Ruzaidi, C.; Heah, C. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Essawy, A.; Aleem, S. Physico-mechanical properties, potent adsorptive and photocatalytic efficacies of sulfate resisting cement blends containing micro silica and nano-TiO2. Constr. Build. Mater. 2014, 52, 1–8. [Google Scholar] [CrossRef]
- Yun, L.; Cheng, H.; Mustafa, M.; Hussin, K. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Falah, M.; Mackenzie, K. Synthesis and properties of novel photoactive composites of P25 titanium dioxide and copper (I) oxide with inorganic polymers. Ceram. Int. 2015, 41, 13702–13708. [Google Scholar] [CrossRef]


| Element/Composite | wt % |
|---|---|
| SiO2 | 52.018 |
| Al2O3 | 44.950 |
| TiO2 | 1.730 |
| Fe2O3 | 0.469 |
| Na2O | 0.295 |
| MgO | 0.192 |
| K2O | 0.158 |
| P2O5 | 0.058 |
| CaO | 0.023 |
| Ce | 0.023 |
| V | 0.021 |
| S | 0.018 |
| Cr | 0.014 |
| Zr | 0.012 |
| Ga | 0.006 |
| Sr | 0.005 |
| Zn | 0.003 |
| Nb | 0.003 |
| Mixture | TiO2% | L/S Ratio | SiO2/Al2O3 | K2O/SiO2 |
|---|---|---|---|---|
| GP | 0 | 0.35, 0.40 and 0.45 | 2.5 | 0.28 |
| GP 5 Ti | 5 | |||
| GP 10 Ti | 10 | – | – | – |
| Composition, % | Spectrum 1 | Spectrum 2 |
|---|---|---|
| O | 53.42 | 36.40 |
| Al | 18.59 | 13.96 |
| Si | 23.42 | 20.89 |
| K | 4.56 | 28.74 |
| Composition, % | Spectrum 2 |
|---|---|
| O | 50.67 |
| Al | 13.43 |
| Si | 19.23 |
| K | 11.02 |
| Ti | 5.65 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Aponte, L.A.; Mejía de Gutiérrez, R.; Maury-Ramírez, A. Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics. Coatings 2017, 7, 233. https://doi.org/10.3390/coatings7120233
Guzmán-Aponte LA, Mejía de Gutiérrez R, Maury-Ramírez A. Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics. Coatings. 2017; 7(12):233. https://doi.org/10.3390/coatings7120233
Chicago/Turabian StyleGuzmán-Aponte, Luis A., Ruby Mejía de Gutiérrez, and Anibal Maury-Ramírez. 2017. "Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics" Coatings 7, no. 12: 233. https://doi.org/10.3390/coatings7120233