Influence of the Distribution of a Spray Paint on the Efficacy of Anti-Graffiti Coatings on a Highly Porous Natural Stone Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stone Material, Anti-Graffiti Systems, and Spray Paint
2.2. Preparation of the Stone Samples and Treatments
2.3. Analytical Investigations
3. Results and Discussion
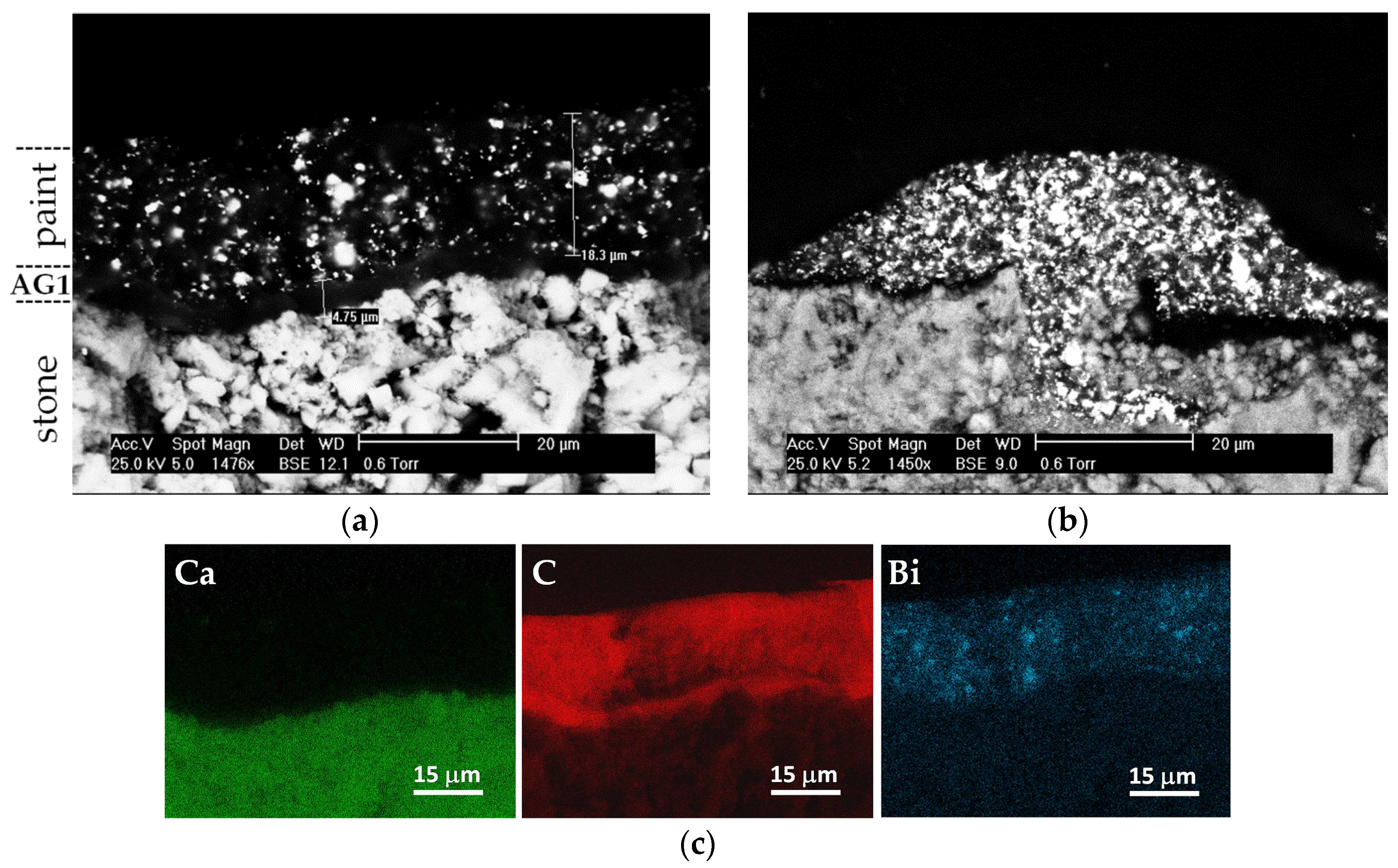
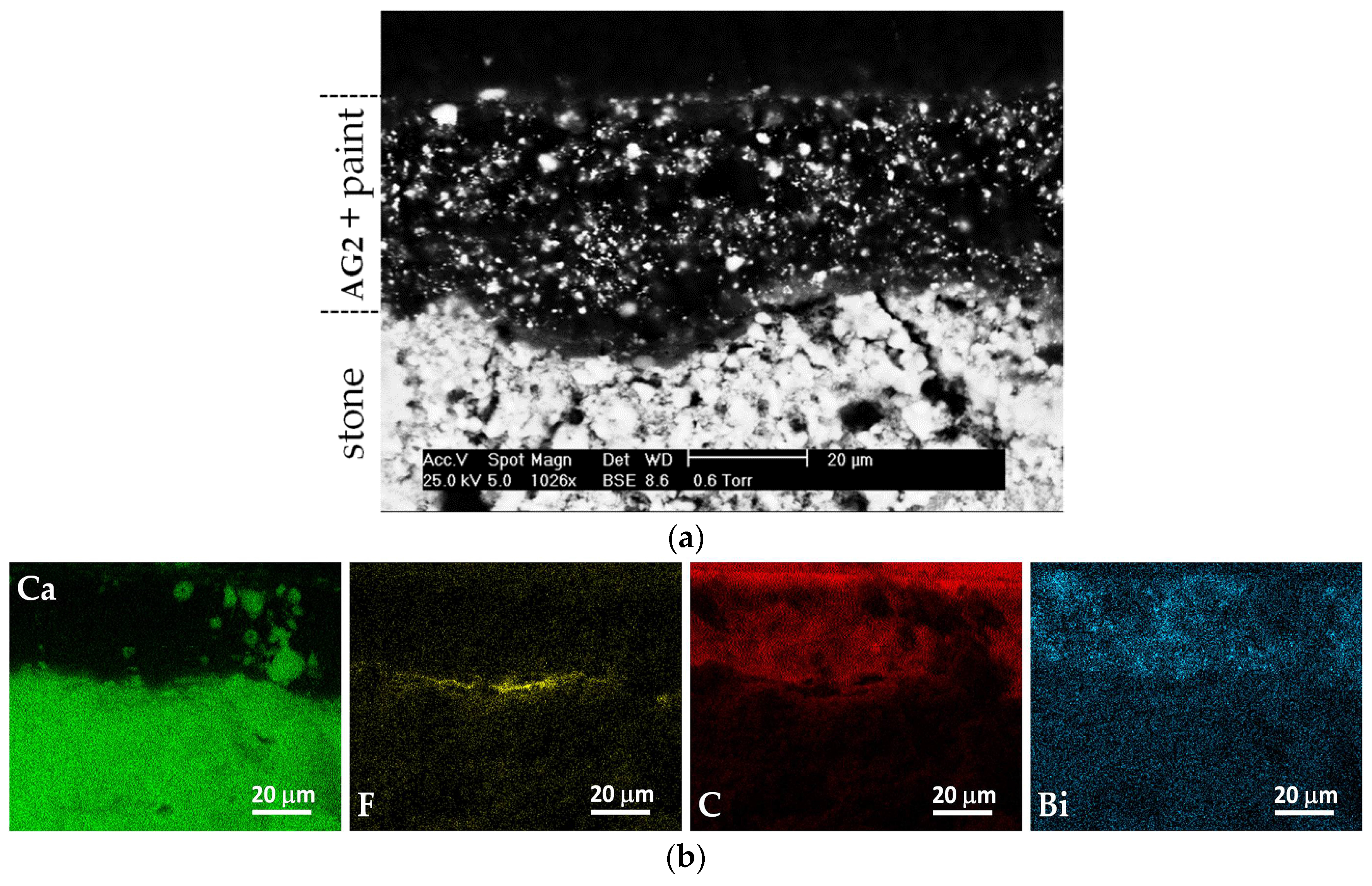
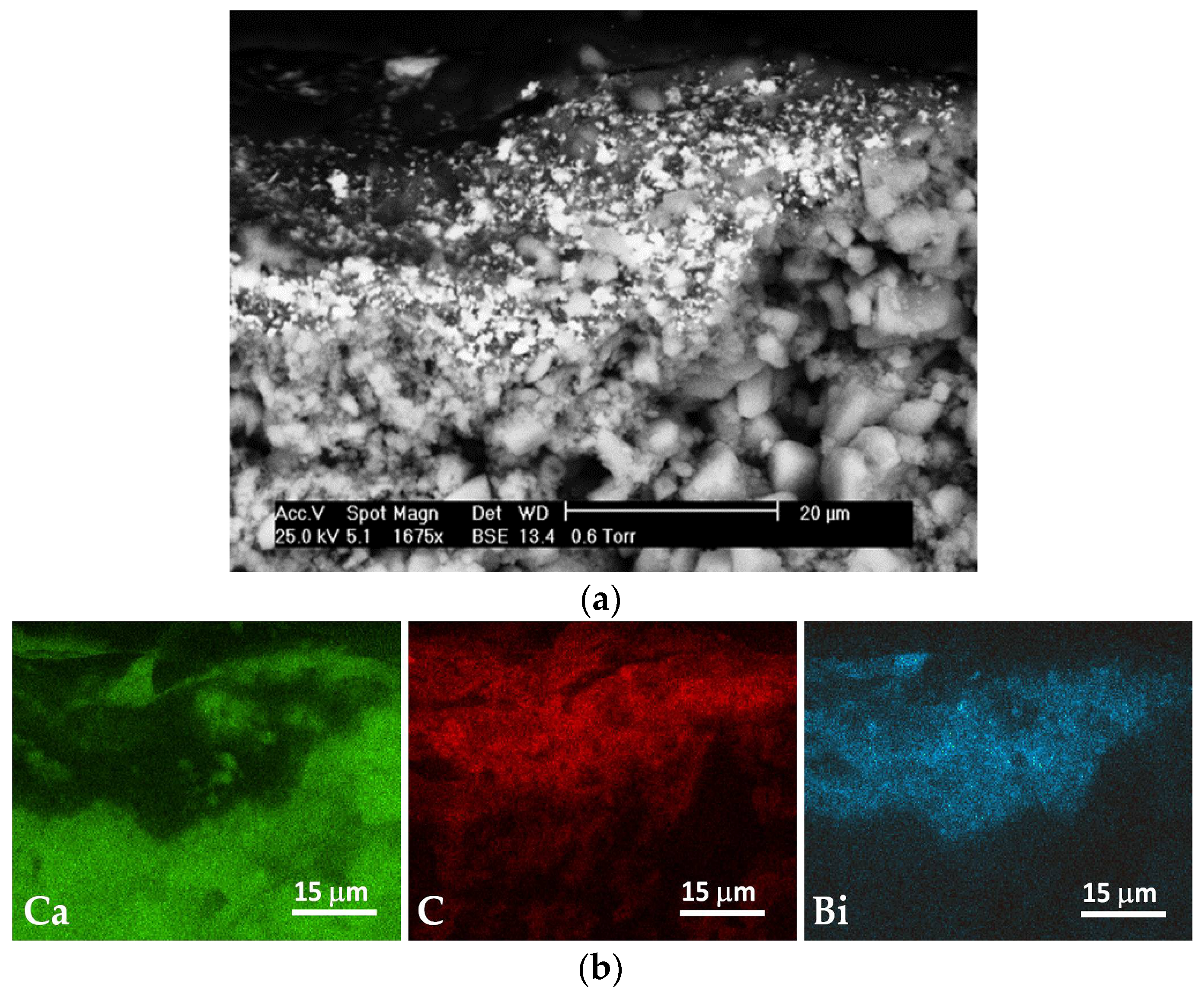
3.1. Morphological Characteristics and Elemental Microanalysis of the Coatings
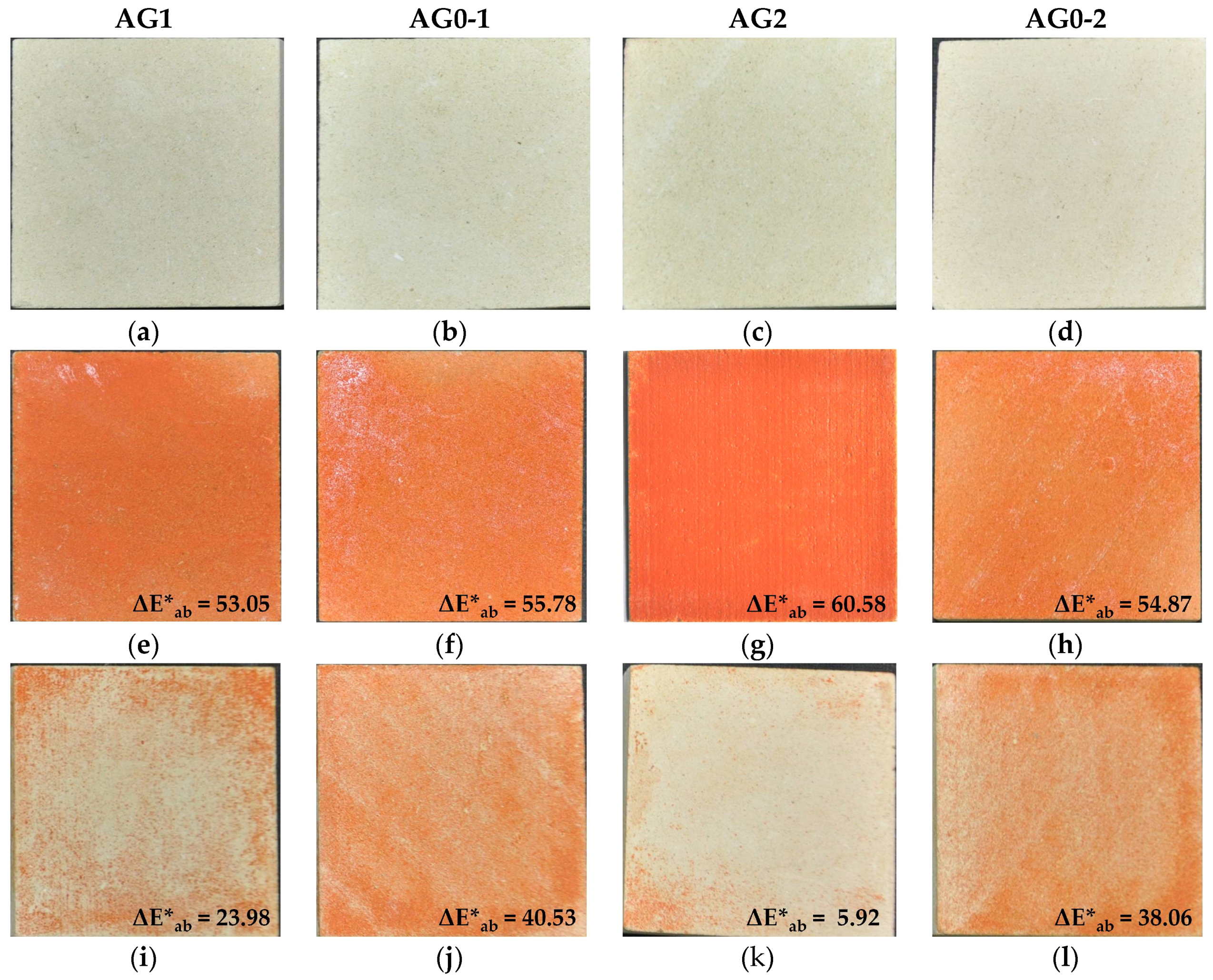
3.2. Distribution of the Paint
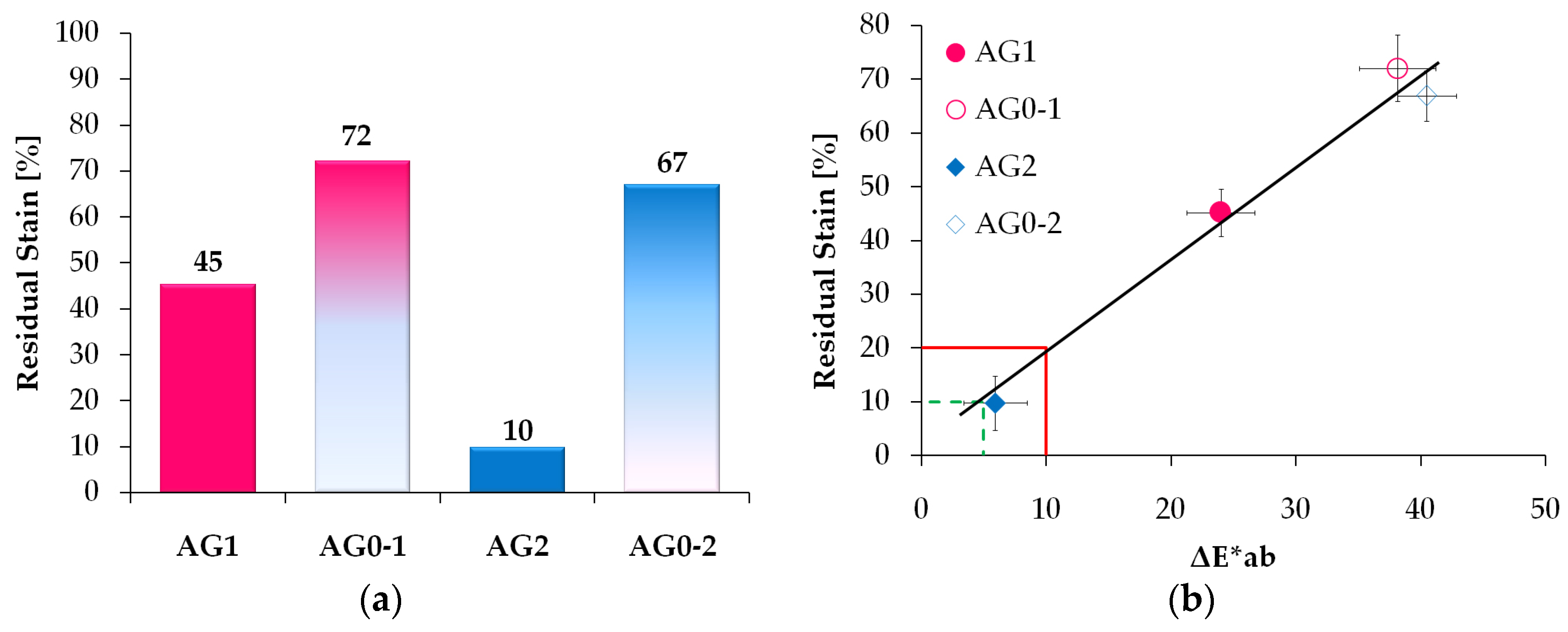
3.3. Efficacy of the Removal Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, J.I. Routledge Handbook of Graffiti and Street Art; Routledge: New York, NY, USA, 2016. [Google Scholar]
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Cocco, O.; Carboni, M.; Carcangiu, G.; Meloni, P.; Murru, M.; Persia, F.; Solla, L. Crime art on the stone: Graffiti vandalism on cultural heritage and the anti-graffiti role in its surfaces protection. Period. Mineral. 2015, 84, 435–452. [Google Scholar]
- Careddu, N.; Akkoyun, O. An investigation on the efficiency of water-jet technology for graffiti cleaning. J. Cult. Herit. 2016, 19, 426–434. [Google Scholar] [CrossRef]
- Von Plehwe-Leisen, E.; Leisen, H.; Auras, M. Efficiency of anti-graffiti systems and their influence on stone surfaces. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; Kwiatkowski, D., Löfvendahl, R., Eds.; ICOMOS: Stockholm, Sweden, 2004; pp. 391–398. [Google Scholar]
- Rabea, A.M.; Mirabedini, S.M.; Mohseni, M. Investigating the surface properties of polyurethane based anti-graffiti coating against UV exposure. J. Appl. Polym. Sci. 2012, 124, 3082–3091. [Google Scholar] [CrossRef]
- Licchelli, M.; Marzolla, S.J.; Poggi, A.; Zanchi, C. Crosslinked fluorinated polyurethanes for the protection of stone surfaces from graffiti. J. Cult. Herit. 2011, 12, 34–43. [Google Scholar] [CrossRef]
- Manvi, G.N.; Singh, A.R.; Jagtap, R.N.; Kothari, D.C. Isocyanurate based fluorinated polyurethane dispersion for anti-graffiti coatings. Prog. Org. Coat. 2012, 75, 139–146. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Sobrados, I.; Blanco-Varela, M.T. Interaction between two anti-graffiti treatments and cement mortar (paste). Cem. Concr. Res. 2010, 40, 723–730. [Google Scholar] [CrossRef]
- Cazaux, F.; Coqueret, X. Fundamental properties and anti-soiling behaviour of vinyl ether functional polydimethylsiloxanes for photocationically cured coatings. Surf. Coat. Int. Part B Coat. Trans. 2001, 84, 127–134. [Google Scholar] [CrossRef]
- Dewasthale, S.; Andrews, C.; Graiver, D.; Narayan, R. Water soluble polysiloxanes. Silicon 2015. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T.; Martínez-Ramírez, S. Freeze-Thaw and UV Resistance in Building Stone Coated with Two Permanent Anti-graffiti Treatments. In Engineering Geology for Society and Territory; Lollino, G., Giordan, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 8, pp. 531–534. [Google Scholar]
- Carmona-Quiroga, P.M.; Rubio, J.; Sánchez, M.J.; Martínez-Ramírez, S.; Blanco-Varela, M.T. Surface dispersive energy determined with IGC-ID in anti-graffiti-coated building materials. Prog. Org. Coat. 2011, 71, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Scheerder, J.; Visscher, N.; Nabuurs, T.; Overbeek, A. Novel, water-based fluorinated polymers with excellent antigraffiti properties. J. Coat. Technol. Res. 2005, 2, 617–625. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T.; Domingo, C.; Martínez-Ramírez, S. Effect of concentration, particle size and the presence of protective coatings in DRIFT spectra of building materials. Vib. Spectrosc. 2009, 50, 312–318. [Google Scholar] [CrossRef]
- Liu, H.; Fu, B.; Li, Y.; Shang, Q.; Xiao, G. Antigraffiti polyurethane coating containing fluorocarbon side chains grafted polymethylsiloxane. J. Coat. Technol. Res. 2013, 10, 361–369. [Google Scholar] [CrossRef]
- Malshe, V.C.; Sangaj, N.S. Fluorinated acrylic copolymers. Part I: Study of clear coatings. Prog. Org. Coat. 2005, 53, 207–211. [Google Scholar] [CrossRef]
- Mager, M.; Schmalstieg, L.; Mechtel, M.; Kraus, H. Organic-inorganic hybrid coatings based on polyfunctional silanols as new monomers in sol-gel processing. Macromol. Mater. Eng. 2001, 286, 682–684. [Google Scholar] [CrossRef]
- Haas, K.H.; Amberg-Schwab, S.; Rose, K. Functionalized coating materials based on inorganic-organic polymers. Thin Solid Films 1999, 351, 198–203. [Google Scholar] [CrossRef]
- Hofacker, S.; Mechtel, M.; Mager, M.; Kraus, H. Sol–Gel: A new tool for coatings chemistry. Prog. Org. Coat. 2002, 45, 159–164. [Google Scholar] [CrossRef]
- Elvira, M.R.; Mazo, M.A.; Tamayo, A.; Rubio, F.; Rubio, J.; Oteo, J.L. Study and characterization of organically modified silica-zirconia anti-graffiti coatings obtained by sol-gel. J. Chem. Chem. Eng. 2013, 7, 120–131. [Google Scholar]
- Rabea, A.M.; Mohseni, M.; Mirabedini, S.M.; Tabatabaei, M.H. Surface analysis and anti-graffiti behavior of a weathered polyurethane-based coating embedded with hydrophobic nanosilica. Appl. Surf. Sci. 2012, 258, 4391–4396. [Google Scholar] [CrossRef]
- Adapala, P.; Gaur, S.; Puri, R.G.; Khanna, A.S. Development and evaluation of nano-silica dispersed polyurethane based coatings for improved anti-graffiti and scratch resistance. Open J. Appl. Sci. 2015, 5, 808–818. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Anti-graffiti nanocomposite materials for surface protection of a very porous stone. Appl. Phys. A 2014, 116, 1525–1539. [Google Scholar] [CrossRef]
- Kronlund, D.; Lindén, M.; Smått, J.H. A sprayable protective coating for marble with water-repellent and anti-graffiti properties. Prog. Org. Coat. 2016, 101, 359–366. [Google Scholar] [CrossRef]
- Meng, B.; Mueller, U.; Garcia, O.; Malaga, K. Performance of a new anti-graffiti agent used for immovable cultural heritage. Int. J. Archit. Herit. 2014, 8, 820–834. [Google Scholar] [CrossRef]
- Moura, A.; Flores-Colen, I.; de Brito, J. Study of the effect of three anti-graffiti products on the physical properties of different substrates. Constr. Build. Mater. 2016, 107, 157–164. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Sánchez-Cortés, S.; Oujja, M.; Castillejo, M.; Blanco-Varela, M.T. Effectiveness of antigraffiti treatments in connection with penetration depth. J. Cult. Herit. 2010, 11, 297–303. [Google Scholar] [CrossRef]
- García, O.; Rz-Maribona, I.; Gardei, A.; Riedl, M.; Vanhellemont, Y.; Santarelli, M.L.; Suput, J.S. Comparative study of the variation of the hydric properties and aspect of natural stone and brick after the application of 4 types of anti-graffiti. Mater. Constr. 2010, 60, 69–82. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Panas, I.; Svensson, J.-E.; Johansson, L.-G.; Blanco-Varela, M.T.; Martínez-Ramírez, S. Protective performances of two anti-graffiti treatments towards sulfite and sulfate formation in SO2 polluted model environment. Appl. Surf. Sci. 2010, 257, 852–856. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Blanco-Varela, M.T.; Martinez-Ramírez, S. Permanent anti-graffiti for artificial construction materials: Lime mortar and brick. In Proceedings of the International Congress on Science and Technology for the Conservation of Cultural Heritage, Santiago de Compostela, Spain, 2–5 October 2012; Rogerio-Candelera, M.A., Lazzari, M., Cano, E., Eds.; Taylor & Francis Group: London, UK, 2013; pp. 311–318. [Google Scholar]
- Neto, E.; Magina, S.; Camões, A.; Begonha, A.; Evtuguin, D.V.; Cachim, P. Characterization of concrete surface in relation to graffiti protection coatings. Constr. Build. Mater. 2016, 102, 435–444. [Google Scholar] [CrossRef]
- Malaga, K.; Mueller, U. Relevance of hydrophobic and oleophobic properties of antigraffiti systems on their cleaning efficiency on concrete and stone surfaces. J. Mater. Civ. Eng. 2013, 25, 755–762. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Jacobs, R.M.J.; Viles, H.A. Weathering of two anti-graffiti protective coatings on concrete paving slabs. Coatings 2017, 7, 1. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Simbirtseva, G.V.; Nazarov, V.G.; Stolyarov, V.P.; Dubois, M.; Peyroux, J. Enhanced anti-graffiti or adhesion properties of polymers using versatile combination of fluorination and polymer grafting. Prog. Org. Coat. 2015, 88, 127–136. [Google Scholar] [CrossRef]
- Rossi, S.; Fedel, M.; Petrolli, S.; Deflorian, F. Behaviour of different removers on permanent anti-graffiti organic coatings. J. Build. Eng. 2016, 5, 104–113. [Google Scholar] [CrossRef]
- Calia, A.; Laurenzi Tabasso, M.; Mecchi, A.M.; Quarta, G. The Study of Stone for Conservation Purposes: Lecce Stone (Southern Italy). Geol. Soc. Lond. Spec. Publ. 2013, 391, 139–156. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Matera, L.; Sileo, M. The assessment of protection treatments on highly porous stones with relation to the sustainability of the interventions on the historical built heritage. In Proceedings of the La Conservazione del Patrimonio Architettonico All’aperto: Superfici, Strutture, Finiture e Contesti, Bressanone, Italy, 10–13 July 2012; Biscontin, G., Driussi, G., Eds.; Edizioni Arcadia Ricerche: Marghera-Venezia, Italy, 2012; pp. 477–487. [Google Scholar]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Zanchi, C. Water-repellent properties of fluoroelastomers on a very porous stone: Effect of the application procedure. Prog. Org. Coat. 2013, 76, 495–503. [Google Scholar] [CrossRef]
- Lettieri, M.; Calia, A.; Licciulli, A.; Marquardt, A.E.; Phaneuf, R.J. Nanostructured TiO2 for stone coating: Assessing compatibility with basic stone’s properties and photocatalytic effectiveness. Bull. Eng. Geol. Environ. 2015. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Surface characterization and effectiveness evaluation of anti-graffiti coatings on highly porous stone materials. Appl. Surf. Sci. 2014, 288, 466–477. [Google Scholar] [CrossRef]
- UNI 10921 Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Prodotti Idrorepellenti—Applicazione su Provini e Determinazione in Laboratorio delle loro Caratteristiche; Ente Italiano di Normazione: Milan, Italy, 2001. (In Italian)
- EN 15886 Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces; CEN (European Committee for Standardization): Brussels, Belgium, 2010.
- EN ISO 11664–4 Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space; CEN (European Committee for Standardization): Brussels, Belgium, 2011.
- Lesar, B.; Pavlič, M.; Petrič, M.; Škapin, A.S.; Humar, M. Wax treatment of wood slows photodegradation. Polym. Degrad. Stab. 2011, 96, 1271–1278. [Google Scholar] [CrossRef]
- Maxová, I.; Šlesinger, R.; Kubová, O. Tests of some antigraffiti systems for preservation of sandstone monuments. In Proceedings of the 7th European Conference “SAUVEUR” SAFEGUARDED CULTURAL HERITAGE-Understanding & Viability for the Enlarged Europe, Prague, Czech Republic, 31 May–3 June 2006.
- Calia, A.; De Benedetto, G.E.; Lettieri, M.; Masieri, M.; Quarta, G. Risultati Preliminari di uno Studio sulla Compatibilità ed Efficacia di Protettivi Anti-Graffiti su Pietra di Lecce. In IGIIC, Lo Stato dell’Arte 3; Nardini Editore: Firenze, Italy, 2005; pp. 52–59. (In Italian) [Google Scholar]
- Carvalhão, M.; Dionísio, A. Evaluation of mechanical soft-abrasive blasting and chemical cleaning methods on alkyd-paint graffiti made on calcareous stones. J. Cult. Herit. 2015, 16, 579–590. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; D’Arienzo, M.; Di Credico, B.; Goidanich, S.; Morazzoni, F.; Simonutti, R.; Toniolo, L. Efficient self-cleaning treatments for built heritage based on highly photo-active and well-dispersible TiO2 nanocrystals. Microchem. J. 2016, 126, 54–62. [Google Scholar] [CrossRef]

| Product | Chemical Composition 1 | Density 1 (g/m3) | pH 1 | Tg 2 (°C) | Tm 2 (°C) |
|---|---|---|---|---|---|
| AG1 | Water emulsion of polymer waxes | 0.980 | 9–10.5 | 41 ± 2 | 65 ± 4 |
| AG2a | Acrylic-fluorinated copolymers in water emulsion | 1.008 | 5 | 53 ± 2 | 72 ± 2 |
| AG2b | Water emulsion of polymer waxes added to acrylic-fluorinated resins | 1.002 | 7 | 35 ± 1 | 58 ± 1 |
| R1 | Dipropyleneglycol methyl ether (>90%) | 0.050 | 6–8 | Not applicable | Not applicable |
| R2 | Mixture of surfactants and solvents | 0.980 | 7 | Not applicable | Not applicable |
| Paint | Methyl-methacrylate resin and pigments | – | – | > 100 | – |
| Samples | Protection | Staining | Cleaning |
|---|---|---|---|
| AG1 | 100 g/m2 AG1 anti-graffiti (by brush) | Orange acrylic paint (2 coats by spraying) | R1 remover 15 min + brushing under running tap water at 60 °C (repeated twice) |
| AG0-1 | – | Orange acrylic paint (2 coats by spraying) | R1 remover 15 min + brushing under running tap water at 60 °C (repeated twice) |
| AG2 | 140 g/m2 AG2a primer (by brush) +24 h in laboratory +80 g/m2 AG2b anti-graffiti (by brush) | Orange acrylic paint (2 coats by spraying) | R2 remover 15 min + brushing under running tap water at 60 °C (repeated twice) |
| AG0-2 | – | Orange acrylic paint (2 coats by spraying) | R2 remover 15 min + brushing under running tap water at 60 °C (repeated twice) |
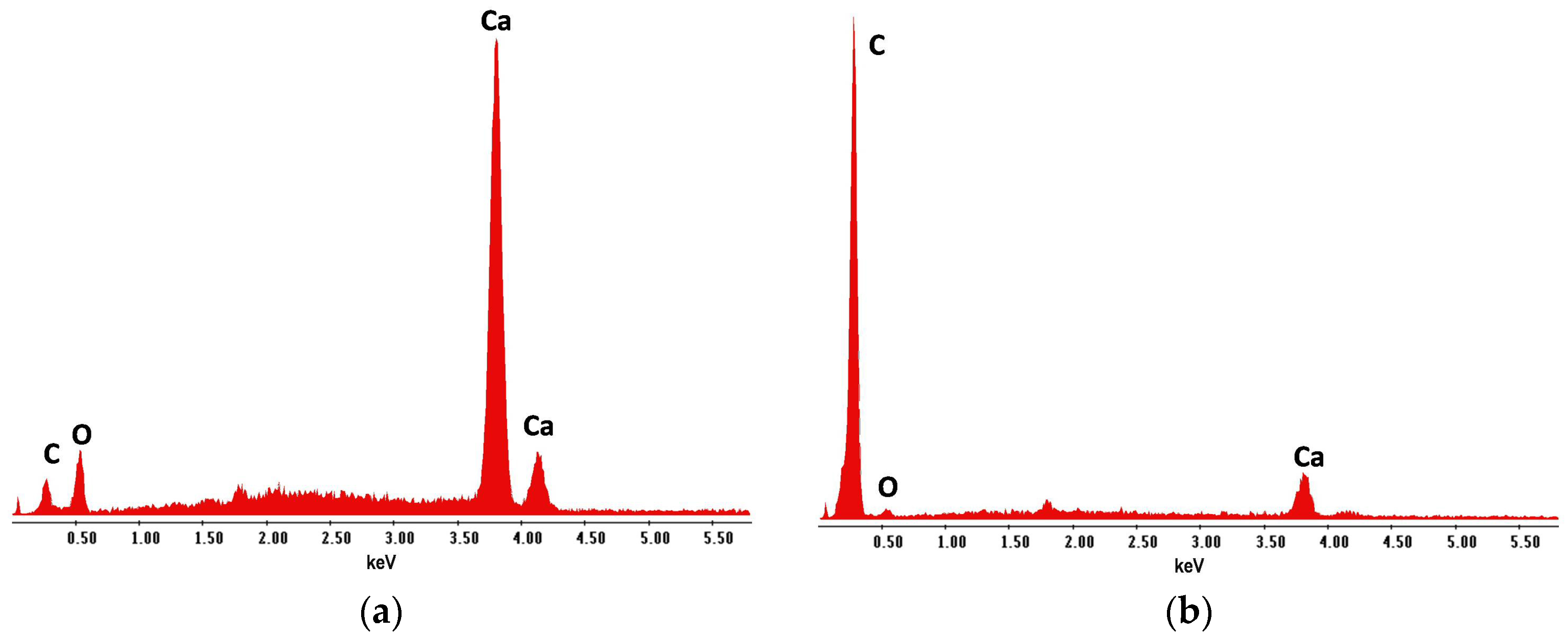
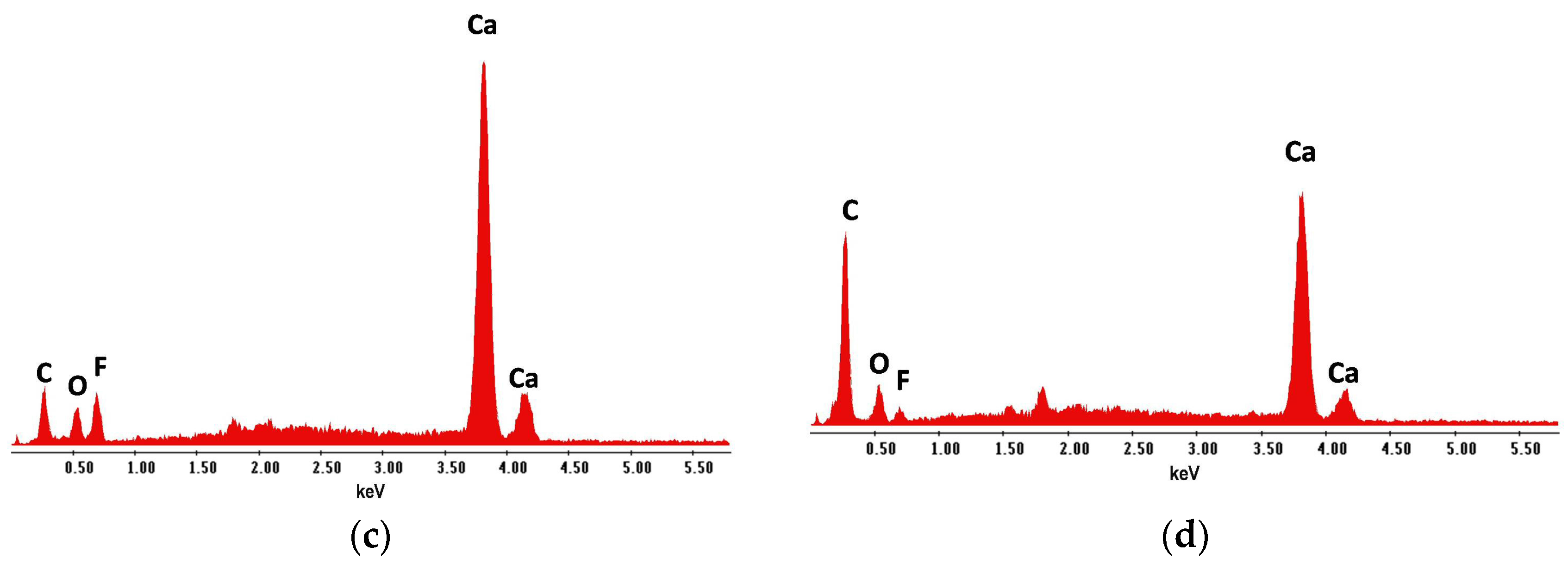
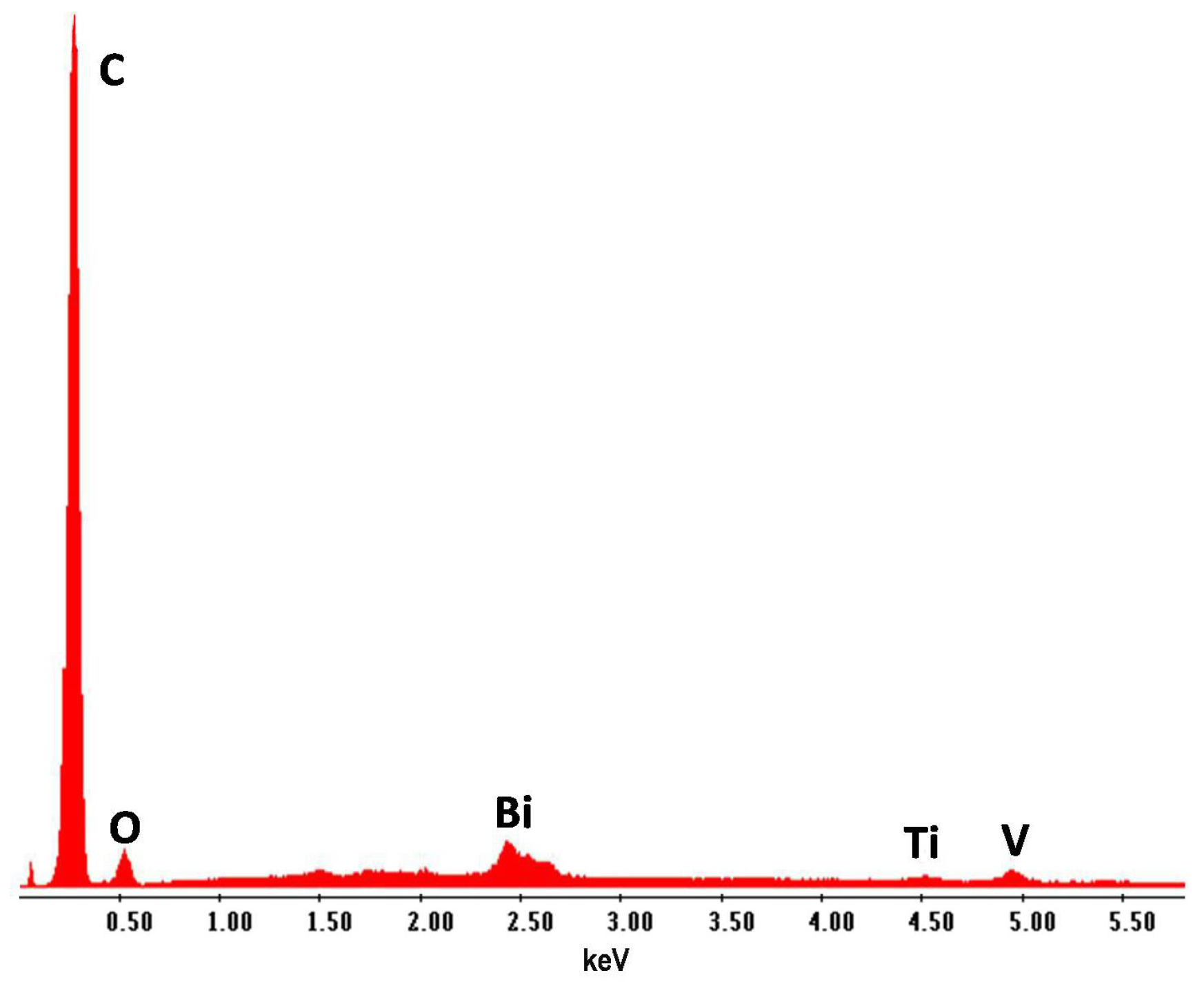
| Coating | Marker Elements 1 | Characteristic X-ray Line Energies (keV) |
|---|---|---|
| – 2 | Ca | 3.69; 4.01 |
| AG1 | C | 0.28 |
| AG2a | F | 0.67 |
| AG2b | C | 0.28 |
| Paint | Bi | 2.42 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masieri, M.; Lettieri, M. Influence of the Distribution of a Spray Paint on the Efficacy of Anti-Graffiti Coatings on a Highly Porous Natural Stone Material. Coatings 2017, 7, 18. https://doi.org/10.3390/coatings7020018
Masieri M, Lettieri M. Influence of the Distribution of a Spray Paint on the Efficacy of Anti-Graffiti Coatings on a Highly Porous Natural Stone Material. Coatings. 2017; 7(2):18. https://doi.org/10.3390/coatings7020018
Chicago/Turabian StyleMasieri, Maurizio, and Mariateresa Lettieri. 2017. "Influence of the Distribution of a Spray Paint on the Efficacy of Anti-Graffiti Coatings on a Highly Porous Natural Stone Material" Coatings 7, no. 2: 18. https://doi.org/10.3390/coatings7020018







