Effect of the Addition of Molybdenum on the Structure and Corrosion Resistance of Zinc–Iron Plating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bath Preparation
2.2. Preparation of Zn–Fe–Mo Platings by Electrodeposition
2.3. Characterization and Evaluation of the Platings
3. Results and Discussions
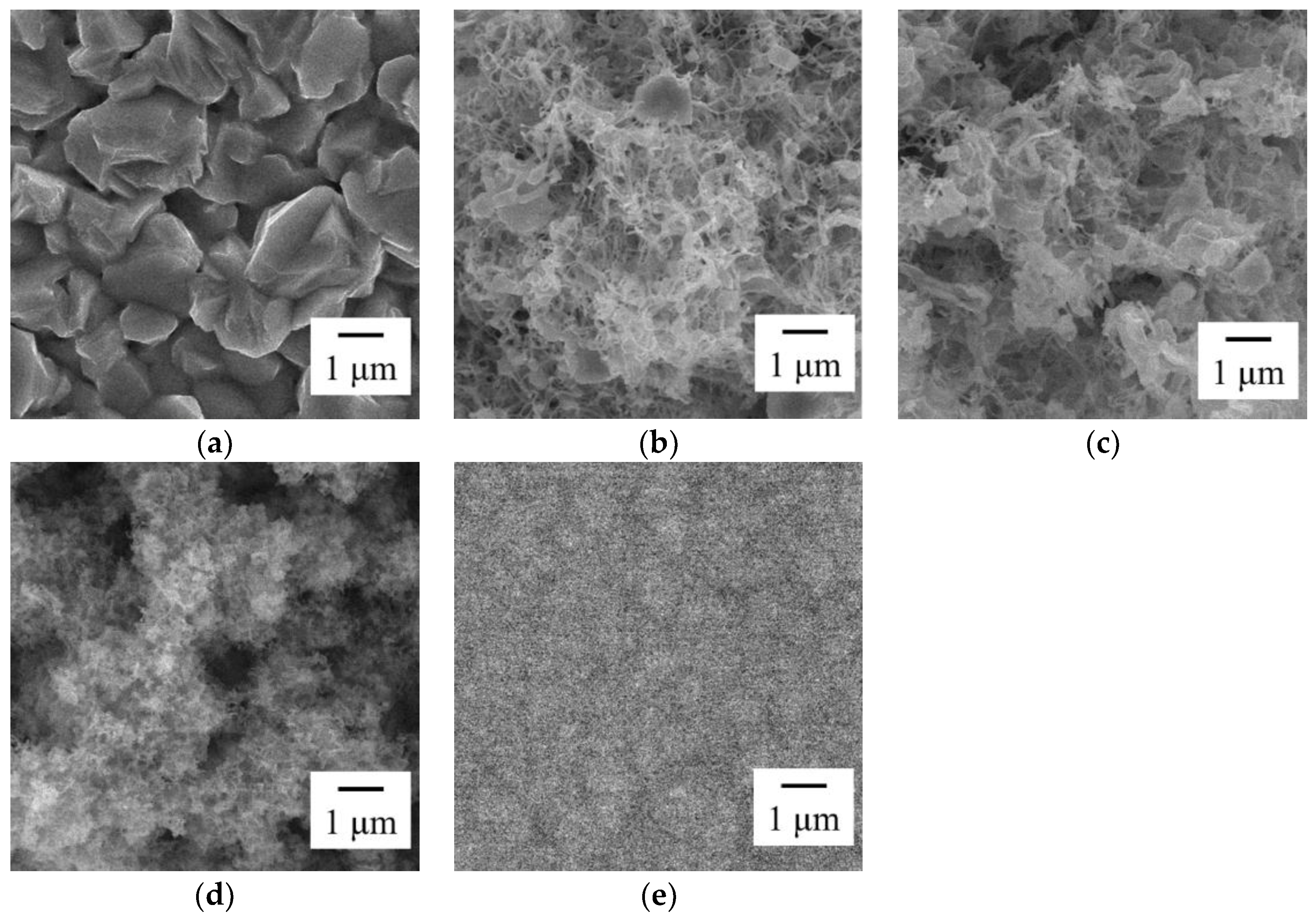
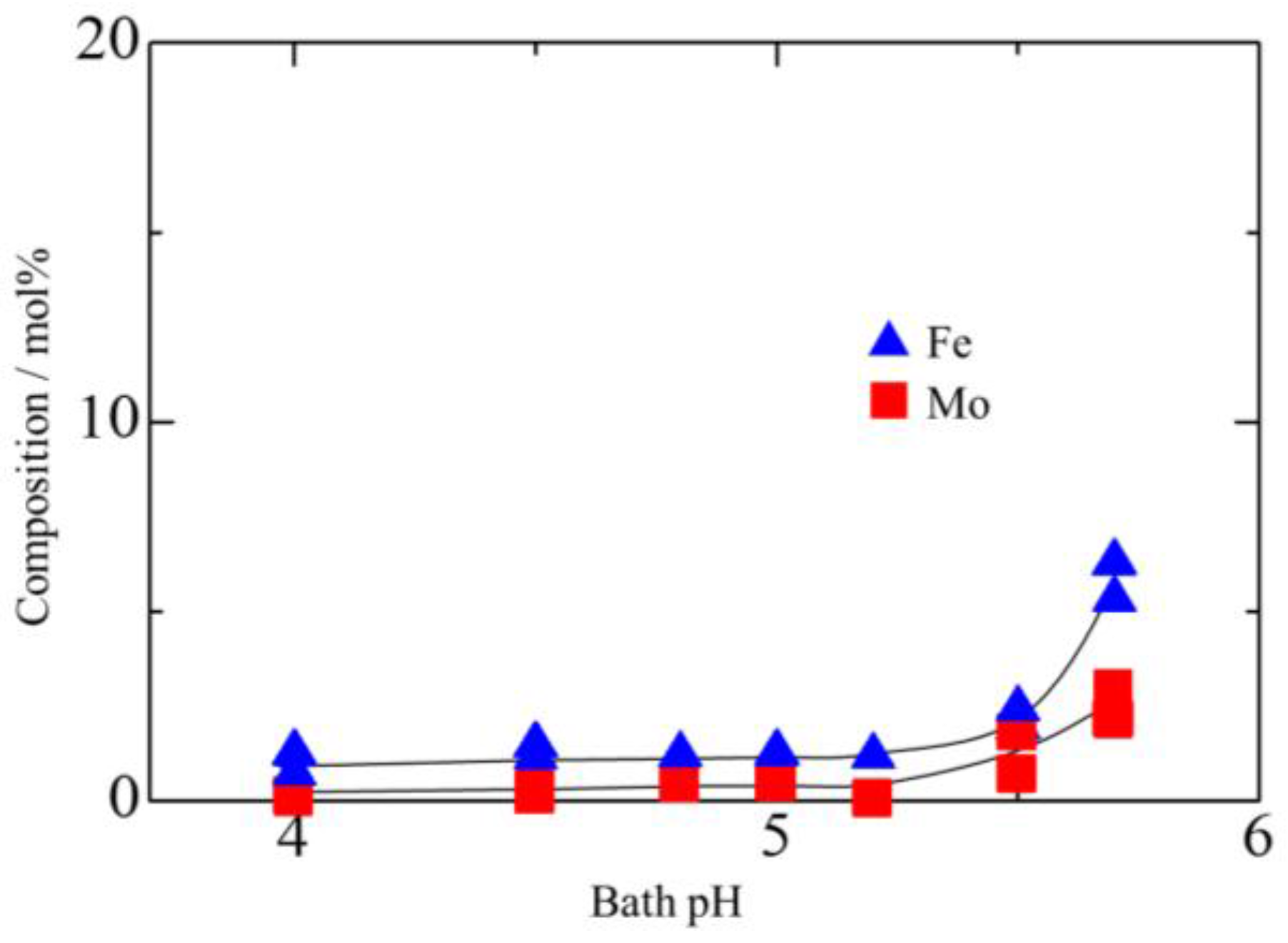
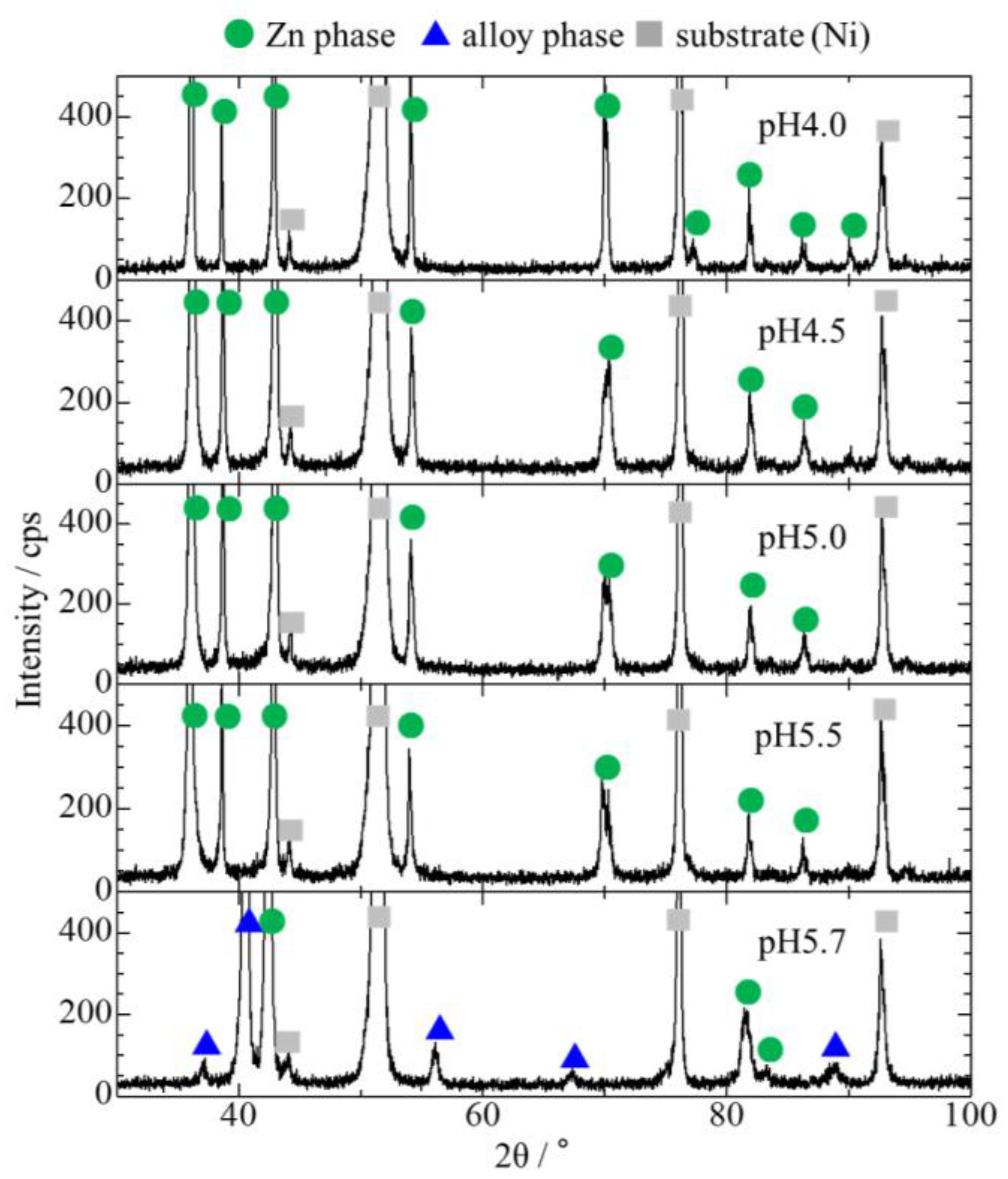
3.1. Effect of Bath pH on Zn–Fe–Mo Platings
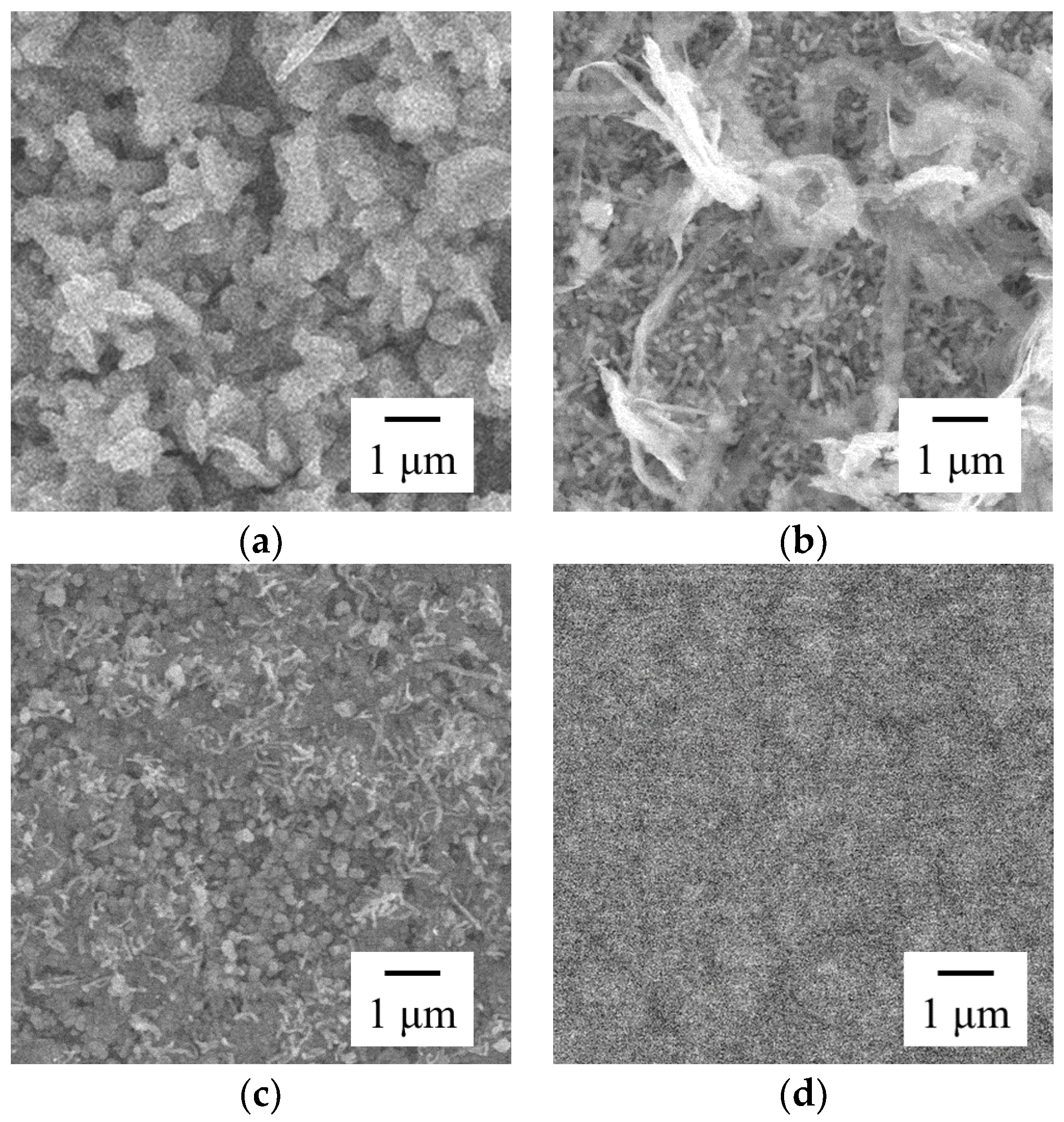
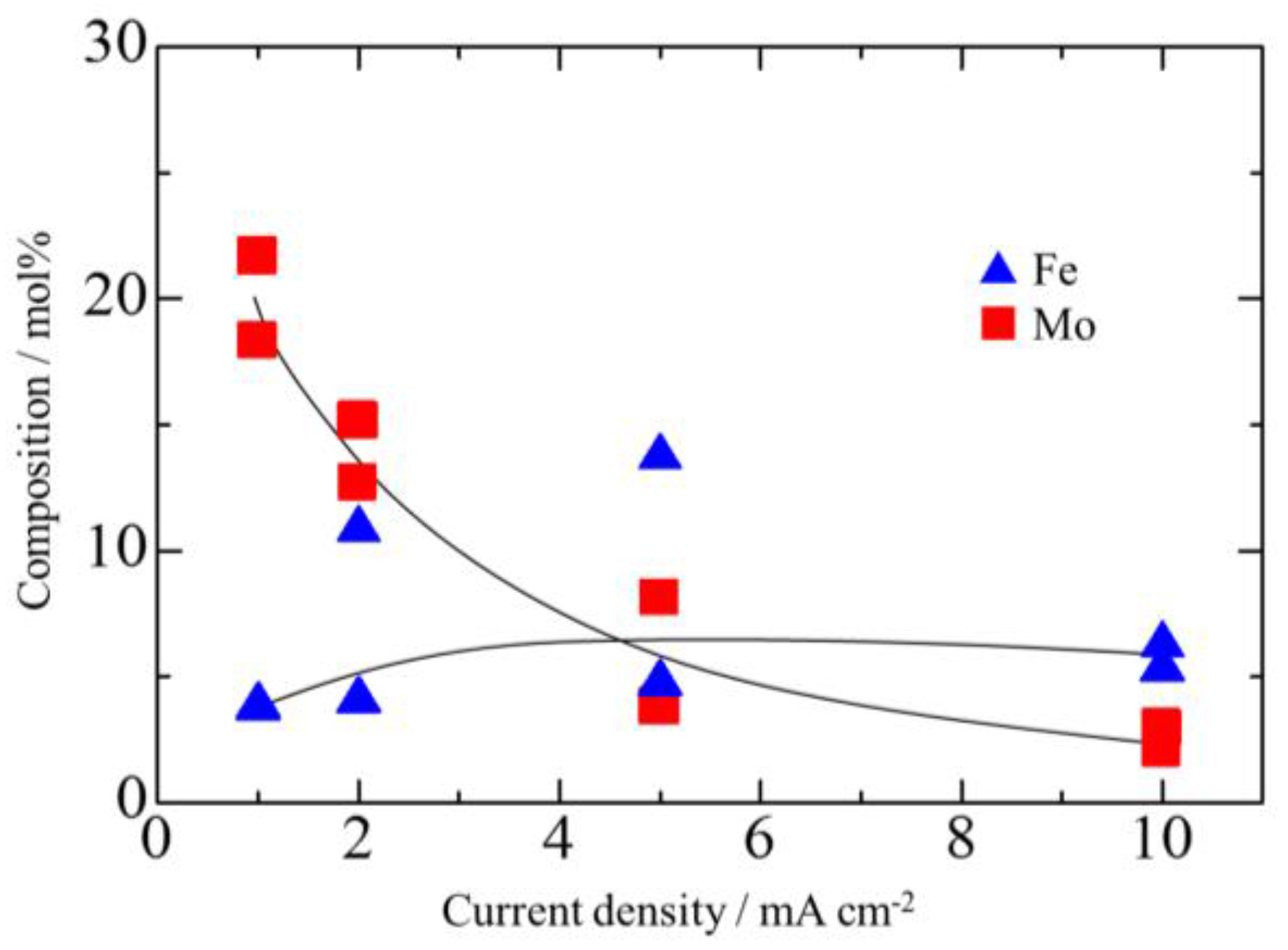
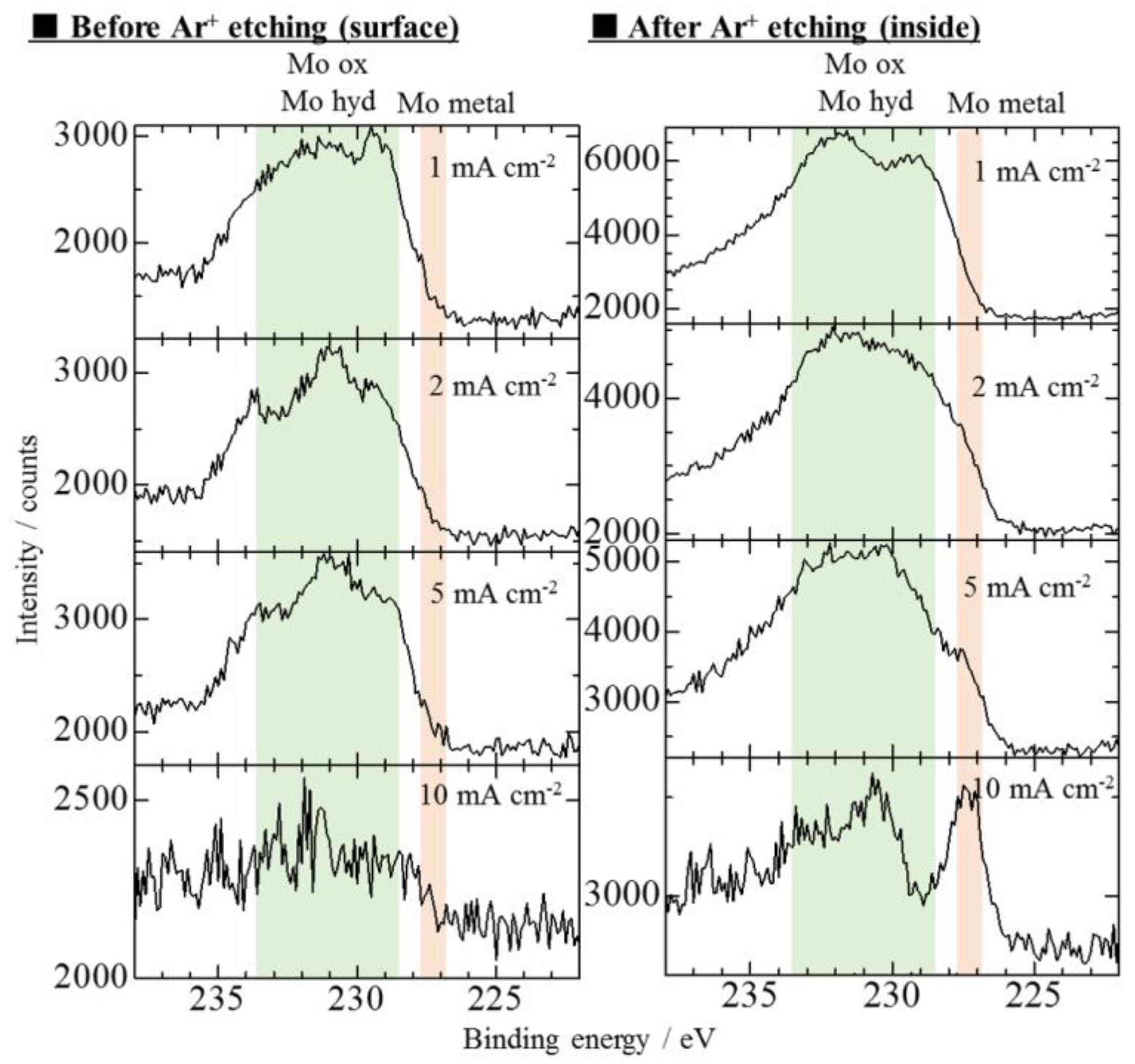
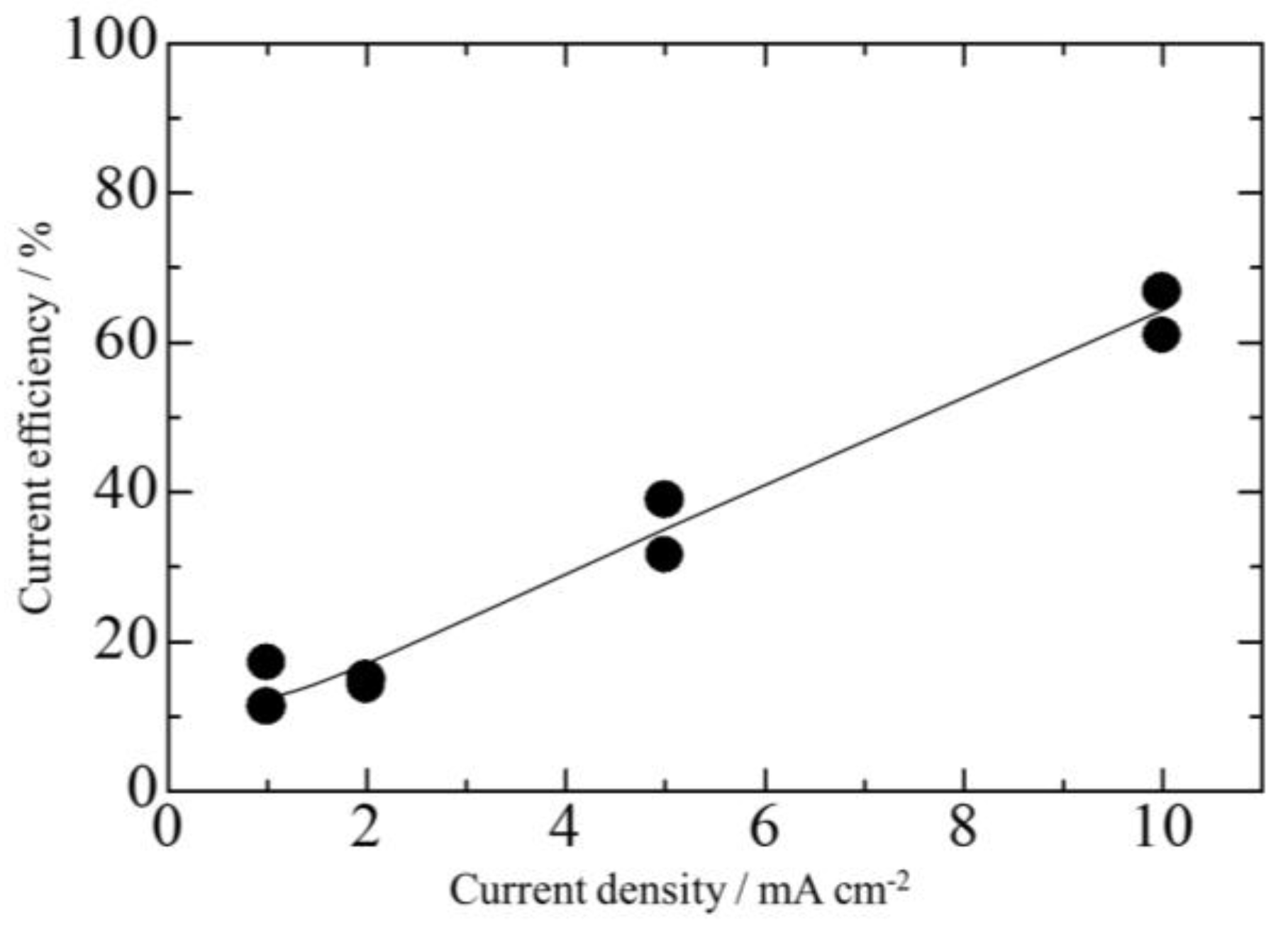
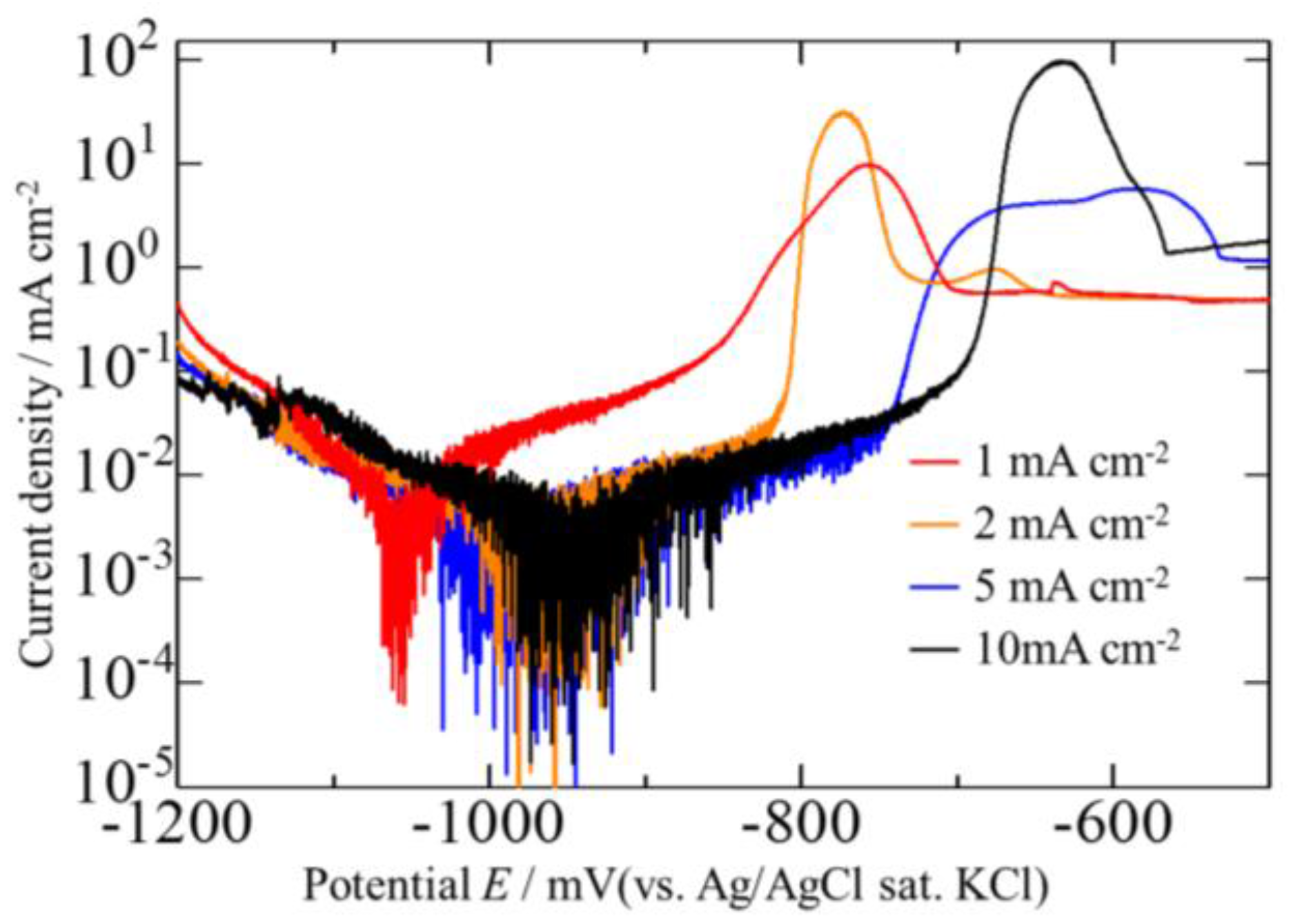
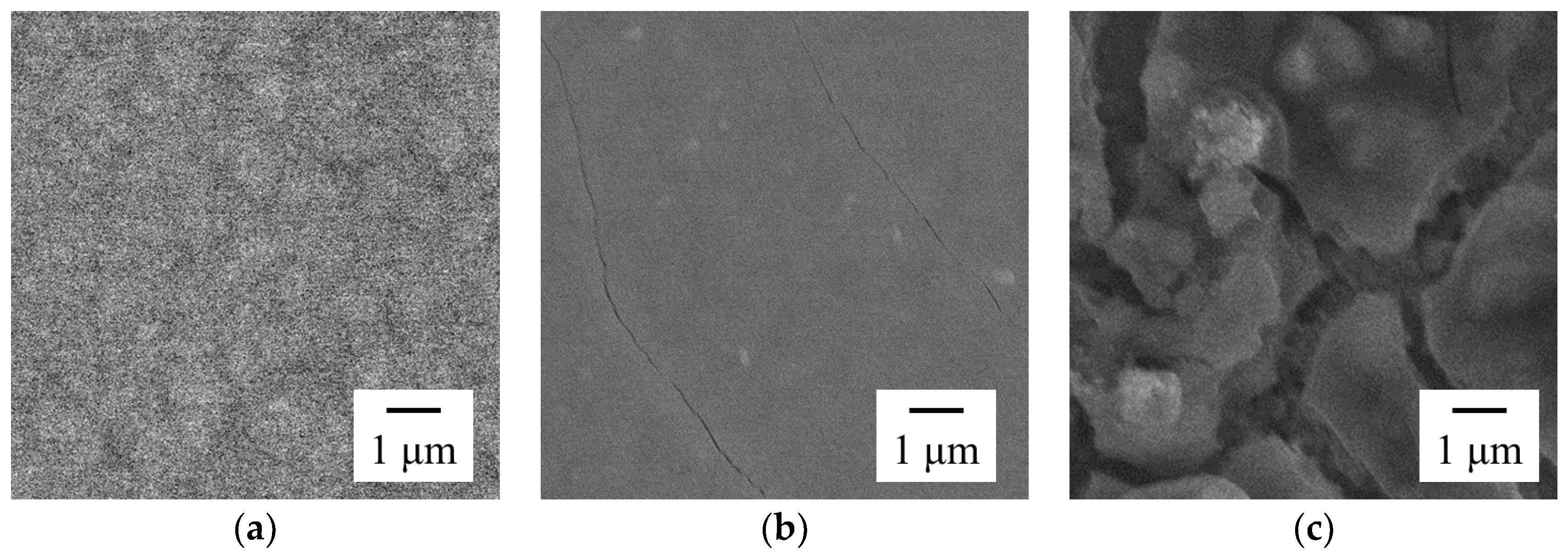
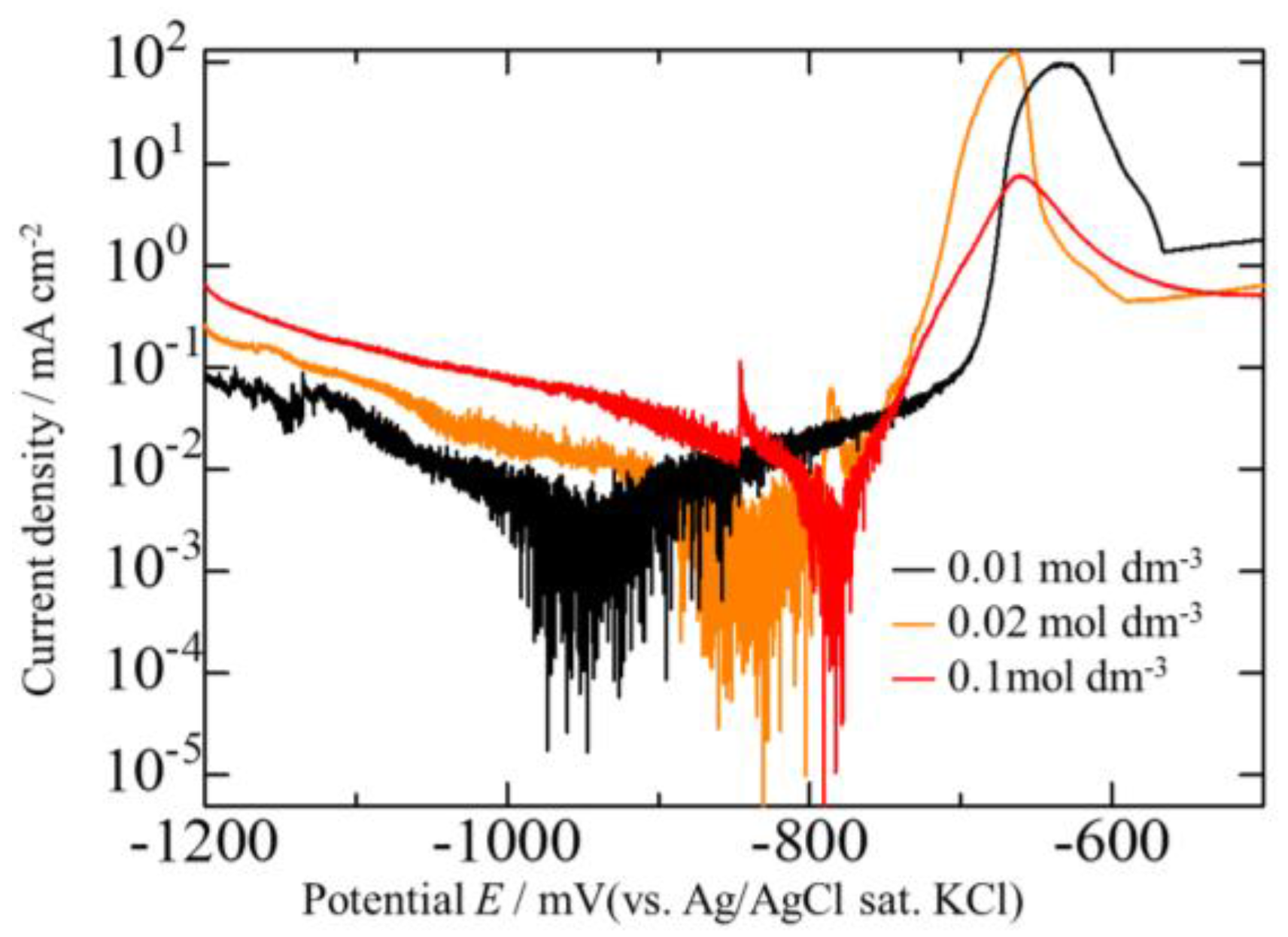
3.2. Effect of Current Density on Zn–Fe–Mo Platings
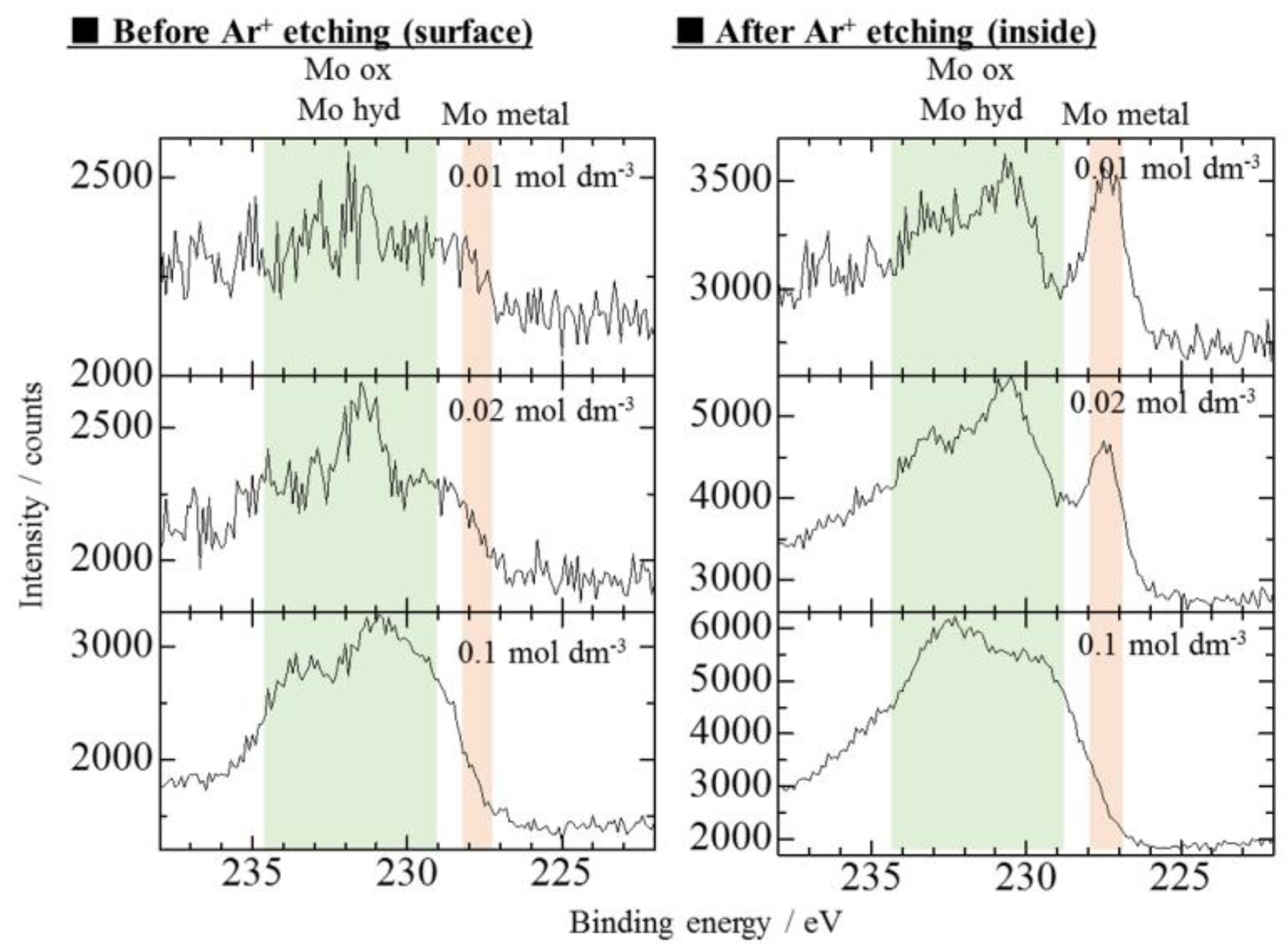
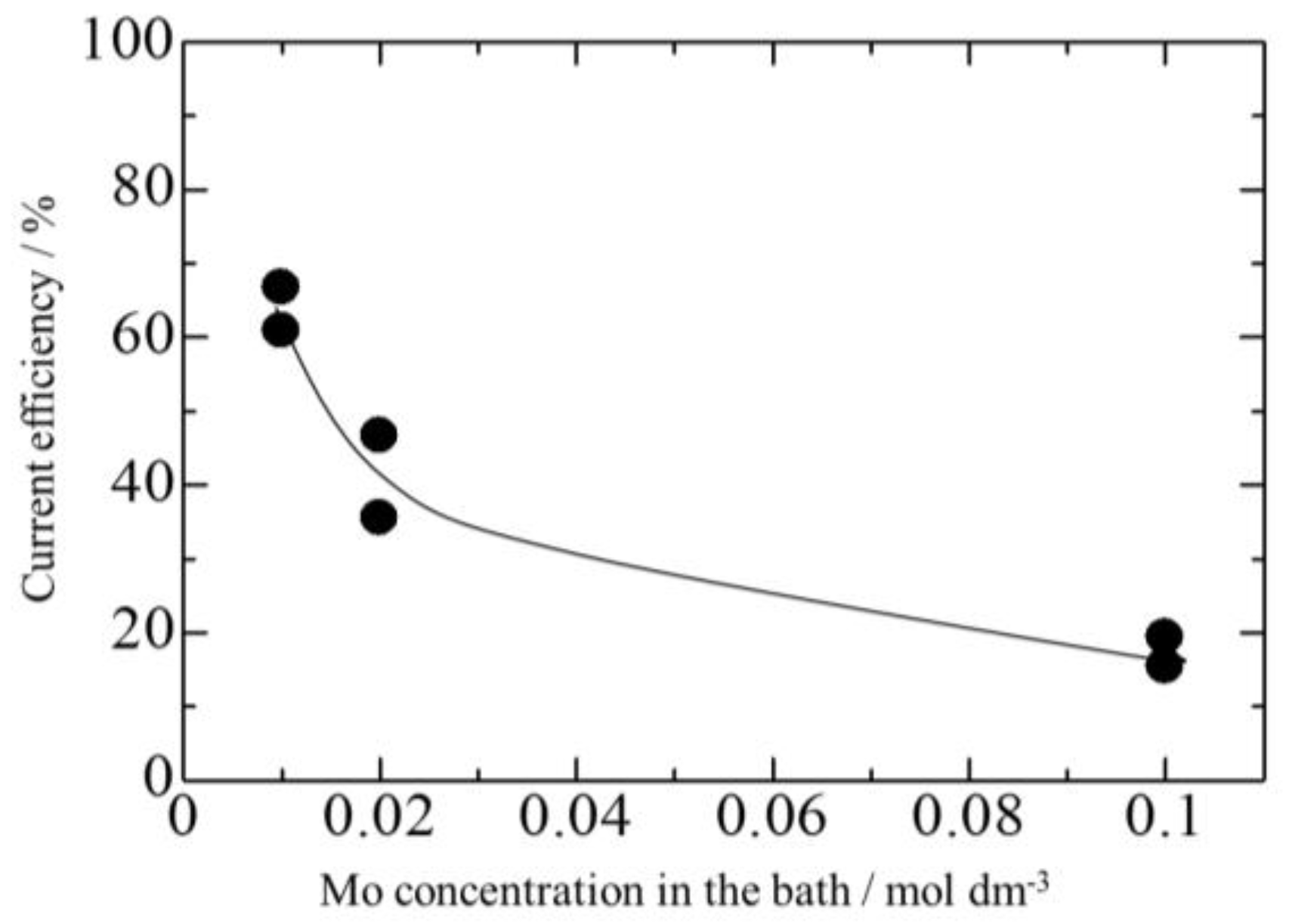
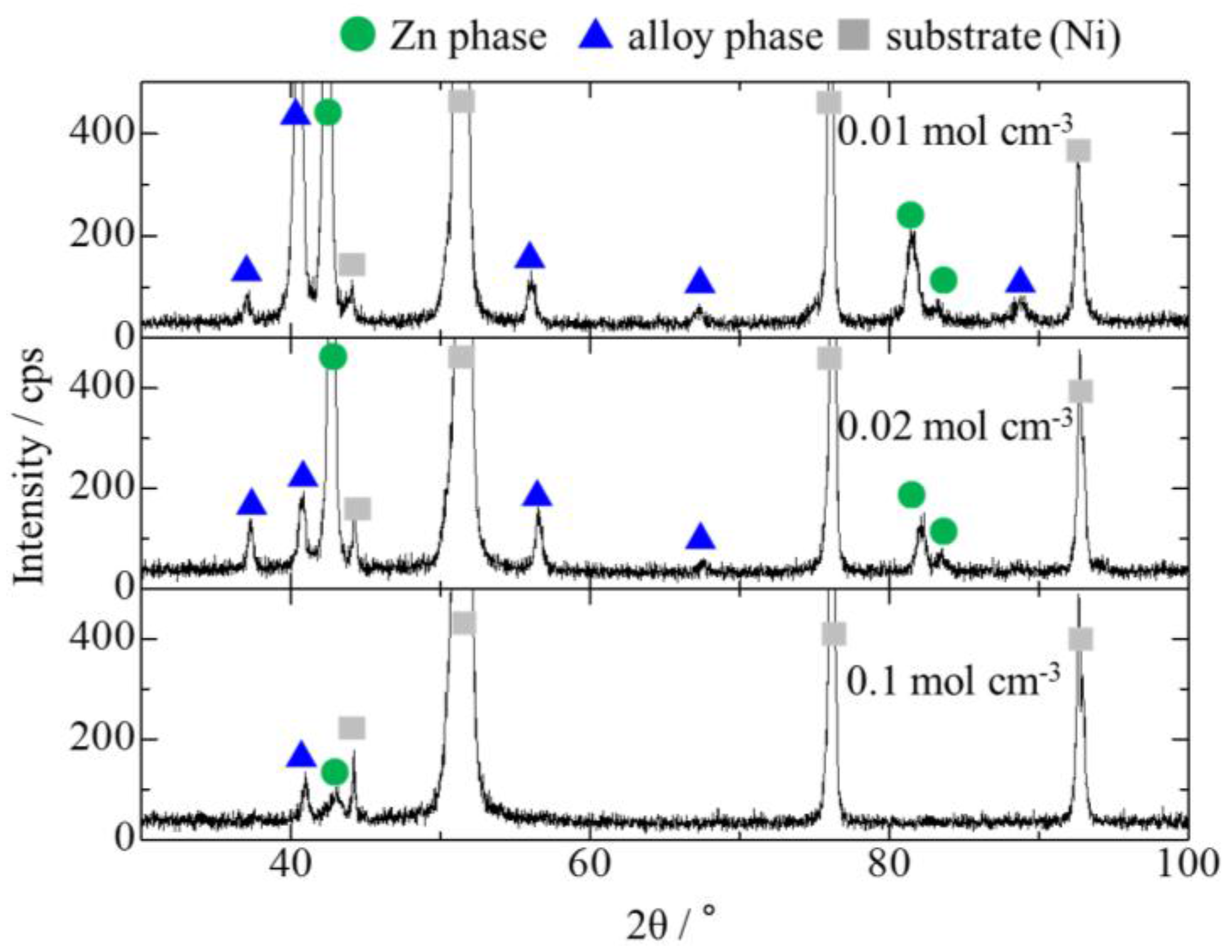
3.3. Effect of Mo Concentration of Plating Bath on Zn-Fe-Mo Platings
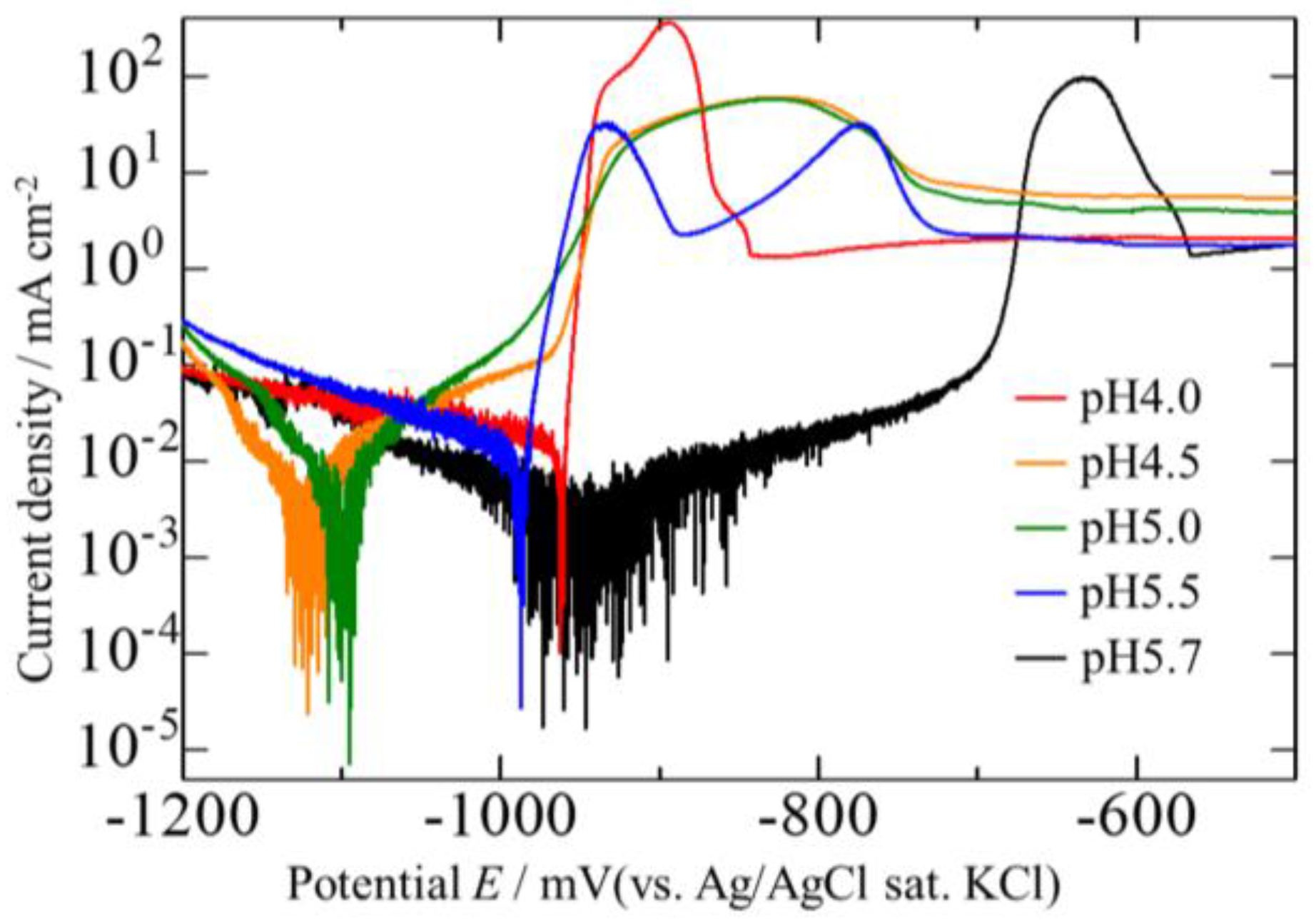
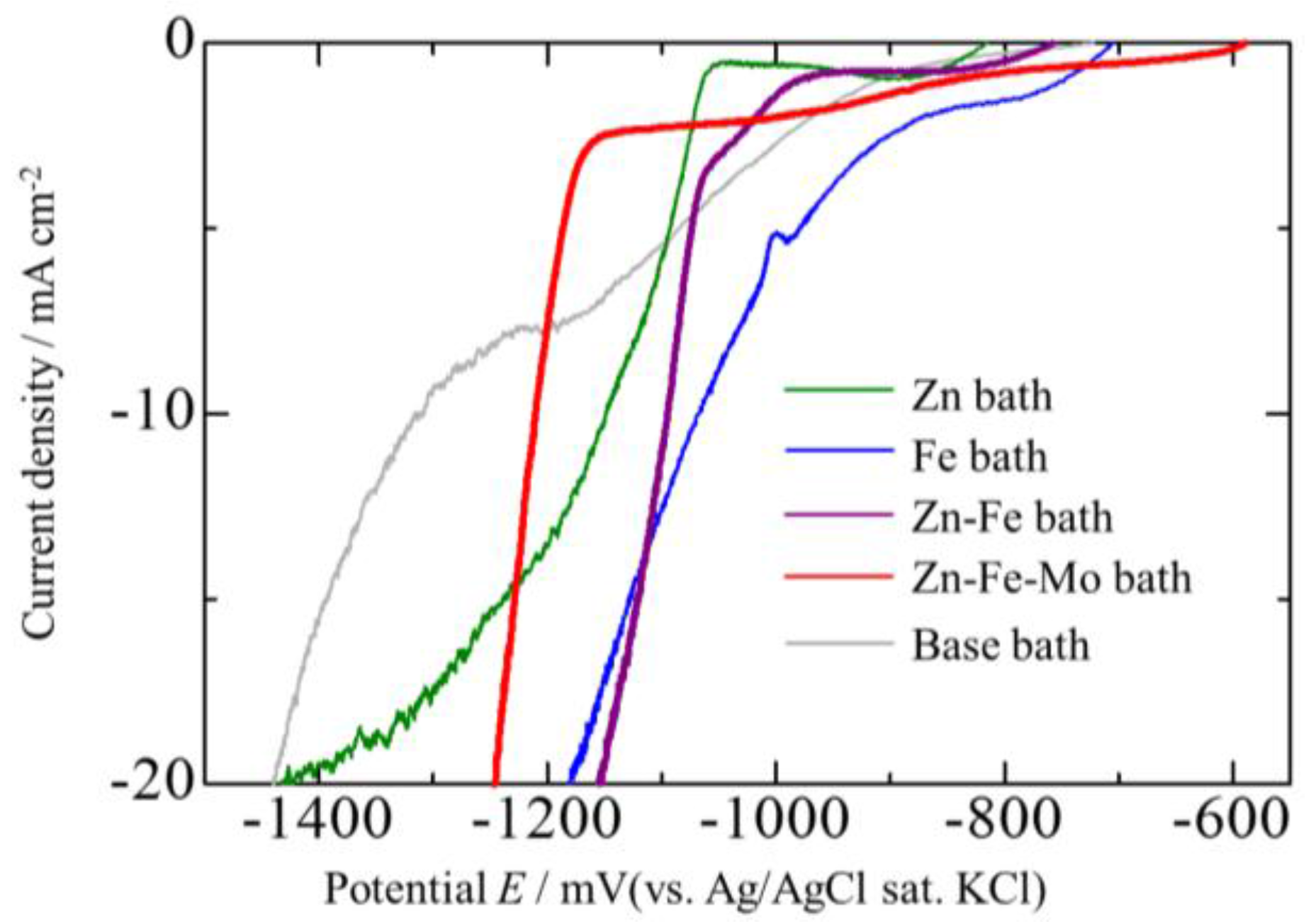
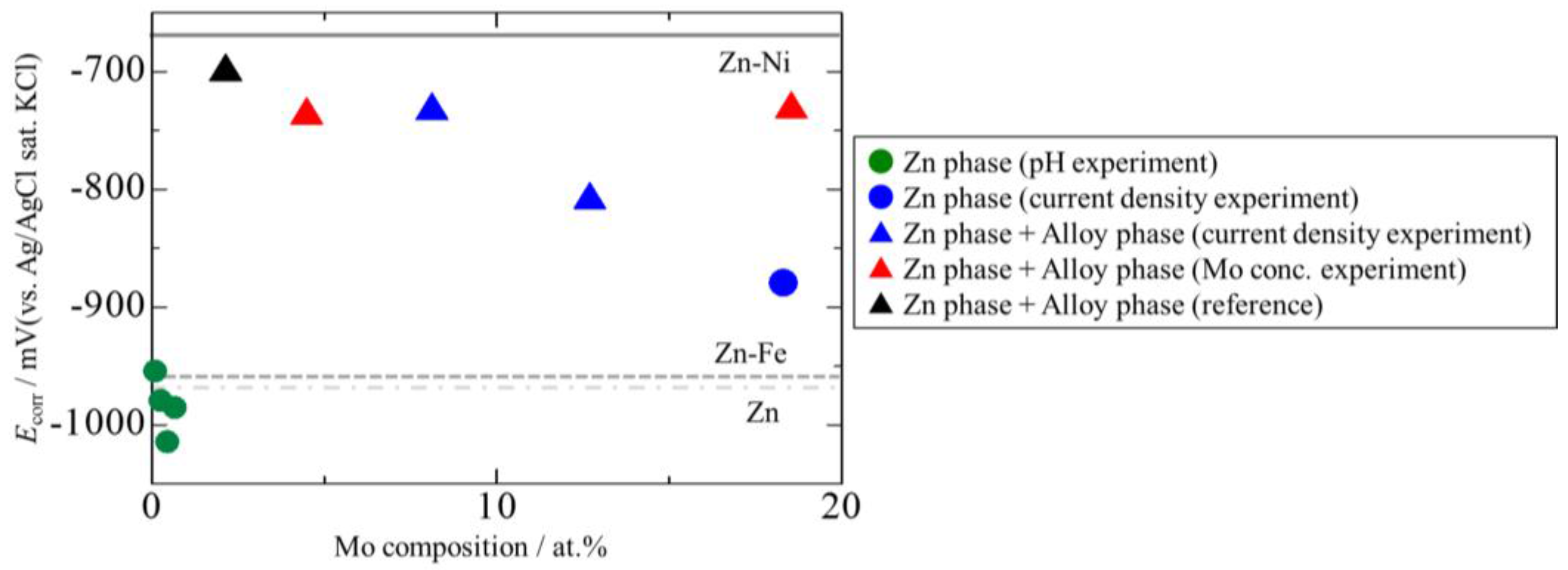
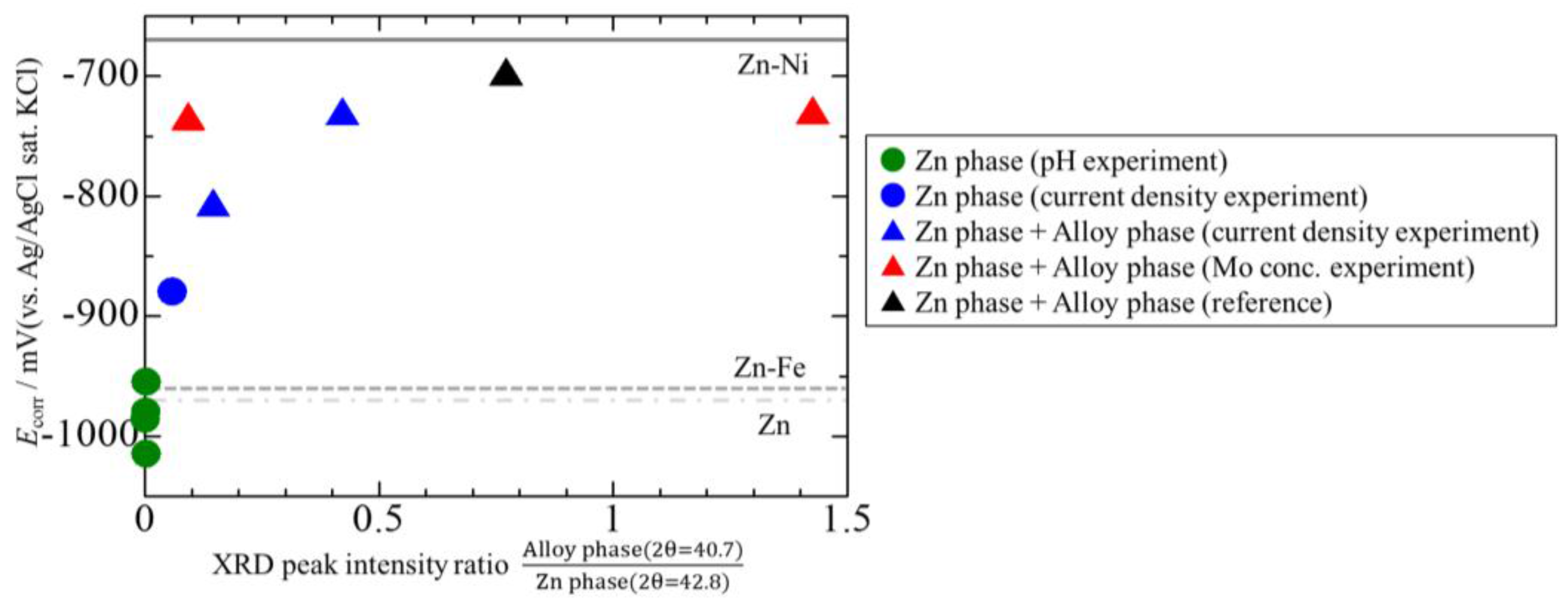
3.4. Corrosion Potentials of the Zn–Fe–Mo Platings
4. Conclusions
- The Zn–Fe–Mo platings obtained by this experiment exhibited high corrosion resistance when the electrically-deposited layer formed a Fe3Mo-based alloy phase.
- The best plating exhibited high corrosion resistance comparable with that of Zn–Ni platings, revealing promise for Zn–Fe–Mo platings as potential alternatives for Zn–Ni platings.
- For the co-deposition of Mo, it is crucial to control the pH to approximately 5.7 to ensure that Mo does not form large polyoxides and Zn does not form hydroxides.
- Co-deposition does not effectively occur at low current densities, and a high Mo concentration in the bath as Mo (IV) cannot be efficiently reduced to Mo (0).
Author Contributions
Conflicts of Interest
References
- Nesic, S.; Postlethwaite, J.; Olsen, S. An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Mackowiak, J.; Short, N.R. Metallurgy of galvanized coatings. Int. Met. Rev. 1979, 24, 1–19. [Google Scholar] [CrossRef]
- Byk, T.V.; Gaevskaya, T.V.; Tsybulskaya, L.S. Effect of electrodeposition conditions on the composition, microstructure, and corrosion resistance of Zn–Ni alloy coatings. Surf. Coat. Technol. 2008, 202, 5817–5823. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, I.H. Structural and corrosion protection properties of electrochemically deposited nano-sized Zn–Ni alloy coatings. Appl. Surf. Sci. 2014, 318, 15–23. [Google Scholar] [CrossRef]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Fe–Zn alloy layer on the corrosion resistance of galvanized steel in chloride containing environments. Corros. Sci. 2007, 49, 3716–3731. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Vitkova, S.; Nemska, S.; Raichevsky, G. Composition of the corrosion products of galvanic alloys Zn–Co and their influence on the protective ability. Surf. Coat. Technol. 2002, 157, 171–178. [Google Scholar] [CrossRef]
- Shibuya, A.; Kurimoto, T.; Korekawa, K.; Noji, K. Corrosion-resistance of electroplated Ni–Zn alloy steel sheet. Tetsu-to-Hagane 1980, 7, 771–778. (In Japanese) [Google Scholar] [CrossRef]
- Gnanamuthu, M.R.; Mohan, S.; Saravanan, G.; Leea, C.W. Comparative study on structure, corrosion and hardness of Zn–Ni alloy deposition on AISI 347 steel aircraft material. J. Alloys Compd. 2012, 513, 449–454. [Google Scholar] [CrossRef]
- Goldenberg, A.; Admani, S.; Janice, J.L.; Jacob, S.E. Belt buckles—Increasing awareness of nickel exposure in children: A case report. Pediatrics 2015, 136, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular mechanisms of nickel allergy. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Qun, Z.L. Electrodeposition of zinc-iron alloy from an alkaline zincate bath. Met. Finish. 1998, 96, 56–57. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Raman, S.G.S.; Seshadri, S.K. Corrosion behaviour of electrodeposited nanocrystalline Ni–W and Ni–Fe–W alloys. Mater. Sci. Eng. 2007, 460–461, 39–45. [Google Scholar] [CrossRef]
- Naka, M.; Hashimoto, K.; Masumoto, T. High corrosion resistance of amorphous Fe–Mo and Fe–W alloys in HCl. J. Non-Cryst. Solids 1978, 29, 61–65. [Google Scholar] [CrossRef]
- Akiyama, T.; Fukushima, H. Recent study on the iron-group metal alloy. ISIJ Int. 1992, 32, 787–798. [Google Scholar] [CrossRef]
- Podlaha, E.J.; Landolt, D. Induced codeposition I. An experimental investigation of Ni–Mo alloys. J. Electrochem. Soc. 1996, 143, 885–892. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, B. The influence of molybdenum on the corrosion resistance of ternary Zn–Co–Mo alloy coatings deposited from citrate–sulphate bath. Corros. Sci. 2015, 91, 330–340. [Google Scholar] [CrossRef]
- Szczygieł, B.; Laszczyńska, A.; Tylus, W. Influence of molybdenum on properties of Zn–Ni and Zn–Co alloy coatings. Surf. Coat. Technol. 2010, 204, 1438–1444. [Google Scholar] [CrossRef]
- Keyvani, A.; Yeganeh, M.; Rezaeyan, H. Electrodeposition of Zn-Co-Mo alloy on the steel substrate from citrate bath and its corrosion behavior in the chloride media. J. Mater. Eng. Perform. 2017, 26, 1958–1966. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Krawczyk, M.S.; Szczygieł, B. The influence of molybdenum on the electrodeposition and properties of ternary Zn–Fe–Mo alloy coatings. Electrochim. Acta 2016, 196, 708–726. [Google Scholar] [CrossRef]
- Winiarski, J.; Leśniewicz, A.; Pohl, P.; Szczygieł, B. The effect of pH of plating bath on electrodeposition and properties of protective ternary Zn–Fe–Mo alloy coatings. Surf. Coat. Technol. 2016, 299, 81–89. [Google Scholar] [CrossRef]
- Gómez, E.; Pelaez, E.; Vallés, E. Electrodeposition of zinc+iron alloys: I. Analysis of the initial stages of the anomalous codeposition. J. Electroanal. Chem. 1999, 469, 139–149. [Google Scholar]
- Gómez, E.; Pellicer, E.; Vallés, E. Influence of the bath composition and the pH on the induced cobalt–molybdenum electrodeposition. J. Electroanal. Chem. 2003, 556, 137–145. [Google Scholar] [CrossRef]
- Kazimierczaka, H.; Ozga, P.; Sochab, R.P. Investigation of electrochemical co-deposition of zinc and molybdenum from citrate solutions. Electrochim. Acta 2013, 104, 378–390. [Google Scholar] [CrossRef]
- Jung, S.M. Quantitative analysis of FeMo alloys by X-ray fluorescence spectrometry. Am. J. Anal. Chem. 2014, 5, 766–774. [Google Scholar] [CrossRef]
- Ma, S.; Xing, J.; Fu, H.; Yi, D.; Zhang, J.; Li, Y.; Zhang, Z.; Zhu, B.; Ma, S. Interfacial morphology and corrosion resistance of Fe–B cast steel containing chromium and nickel in liquid zinc. Corr. Sci. 2011, 53, 2826–2834. [Google Scholar] [CrossRef]
- Chassaing, E.; Quang, K.V.; Wiart, R. Mechanism of nickel-molybdenum alloy electrodeposition in citrate electrolytes. J. Appl. Electrochem. 1989, 19, 839–844. [Google Scholar] [CrossRef]
- Fukushima, H.; Akiyama, T.; Akagi, S.; Higashi, K. Role of iron-group metais in the induced codeposition of molybdenum from aqueous olution. Trans. JIM 1979, 20, 358–364. [Google Scholar] [CrossRef]
- Kubota, A.; Tashiro, Y.; Yamasaki, K.; Nakano, H.; Oue, S.; Kobayashi, S.; Akiyama, T.; Fukushima, H. Electrodeposition behavior and properties of iron-group metal alloys with W from ammoniacal citrate baths. Tetsu-to-Hagane 2000, 86, 116–122. (In Japanese) [Google Scholar] [CrossRef]


| Type of Bath | Composition of Bath (mol dm−3) | ||||
|---|---|---|---|---|---|
| ZnSO4 | FeSO4 | Na2MoO4 | C6H5Na3O7 | Na2SO4 | |
| Base | – | – | – | 0.2 | 0.1 |
| Zn | 0.2 | – | – | 0.2 | 0.1 |
| Fe | – | 0.2 | – | 0.2 | 0.1 |
| Zn–Fe | 0.2 | 0.2 | – | 0.2 | 0.1 |
| Zn–Fe–Mo | 0.2 | 0.2 | 0.01–0.1 | 0.2 | 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosugi, D.; Hagio, T.; Kamimoto, Y.; Ichino, R. Effect of the Addition of Molybdenum on the Structure and Corrosion Resistance of Zinc–Iron Plating. Coatings 2017, 7, 235. https://doi.org/10.3390/coatings7120235
Kosugi D, Hagio T, Kamimoto Y, Ichino R. Effect of the Addition of Molybdenum on the Structure and Corrosion Resistance of Zinc–Iron Plating. Coatings. 2017; 7(12):235. https://doi.org/10.3390/coatings7120235
Chicago/Turabian StyleKosugi, Daichi, Takeshi Hagio, Yuki Kamimoto, and Ryoichi Ichino. 2017. "Effect of the Addition of Molybdenum on the Structure and Corrosion Resistance of Zinc–Iron Plating" Coatings 7, no. 12: 235. https://doi.org/10.3390/coatings7120235






